Abstract
Objective
To describe symptoms, physical exam findings, and set point viral load associated with acute HIV seroconversion in a heterosexual cohort of discordant couples in Zambia.
Design
We followed HIV serodiscordant couples in Lusaka, Zambia from 1995–2009 with HIV testing of negative partners and symptom inventories 3-monthly, and physical examinations annually.
Methods
We compared prevalence of self-reported or treated symptoms (malaria syndrome, chronic diarrhea, asthenia, night sweats, and oral candidiasis) and annual physical exam [PE] findings (unilateral or bilateral neck, axillary, or inguinal adenopathy; and dermatosis) in seroconverting versus HIV-negative or HIV-positive intervals, controlling for repeated observations, age, and sex. A composite score comprised of significant symptoms and PE findings predictive of seroconversion versus HIV-negative intervals was constructed. We modeled the relationship between number of symptoms and PE findings at seroconversion and log set-point viral load [VL] using linear regression.
Results
2,388 HIV-negative partners were followed for a median of 18 months; 429 seroconversions occurred. Neither symptoms nor PE findings were reported for most seroconverters. Seroconversion was significantly associated with malaria syndrome among non-diarrheic patients (adjusted odds ratio [aOR]=4.0) night sweats (aOR=1.4), and bilateral axillary (aOR = 1.6), inguinal (aOR=2.2), and neck (aOR=2.2) adenopathy relative to HIV-negative intervals. Median number of symptoms was positively associated with set-point VL (p<0.001).
Conclusions
Though most acute and early infections were asymptomatic, malaria syndrome was more common and more severe during seroconversion compared with HIV-negative and HIV-positive intervals. When present, symptoms and physical exam findings were non-specific and associated with higher set point viremia.
Keywords: HIV, seroconversion syndrome, set point HIV viral load
INTRODUCTION
Identifying acute HIV infection is of interest clinically, for studies or pathogenesis, for HIV prevention and vaccine development, and for surveillance [1]. Manifestations of chronic HIV infection differ substantially in Africa compared with developed countries [2,3], and differ between East-Central and Southern African cohorts [4–6]. Similarly, there are several factors impacting early infection that differ based on population genetics and the subtype of virus that predominates within a particular region. Studies in newly infected Zambian subjects have identified a unique set of favorable HLA class I alleles that modulate both peak and early set point viral load [7,8] and, mutations, selected in a chronically infected partner, that allow escape from immune pressure directed by these alleles, have also been shown to reduce early set-point viral load in the partner to whom they transmit [9]. In the Zambian cohort under study here, genotypic analyses of the viruses that initiate infection and the viral quasispecies in their transmitting partner, have shown that in approximately 90% of transmissions a single genetic variant establishes infection [10,11]. Despite this, in a recent study of primary HIV-1 subtype C infection, a high proportion of individuals who maintained high plasma HIV-1 RNA load after acute infection were identified [12]. Thus, understanding the early events of infection, including initial viral pathogenesis and early host immune responses may enable new vaccine strategies.
To date, most descriptions of the clinical features of seroconverting persons have been reported from countries with predominately subtype B HIV epidemics [13,14], and the majority of previous studies of acute infection recruited symptomatic study populations, with few notable exceptions [15], and thus give a biased view of acute infection. We used data from a large cohort of HIV-discordant couples in Lusaka, Zambia, where HIV-subtype C predominates, to characterize self-reported and treated symptoms and physical exam findings associated with HIV seroconversion. We further analyzed the relationship between the number of symptoms and physical exam findings at HIV seroconversion and set point HIV viral load.
METHODS
Patient population
The Zambia-Emory HIV Research Project (ZEHRP) provides HIV counseling and testing to couples in Zambia; from 1995 – 2009, couples in whom one partner was HIV-positive and one partner was HIV-negative (i.e., HIV serodiscordant couples) were enrolled in an ongoing prospective cohort of discordant couples [16–18]. As part of the follow-up activities in the research studies, HIV-negative partners attended a study visit every 3 months [19]. At each visit, chart review and interview were used to inventory self-reported or treated symptoms that occurred since the last 3-month visit. HIV testing was conducted at each 3-month visit, using screening and confirmatory rapid tests, followed by another rapid test for those with discrepant or indeterminate confirmatory results [20]. Once yearly, and at any visit where a new infection was detected, a complete physical examination was performed. Viral load testing began in 1998 when facilities for plasma banking were installed.
