Abstract
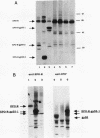
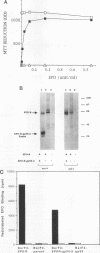
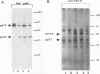
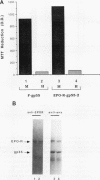
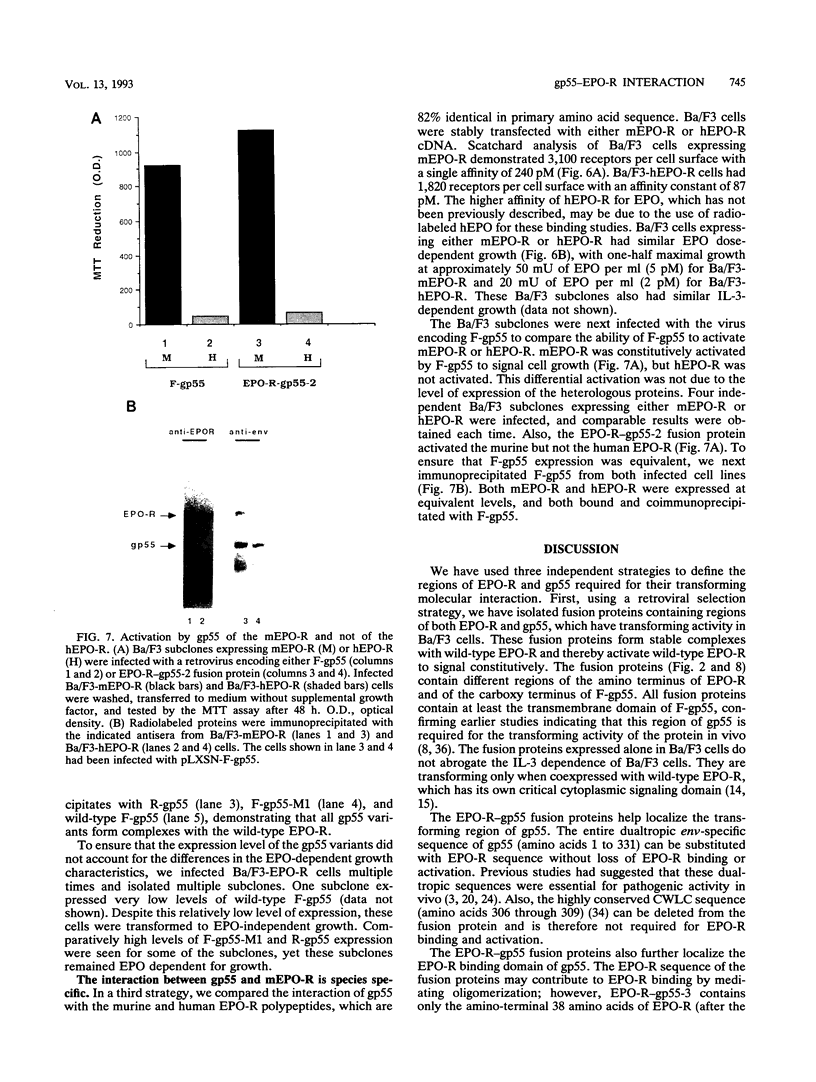
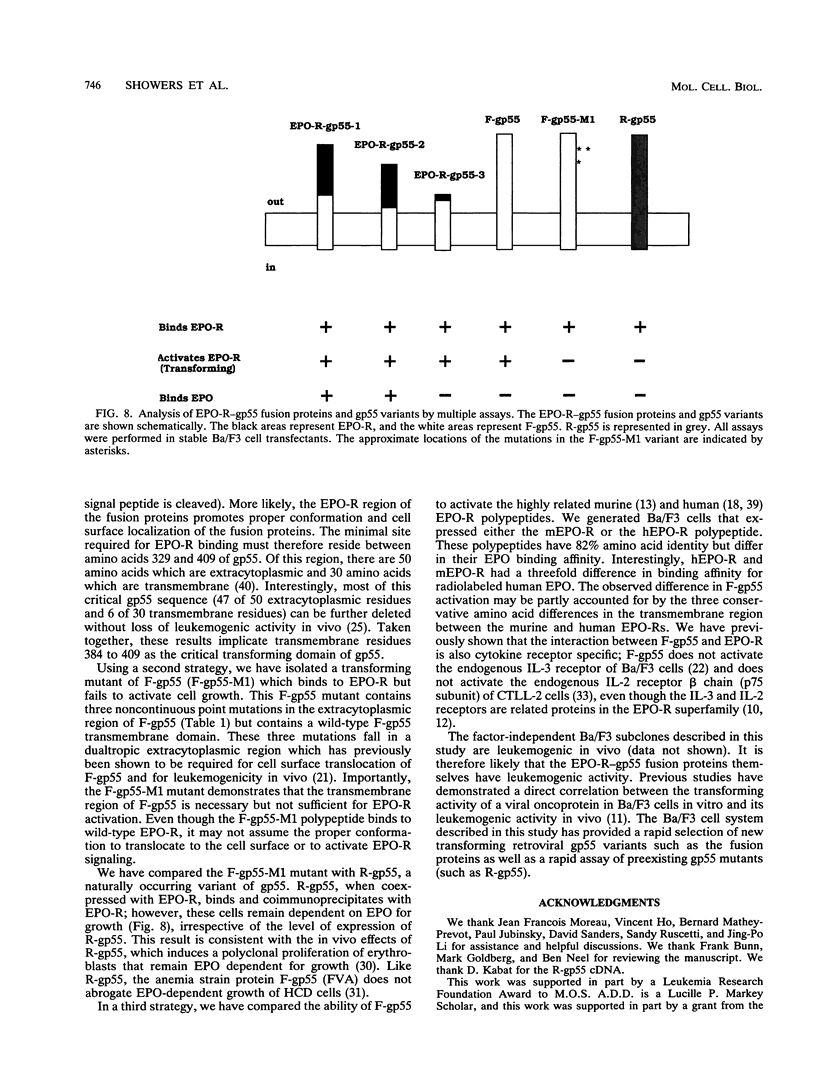
The Friend spleen focus-forming virus (SFFV) gp55 glycoprotein binds to the erythropoietin receptor (EPO-R), causing constitutive receptor signaling and the first stage of Friend erythroleukemia. We have used three independent strategies to further define this transforming molecular interaction. First, using a retroviral selection strategy, we have isolated the cDNAs encoding three fusion polypeptides containing regions of both EPO-R and gp55. These fusion proteins, like full-length gp55, transformed the Ba/F3 factor-dependent hematopoietic cell line and localized the transforming activity of gp55 to its transmembrane domain. Second, we have isolated a mutant of gp55 (F-gp55-M1) which binds, but fails to activate, EPO-R. We have compared the transforming activity of this gp55 mutant with the EPO-R-gp55 fusion proteins and with other variants of gp55, including wild-type polycythemia Friend gp55 and Rauscher gp55. All of the fusion polypeptides and mutant gp55 polypeptides were expressed at comparable levels, and all coimmunoprecipitated with wild-type EPO-R, but only the Friend gp55 and the EPO-R-gp55 fusion proteins constitutively activated wild-type EPO-R. Third, we have examined the specificity of the EPO-R-gp55 interaction by comparing the differential activation of murine and human EPO-R by gp55. Wild-type gp55 had a highly specific interaction with murine EPO-R; gp55 bound, but did not activate, human EPO-R.