Measures
For the purpose of this analysis, we considered 5 self-reported or treated symptoms (malaria syndrome, chronic diarrhea, asthenia, night sweats, and oral candidiasis) and 7 physical exam findings (neck, axillary, or inguinal adenopathy, each unilateral or bilateral; and dermatosis) as possible predictors of HIV seroconversion. Malaria syndrome was defined as any febrile-like illness treated with anti-malarials. The selection of these factors was based on previously reported descriptions of the HIV seroconversion syndrome in countries with predominantly subtype B epidemics [21–27].
HIV viral load was determined with the Amplicor HIV-1 Monitor test, v1.5 (Roche Molecular Diagnostics, USA). HIV subtype was determined as previously described [28]. Briefly, sequences of eight partially overlapping regions in gag, three in gp120, one in gp41, and one in the LTR were subjected to preliminary phylogenetic tree analyses to identify all circulating HIV-1 group M subtypes. Full-length and nonrecombinant reference sequences representing these subtypes were then obtained from the Los Alamos HIV Sequence Database and subjected to pairwise sequence comparisons in the genomic regions corresponding to the PCR amplification products.
Analysis
We conducted four primary analyses: (1) an analysis of self-reported or treated symptoms associated with HIV seroconversion (defined as being the first 3-month interval in which one or more positive HIV rapid test results were reported) relative to HIV-negative and HIV-positive intervals; (2) an analysis of physician-documented physical exam findings and ESR associated with HIV seroconversion relative to HIV-negative and HIV-positive intervals; (3) an evaluation of a clinical scoring system predictive of seroconversion; and (4) an analysis of the relationship between number of symptoms and physical exam findings at HIV seroconversion, and set point HIV RNA concentration (i.e., HIV viral load). For the analysis of symptoms associated with HIV seroconversion (considering both HIV-negative and HIV-positive intervals as referent groups, separately), we conducted logistic regression analyses with an outcome of HIV seroconversion, and the 5 self-reported symptoms as independent variables. Models controlled for age, sex, and repeated observations. PROC GENMOD (SAS Institute, Cary NC) was used to control for repeated observations of individuals, and robust variance estimates were used to calculate 95% confidence intervals for the adjusted odds ratios. Because we considered all of the independent variables to be plausibly associated with our outcome of seroconversion, we did not reduce the model by removing non-significant factors. We checked for all 2-way associations using a forward selection procedure with an entry criterion of α = 0.002 (reflecting a Bonferroni-type correction for testing of 28 possible 2-way interactions). For the analysis of physician-documented physical exam findings associated with HIV seroconversion, a similar analytic approach was used, but the total number of intervals of observation was lower because physical examinations occurred only annually and at the time of HIV seroconversion.
Index of significantly associated symptoms and physical exam findings
Symptoms and physical exam findings found to be significant in the multivariate models of seroconverting versus HIV-negative intervals were used to construct an additive score. This analysis was limited to data from visits with both symptom inventories and physical exams. Each symptom or physical exam finding contributed 1 point to the score. For each cutoff level of number of symptoms, sensitivity, specificity, positive predictive value, and negative predictive value were calculated.
For the analysis of the relationship between number of symptoms and physical exam findings and set-point HIV viral load, we performed new HIV viral load tests on stored specimens. We considered a viral load to represent the set-point viral load if it met three criteria: (1) taken > 30 days after the first positive HIV antibody test; (2) taken < 3 years after the first positive HIV antibody test; and (3) taken before the initiation of antiretroviral medications. Where multiple candidate set-point viral load values were available, we used the earliest value that met all criteria. PROC GLM (SAS Institute, Cary NC) was used to conduct an analysis of variance, with the outcome being the log10 of the viral load, and the independent variable being the number of self-reported symptoms, controlling for sex and year of first positive HIV test. The results are reported as a p-value from the Type III analysis. Type III partial sum of squares were chosen because our data were balanced, and all variables were included in the linear model.
RESULTS
Cohort
A total of 2,388 HIV-negative partners were followed for a median of 18 months (IQR, 6–42 months for those who never seroconverted, and 6–36 months for seroconverters). A total of 429 seroconversions occurred, and of those, 387 were diagnosed based on an HIV antibody test, 41 were diagnosed based on a positive p24 test prior to development of a positive antibody test, and 1 was diagnosed by a positive HIV RNA test prior to development of a positive antibody test. 417 (97%) had a 3-monthly visit with complete symptom data and 361 (84%) had a physical exam at the time of detection of the new infection. 291 seroconverters (68%) had a followup viral load which met criteria for a set-point viral load, and associated symptom and physical exam data. Nonseroconverters contributed 15,754 3-monthly visits with complete symptom data and 7,833 interval visits with complete physical examination data. Subsequent seroconverters contributed 2,920 HIV-negative interval visits with complete symptom data, and 1,971 HIV-negative interval visits with complete physical examination data. HIV-positive subjects contributed 12,857 HIV-positive interval visits with complete symptom data, and 7,724 HIV-positive interval visits with complete physical examination data. Seroconverters contributed 5,332 HIV-positive post-seroconversion interval visits with complete symptom data, and 1,550 HIV-positive interval visits with complete physical examination data.
The median age of seroconverters was 28, which was significantly different from the median age of HIV-negative (31) and HIV-positive intervals (30). Women were significantly younger than men for all intervals. Median erythrocyte sedimentation rate (ESR) for seroconverting intervals was 29, which was significantly different from the median ESR of HIV-negative (13) and HIV-positive intervals (33). Women had consistently and significantly higher ESR for all intervals compared with men.
Of the 429 seroconverters included in the analysis, subtype data were available for 402 (93.7% of all seroconverters). Among those with available data, 391 (97.3%), were subtype C, 4 (1.0%) were subtype A, 2 (0.5%) were subtype G, 2 (0.5%) were subtype J, 1 (0.2%) was subtype C/G, 1 (0.2%) was subtype D, and 1 (0.2%) was recombinant.
Prevalence of symptoms and physical exam findings
Symptoms, documented by self-report or treatment, were uncommon at most follow-up seroconversion visits or non-seroconversion visits (Table 1). Most symptoms were not severe enough to prompt a clinic visit for treatment. Some symptoms (night sweats, chronic diarrhea, oral candida) were early manifestations of HIV, with frequency and proportion treated similar among HIV-positive intervals compared with seroconvertors, and greater among seroconvertors compared with HIV-negative intervals. Malaria syndrome and asthenia were more common among seroconvertors compared with both HIV-negative and HIV-positive intervals. More than half of malaria syndrome events required treatment among seroconvertors. In contrast, more than half of malaria syndrome events among HIV-negative and HIV-positive intervals did not require treatment. All symptoms were more common among HIV-positive men than among HIV-positive women. A higher prevalence of symptoms among men was noted for night sweats and malaria in all serostatus groups, while oral candida was more common among HIV-negative women than among HIV-negative men.
Table 1.
Age, ESR, symptoms, and physical examination findings by HIV interval status and gender, Zambia, 1995–2009 (N = 429 seroconverters, N = 1959 HIV-negative partners, N = 1959 HIV-positive partners).
| HIV-negative intervals | Seroconverting intervals | HIV-positive intervals | p values* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Men | Women | Total | Men | Women | Total | Men | Women | |||||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Age | 31 | 25–37 | 34 | 29–40 | 27 | 22–33 | 28 | 24–34 | 31 | 36–28 | 25 | 22–30 | 30 | 25–35 | 33 | 39–28 | 27 | 22–32 | a, b, c, d, e |
| ESR* | 13 | 6–27 | 9 | 5–18 | 22 | 12–39 | 29 | 14–55 | 20 | 9–39 | 38 | 20–70 | 34 | 17–64 | 25 | 12–47 | 42 | 23–72 | a, b, c, d, e |