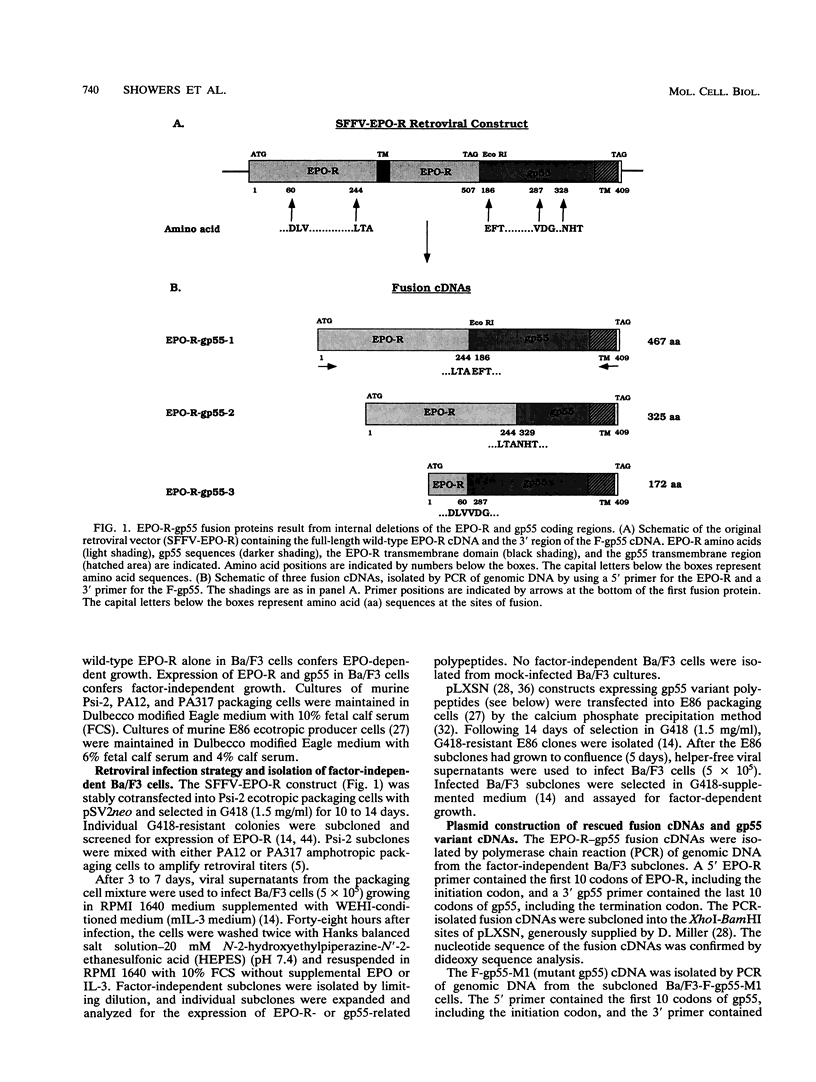
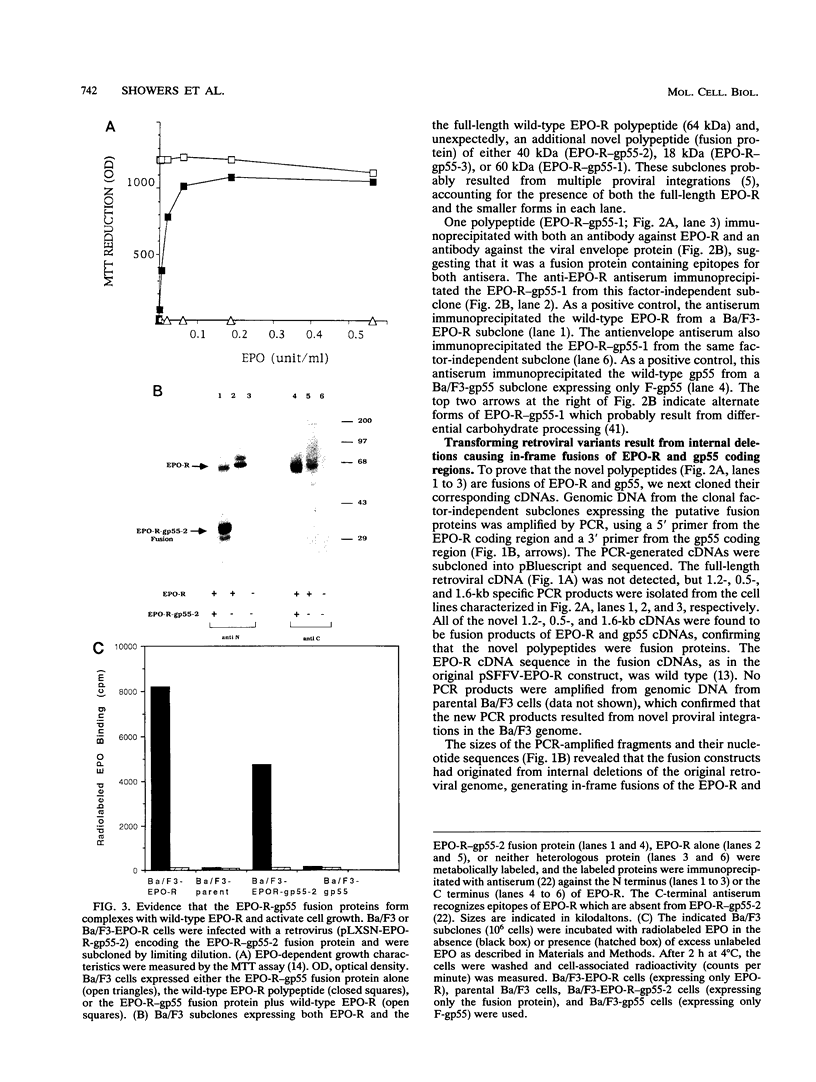
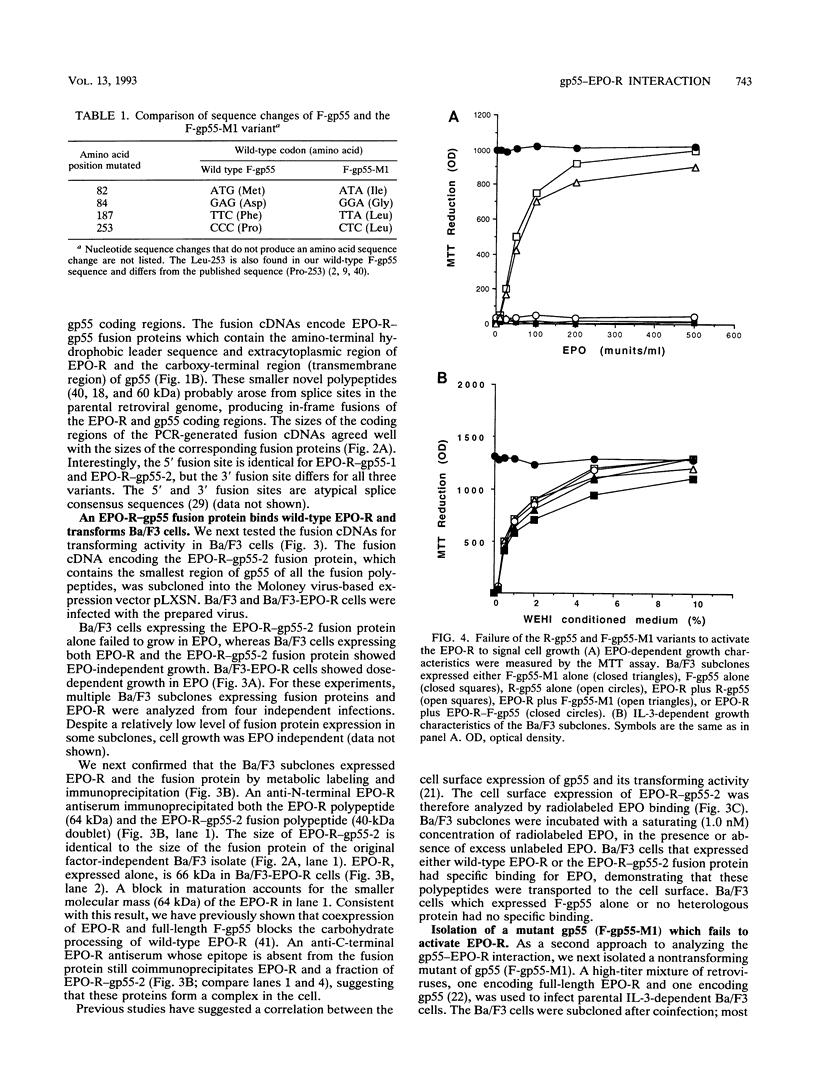
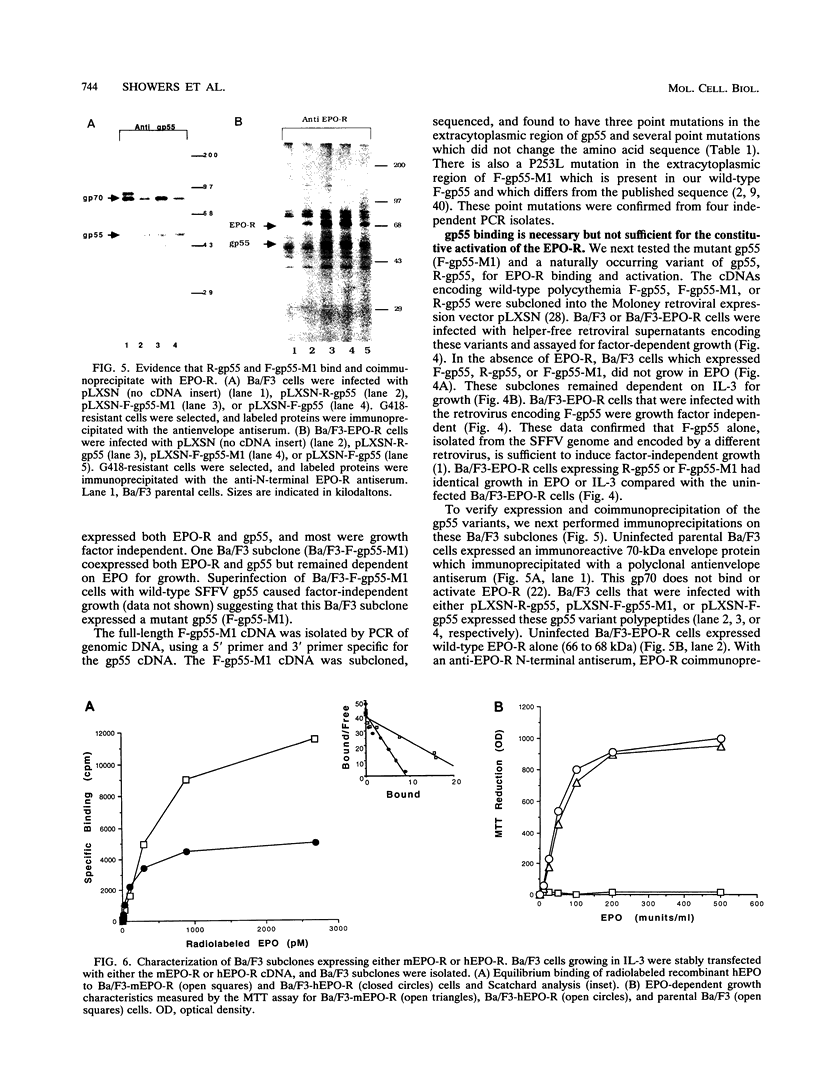
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S., Suda Y., Furuta Y., Yagi T., Takeda N., Watanabe N., Nagayoshi M., Ikawa Y. Env-derived gp55 gene of Friend spleen focus-forming virus specifically induces neoplastic proliferation of erythroid progenitor cells. EMBO J. 1990 Jul;9(7):2107–2116. doi: 10.1002/j.1460-2075.1990.tb07379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanuma H., Watanabe N., Nishi M., Ikawa Y. Requirement of the single base insertion at the 3' end of the env-related gene of Friend spleen focus-forming virus for pathogenic activity and its effect on localization of the glycoprotein product (gp55). J Virol. 1989 Nov;63(11):4824–4833. doi: 10.1128/jvi.63.11.4824-4833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David Y., Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991 Sep 6;66(5):831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Boswell B. A., Kabat D. Molecular cloning of biologically active Rauscher spleen focus-forming virus and the sequences of its env gene and long terminal repeat. J Virol. 1984 Sep;51(3):695–705. doi: 10.1128/jvi.51.3.695-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall N., Lacombe C., Muller O., Gisselbrecht S., Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells). Cross-linking of erythropoietin with the spleen focus-forming virus envelope protein. J