| Proportion of HIV-negative intervals | Proportion of seroconverting intervals | Proportion of HIV-positive intervals | |||||||||||||||||
| Symptoms | N = 18674 | % | N = 9900 | % | N = 8774 | % | N = 417 | % | N = 189 | % | N = 228 | % | N = 18189 | % | N = 7844 | % | N = 10345 | % | |
| Malaria syndrome | a, b, c, d, e | ||||||||||||||||||
| Treated† | 1379 | 7.4 | 681 | 6.9 | 698 | 8 | 100 | 24 | 54 | 28.6 | 46 | 20.2 | 2043 | 11.2 | 904 | 11.5 | 1139 | 11 | |
| Self-reported‡ | 1801 | 9.6 | 1015 | 10.3 | 786 | 9 | 93 | 22.3 | 49 | 25.9 | 44 | 19.3 | 2101 | 11.6 | 1114 | 14.2 | 987 | 9.5 | |
| Asthenia | a, b, e | ||||||||||||||||||
| Treated | 101 | 0.5 | 57 | 0.6 | 44 | 0.5 | 6 | 1.4 | 2 | 1.1 | 4 | 1.8 | 228 | 1.3 | 97 | 1.2 | 131 | 1.3 | |
| Self-reported | 1053 | 5.6 | 585 | 5.9 | 468 | 5.3 | 50 | 12.0 | 25 | 13.2 | 25 | 11.0 | 1465 | 8.1 | 769 | 9.8 | 696 | 6.7 | |
| Night sweats | a, c , d, e | ||||||||||||||||||
| Treated | 166 | 0.9 | 140 | 1.4 | 26 | 0.3 | 5 | 1.2 | 3 | 1.6 | 2 | 0.9 | 470 | 2.6 | 310 | 4.0 | 160 | 1.5 | |
| Self-reported | 611 | 3.3 | 463 | 4.7 | 148 | 1.7 | 30 | 7.2 | 21 | 11.1 | 9 | 3.9 | 985 | 5.4 | 637 | 8.1 | 348 | 3.4 | |
| Chronic diarrhea | a, e | ||||||||||||||||||
| Treated | 29 | 0.2 | 21 | 0.2 | 8 | 0.1 | 1 | 0.2 | 0 | 0.0 | 1 | 0.4 | 212 | 1.2 | 132 | 1.7 | 80 | 0.8 | |
| Self-reported | 49 | 0.3 | 28 | 0.3 | 21 | 0.2 | 8 | 1.9 | 3 | 1.6 | 5 | 2.2 | 194 | 1.1 | 109 | 1.4 | 85 | 0.8 | |
| Oral Candidiasis | a, c, e | ||||||||||||||||||
| Treated | 5 | 0.0 | 5 | 0.1 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.4 | 87 | 0.5 | 51 | 0.7 | 36 | 0.3 | |
| Self-reported | 38 | 0.2 | 11 | 0.1 | 27 | 0.3 | 2 | 0.5 | 0 | 0.0 | 2 | 0.9 | 98 | 0.5 | 50 | 0.6 | 48 | 0.5 | |
| Physical examination findings | N = 9804 | % | N = 4846 | % | N = 4958 | % | N = 361 | % | N = 166 | % | N = 195 | % | N = 9274 | % | N = 4479 | % | N = 4795 | % | |
| Bilateral adenopathy | |||||||||||||||||||
| Axillary | 252 | 2.6 | 153 | 3.2 | 99 | 2.0 | 25 | 6.9 | 18 | 10.8 | 7 | 3.6 | 1705 | 18.4 | 1119 | 25.0 | 586 | 12.2 | a, b ,c, d, e |
| Inguinal | 1241 | 12.7 | 932 | 19.2 | 309 | 6.2 | 95 | 26.3 | 59 | 35.5 | 36 | 18.5 | 3539 | 38.2 | 2380 | 53.1 | 1159 | 24.2 | a, b, c, d, e |
| Neck | 86 | 0.9 | 39 | 0.8 | 47 | 0.9 | 12 | 3.3 | 5 | 3.0 | 7 | 3.6 | 538 | 5.8 | 275 | 6.1 | 263 | 5.5 | a, b |
| Unilateral adenopathy | |||||||||||||||||||
| Axillary | 469 | 4.8 | 392 | 8.1 | 77 | 1.6 | 21 | 5.8 | 14 | 8.4 | 7 | 3.6 | 1140 | 12.3 | 860 | 19.2 | 280 | 5.8 | b, c, d, e |
| Inguinal | 799 | 8.1 | 686 | 14.2 | 113 | 2.3 | 21 | 5.8 | 16 | 9.6 | 5 | 2.6 | 873 | 9.4 | 595 | 13.3 | 278 | 5.8 | b, c, d, e |
| Neck | 206 | 2.1 | 137 | 2.8 | 69 | 1.4 | 11 | 3.0 | 4 | 2.4 | 7 | 3.6 | 690 | 7.4 | 481 | 10.7 | 209 | 4.4 | b, c, e |
| Dermatosis | 564 | 5.8 | 409 | 8.4 | 155 | 3.1 | 14 | 3.9 | 6 | 3.6 | 8 | 4.1 | 795 | 8.6 | 498 | 11.1 | 297 | 6.2 | b, c, e |
a. Significant difference between HIV-negative and seroconverting intervals (p<0.05)
b. Significant difference between HIV-positive and seroconverting intervals (p<0.05)
c. Significant difference between men and women among HIV-negative intervals (p<0.05)
d. Significant difference between men and women among seroconverting intervals (p<0.05)
e. Significant difference between men and women among HIV-positive intervals (p<0.05)
Wilcoxon tests for continuous variables; chi-square tests for categorical variables
For ESR data, N = 956 HIV-negative intervals; N = 684 Seroconverting intervals; N = 956 HIV-positive intervals
Treated malaria = treated suspected or treated confirmed malaria
Self-reported malaria = self-reported suspected or self-reported confirmed malaria
Similarly, significant physical examination findings were not noted for most visits, whether seroconverting or non-seroconverting visits. Prevalence of adenopathy was lowest among HIV-negative intervals, intermediate among seroconverting intervals, and highest in HIV-positive intervals. (Table 1). Most adenopathies were significantly more common in men versus women regardless of serostatus interval.
Symptoms and physical examination findings associated with seroconversion: Relative to HIV-negative intervals in multivariate models, seroconversion was associated with malaria syndrome among non-diarrheic patients, night sweats, and bilateral axillary, inguinal, and neck adenopathy >1 cm. Relative to HIV-positive intervals in multivariate models, seroconversion was associated with asthenia, and malaria syndrome. Seroconverters were less likely than chronically infected men and women to have unilateral axillary and inguinal adenopathy, bilateral axillary and inguinal adenopathy, and dermatosis (Table 2).
Table 2.
Logistic regression models describing symptoms and physical examination findings, respectively, associated with HIV seroconversion intervals, Zambia, 1995–2009.
| Total | |||
|---|---|---|---|
| Adjusted odds ratio* | 95% CI | ||
| Lower | Upper | ||
| Symptoms Associated with Seroconversion vs HIV-negative intervals | |||
| Asthenia | 1.6 | 0.7 | 3.3 |
| Chronic diarrhea | 3.3 | 1.5 | 7.0 |
| Night sweats | 1.4 | 1.0 | 2.1 |
| Oral Candidiasis | 0.6 | 0.2 | 2.4 |
| Malaria syndrome | 3.9 | 3.2 | 4.7 |
| Physical Examination Findings Associated with Seroconversion vs HIV-negative intervals | |||
| Unilateral adenopathy | |||
| Axillary | 1.1 | 0.7 | 1.7 |
| Inguinal | 0.9 | 0.6 | 1.5 |
| Neck | 1.1 | 0.6 | 2.1 |
| Bilateral adenopathy | |||
| Axillary | 1.6 | 1.0 | 2.5 |
| Inguinal | 2.2 | 1.6 | 2.9 |
| Neck | 2.2 | 1.2 | 4.0 |
| Dermatosis | 0.6 | 0.4 | 1.1 |
| Symptoms Associated with Seroconversion vs HIV-positive intervals | |||
| Asthenia | 1.3 | 1.0 | 1.7 |
| Chronic diarrhea | 0.8 | 0.4 | 1.6 |
| Night sweats | 0.8 | 0.5 | 1.1 |
| Oral Candidiasis | 0.6 | 0.2 | 1.8 |
| Malaria syndrome | 2.9 | 2.4 | 3.6 |
| Physical Examination Findings Associated with Seroconversion vs HIV-positive intervals | |||
| Unilateral adenopathy | |||
| Axillary | 0.5 | 0.3 | 0.7 |
| Inguinal | 0.6 | 0.4 | 0.9 |
| Neck | 0.6 | 0.3 | 1.1 |
| Bilateral adenopathy | |||
| Axillary | 0.4 | 0.2 | 0.6 |
| Inguinal | 0.8 | 0.6 | 1.0 |
| Neck | 1.0 | 0.5 | 1.7 |
| Dermatosis | 0.5 | 0.3 | 0.9 |
Models adjusted for age, sex, and repeated observation of individuals
Evaluation of index of symptoms and physical examination findings as a predictor of seroconversion
The additive score comprised significant symptoms and physical exam findings from the multivariate analysis of seroconversion versus HIV-negative intervals: chronic diarrhea, night sweats, malaria syndrome, and any bilateral adenopathy. Of 7,701 total intervals included, 4,510 (58.6%) reported no symptoms, 3,191 (41.4%) reported 1 or more symptoms, 706 (9.2%) reported 2 or more symptoms, 79 (1.0%) reported 3 or more symptoms, and 6 (0.1%) reported all 4 symptoms. Of the total intervals, 350 were seroconverting intervals and 7,351 were non-seroconversion intervals. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for the score at each possible cutoff of number of symptoms. Within 226 seroconverting intervals, 1 or more symptoms were reported. Sensitivity for this cutoff level was 64.6%, specificity was 59.7%, the positive predictive value was 7.1%, and the negative predictive value was 97.3%. Sensitivity, specificity, PPV, and NPV were also determined at each cutoff of number of symptoms and by gender. Men had a higher sensitivity at all cutoffs relative to women (73.0% vs. 57.6% for 1 or more symptoms, respectively), and PPV varied by gender and cutoff (Table 3).