Biol Chem. 1991 Aug 25;266(24):16015–16020. [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Jackson P. K., Bernards A., Baltimore D. Nonmyristoylated Abl proteins transform a factor-dependent hematopoietic cell line. Mol Cell Biol. 1992 Apr;12(4):1864–1871. doi: 10.1128/mcb.12.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliniak B. C., Kabat D. Leukemogenic membrane glycoprotein encoded by Friend spleen focus-forming virus: transport to cell surfaces and shedding are controlled by disulfide-bonded dimerization and by cleavage of a hydrophobic membrane anchor. J Virol. 1989 Sep;63(9):3561–3568. doi: 10.1128/jvi.63.9.3561-3568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoatlin M. E., Kozak S. L., Lilly F., Chakraborti A., Kozak C. A., Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. S., D'Andrea A. D., Haines L. L., Wong G. G. Human erythropoietin receptor: cloning, expression, and biologic characterization. Blood. 1990 Jul 1;76(1):31–35. [PubMed] [Google Scholar]
- Kilpatrick D. R., Srinivas R. V., Compans R. W. The spleen focus-forming virus envelope glycoprotein is defective in oligomerization. J Biol Chem. 1989 Jun 25;264(18):10732–10737. [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991 May;65(5):2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Longmore G. D., Lodish H. F. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogene. Cell. 1991 Dec 20;67(6):1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Kabat D. A weakly pathogenic Rauscher spleen focus-forming virus mutant that lacks the carboxyl-terminal membrane anchor of its envelope glycoprotein. J Virol. 