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive values for different cutoff levels of scores for physical exam findings and symptoms, Zambia, 1995–2009.
| All | |||||
|---|---|---|---|---|---|
| Number of symptoms and PE findings (cutoffs) | Proportion of seroconverting intervals in cutoff | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| 0 | 124/4510 | ||||
| ≥1 | 226/3191 | 64.6 | 59.7 | 7.1 | 97.3 |
| ≥2 | 81/706 | 23.1 | 91.5 | 11.5 | 96.2 |
| ≥3 | 11/79 | 3.1 | 99.1 | 13.9 | 95.6 |
| 4 | 2/6 | 0.6 | 99.9 | 33.3 | 95.5 |
| Men | |||||
|---|---|---|---|---|---|
| Number of symptoms and PE findings (cutoffs) | Proportion of seroconverting intervals in cutoff | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| 0 | 43/1909 | ||||
| ≥ 1 | 116/1833 | 73.0 | 52.1 | 6.3 | 97.7 |
| ≥ 2 | 48/511 | 30.2 | 87.1 | 9.4 | 96.6 |
| ≥ 3 | 10/63 | 6.3 | 98.5 | 15.9 | 95.9 |
| 4 | 1/4 | 0.6 | 99.9 | 25.0 | 95.8 |
| Women | |||||
|---|---|---|---|---|---|
| Number of symptoms and PE findings (cutoffs) | Proportion of seroconverting intervals in cutoff | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| 0 | 81/2601 | ||||
| ≥ 1 | 110/1358 | 57.6 | 66.9 | 8.1 | 96.9 |
| ≥ 2 | 33/195 | 17.3 | 95.7 | 16.9 | 95.8 |
| ≥ 3 | 1/16 | 0.5 | 99.6 | 6.3 | 95.2 |
| 4 | 1/2 | 0.5 | 100.0 | 50.0 | 95.2 |
Physical exam findings and symptoms include chronic diarrhea, malaria syndrome, night sweats, and any bilateral adenopathy
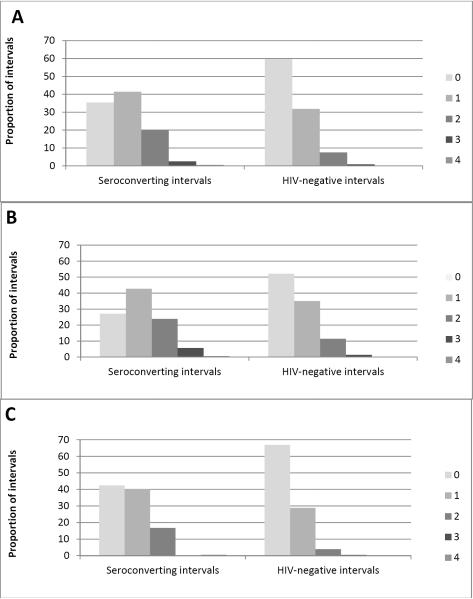
Proportion of intervals for each seroconverting and HIV-negative intervals were calculated by number of symptoms and physical exam findings. While most (60%) HIV-negative intervals exhibited no associated symptoms, 65% of seroconverting intervals exhibited ≥1 symptom, with more men reporting ≥1 symptom compared with women (73% vs. 58%, respectively) (Figure 1).
Figure 1.
Proportion of seroconverting or HIV-negative intervals by combined number of symptoms and physical exam findings for (A) all, (B) men and (C) women. Symptoms include chronic diarrhea, malaria syndrome, night sweats, and any bilateral adenopathy.
Association of symptoms of seroconversion and set point viral load
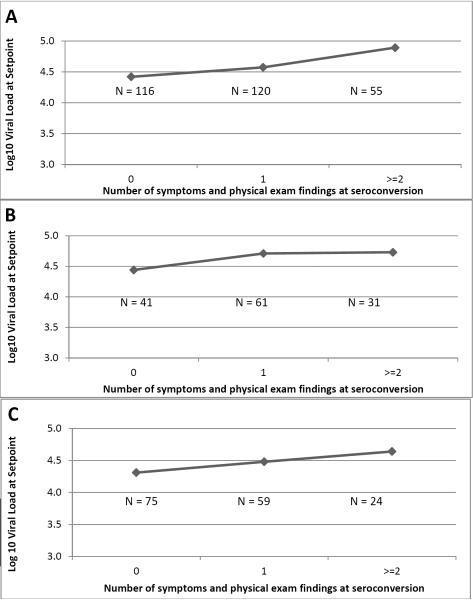
The mean number of significant symptoms and physical examination findings (malaria syndrome, chronic diarrhea, night sweats, and any bilateral adenopathy) exhibited was 0.8 (range, 0–4). Increasing number of these symptoms and physical examination findings was positively associated with a higher set point viral load for all seroconverters and for both men and women (Figure 2).
Figure 2.
Relationship between combined number of symptoms and physical exam findings and setpoint viral load among (A) all seroconverters (N = 291), (B) men (N = 133), and (C) women (N = 158). Physical exam findings and symptoms include chronic diarrhea, malaria syndrome, night sweats, and any bilateral adenopathy. Number of symptoms were are significantly different by setpoint viral load (Kruskal-Wallis Test p < 0.001) (A–C).
DISCUSSION
HIV seroconversion illness has been frequently described in countries with predominantly subtype B epidemics, but much less is reported about the clinical circumstances surrounding seroconversion in subtype C epidemics. Our report adds to the existing literature on this topic by examining data from a prospective cohort of discordant couples undergoing regular HIV screening, allowing comparison of symptoms and signs in HIV-negative, seroconverting, and HIV-positive intervals in both men and women. We have also further previous reports by considering some clinical features in a graded way.
Although malaria-like illness and adenopathy were more common in HIV seroconverters than non-seroconverters in Zambia, most seroconverters in Zambia were not symptomatic. This corroborates findings in cohort studies from A and D clade areas in Uganda [13] and from US cohorts of gay men with B clade HIV [15]. Reports of seroconversions identified during screening of at-risk patients seeking clinical care in the West not surprisingly have much higher rates of symptoms, since these symptoms are what prompted them to seek treatment. In these series, fever, rash, athralgias, malaise, fatigue, and night sweats are reported by half to 89% of patients [25] [24], with a `flu-like illness' often including many of these symptoms [29]. In a study of acutely infected men seeking treatment for sexually transmitted diseases in Malawi, participants reported fever (56%), headache (44%), body ache (44%), or abdominal pain (44%), while chronically infected men more often reported genital ulcer disease (81%) and inguinal adenopathy (44%)[21]. In a Kenyan cohort study, 50 of 72 seroconvertors enrolled sought health care prior to diagnosis, of whom 29 were treated for malaria.[30] Manifestations of chronic HIV infection differ in East-Central vs. Southern Africa [4], perhaps reflecting varying prevalences of pathogens such as tuberculosis in the environment. Our finding that most seroconversions in our Zambian cohort were asymptomatic may be because subtype C seroconversion are less likely to be symptomatic, because our sampling was not related to care-seeking behavior, or both.