1985 Mar;53(3):990–993. doi: 10.1128/jvi.53.3.990-993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar M. K., Cho C. L., Fox M. T., Eckner K. L., Kozak S., Kabat D., Geib R. W. Mutations in the env gene of friend spleen focus-forming virus overcome Fv-2r-mediated resistance to Friend virus-induced erythroleukemia. J Virol. 1992 Jun;66(6):3652–3660. doi: 10.1128/jvi.66.6.3652-3660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Janesch N. J., Chakraborti A., Sawyer S. T., Hankins W. D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990 Mar;64(3):1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Sitbon M., d'Auriol L., Ellerbrok H., André C., Nishio J., Perryman S., Pozo F., Hayes S. F., Wehrly K., Tambourin P. Substitution of leucine for isoleucine in a sequence highly conserved among retroviral envelope surface glycoproteins attenuates the lytic effect of the Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5932–5936. doi: 10.1073/pnas.88.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Compans R. W. Membrane association and defective transport of spleen focus-forming virus glycoproteins. J Biol Chem. 1983 Dec 10;258(23):14718–14724. [PubMed] [Google Scholar]
- Srinivas R. V., Kilpatrick D. R., Tucker S., Rui Z., Compans R. W. The hydrophobic membrane-spanning sequences of the gp52 glycoprotein are required for the pathogenicity of Friend spleen focus-forming virus. J Virol. 1991 Oct;65(10):5272–5280. doi: 10.1128/jvi.65.10.5272-5280.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Nishi M., Ikawa Y., Amanuma H. A deletion in the Friend spleen focus-forming virus env gene is necessary for its product (gp55) to be leukemogenic. J Virol. 1990 Jun;64(6):2678–2686. doi: 10.1128/jvi.64.6.2678-2686.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Nishi M., Ikawa Y., Amanuma H. Conversion of Friend mink cell focus-forming virus to Friend spleen focus-forming virus by modification of the 3' half of the env gene. J Virol. 1991 Jan;65(1):132–137. doi: 10.1128/jvi.65.1.132-137.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Penny L. A., Deaven L. L., Forget B. G., Jenkins R. B. The gene for the human erythropoietin receptor: analysis of the coding sequence and assignment to chromosome 19p. Blood. 1990 Jul 1;76(1):24–30. [PubMed] [Google Scholar]
- Wolff L., Scolnick E., Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3' gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Zimmers T., Neumann D., Longmore G., Yoshimura Y., Lodish H. F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992 Jun 5;267(16):11619–11625. [PubMed] [Google Scholar]
- Zon L. I., Moreau J. F., Koo J. W., Mathey-Prevot B., D'Andrea A. D. The erythropoietin receptor transmembrane region is necessary for activation by the Friend spleen focus-forming virus gp55 glycoprotein. Mol Cell Biol. 1992 Jul;12(7):2949–2957. doi: 10.1128/mcb.12.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]