Malaria syndrome was the most commonly exhibited symptom among all intervals in our cohort, with a significantly greater proportion of seroconverters exhibiting malaria syndrome versus HIV-negative or -positive intervals. Malaria syndrome may also be a culturally familiar – or culturally acceptable – marker for a collection of other symptoms suggestive of HIV infection, including fever, chills, and acute diarrhea [31]. A study of the relationship between falciparum malaria syndrome and HIV seropositivity in Zambia found that over 10% of patients presenting for suspect malaria syndrome were HIV seropositive without evidence of malaria syndrome infection [31]. The incidence of malaria syndrome was significantly higher for men versus women for all intervals, and notably so among seroconverting intervals, indicating that malaria syndrome may be more strongly associated with primary HIV infection in men versus women. An early study of malaria and HIV in Kigali, Rwanda showed that HIV antibodies were more prevalent in women from the urban center than in those from the outskirts while malaria parasites showed the opposite prevalence pattern; after stratifying by location, there was no association between HIV and the presence or degree of malaria parasitemia [32]. More recently however, malaria parasitemia has been reported to be associated with prevalent HIV infection in Malawi.[33]
Chronic diarrhea, either treated or self-reported, was seen more frequently in seroconverting and HIV-positive intervals versus HIV-negative intervals in bivariate analysis. In an earlier analysis of 74 seroconverting men in this cohort, acute diarrhea was more likely to be treated (18%) or reported (31%) compared with HIV-negative men (8% treated, 21% reported) or HIV-positive men (14% treated, 26% reported)[34].
Adenopathy has been commonly reported as a physical examination finding in patients with acute HIV infection [1,23,35,36]. We found that bilateral, but not unilateral, adenopathy was significantly more common in HIV seroconverters than HIV-negative intervals. All adenopathies were more common among HIV-positive intervals compared with seroconvertors, and – with the exception of neck nodes – were more common among men compared with women regardless of serostatus. Physical examination for adenopathies may be less sensitive in women due to increased amounts of adipose tissue and anatomical differences in the inguinal area.
ESR is a non-specific and evidently early marker for inflammation, as it was elevated in seroconvertors compared with HIV-negative intervals and only marginally higher in HIV-positive compared with seroconversion intervals. As previously reported, ESR was higher in women compared with men regardless of serostatus [4,37,38].
Scoring systems combining signs, symptoms, and behaviors have been evaluated in two publications from Africa [39,40]. Our study study, a cutoff of 1 or more symptoms and physical exam findings has a sensitivity of 64.6% and a specificity of 59.7%, but a very low positive predictive value of 7.1%. By gender, a cutoff of 1 or more symptoms and physical exam findings had a higher sensitivity in men versus women (73.0% vs. 57.6%). Even small increases in positive predictive value result in considerable decreases in sensitivity, leading to the accurate identification of fewer recent HIV seroconversions. Decreases in sensitivity must be weighed against increases in specificity and positive predictive value, which confer increased cost effectiveness.
We found significantly increasing set point viral load with increasing number of symptoms/physical exam findings, and noted that this relationship may be slightly more prominent for women. In a prospective cohort in San Francisco, increased number of symptoms associated with acute HIV infection was also associated with high initial viral load [41]. Importantly, higher number and severity of acute symptoms have also been shown to increase disease progression and mortality [40,42,43].
Our analysis was subject to several important limitations. First, we designated 3-monthly followup visits as seroconversion intervals or non-seroconversion intervals; transient signs may have been missed and symptoms occurring early in the 3 month period may have been subject to differential recall bias. This would artificially lower the prevalence of reported symptoms [44]. Though our intent was to blind clinicians to patient's serostatus, physical examinations conducted at a non-annual visit might have been conducted with knowledge that an HIV seroconversion was likely, leading to ascertainment bias. If such bias occurred, it would tend to over represent the associations of physical examination findings with seroconversion. Our results are not generalizable to all persons in Zambia, or to other geographic areas.
Our study also had significant strengths. We collected data from a large, stable cohort of discordant couples, and were therefore able to consistently collect data from a large group of HIV-negative persons with high risk for HIV infection. Screening for HIV infection was performed at regular intervals, independent of clinical suspicion of HIV infection, so ascertainment of seroconversion was unbiased. Our study thus allowed comparison of non-specific signs and symptoms of acute and chronic HIV to the `background' rate found in HIV-negative intervals, and to compare men and women.
CONCLUSIONS
According to our data, collection of self-reported symptoms and physical examination data are not likely to be productive strategies for targeting HIV screening in Zambia, though acute HIV infection may be an important differential for patients presenting with malaria syndrome and bilateral adenopathy. Implementation of existing approaches, including routine HIV counseling and testing of cohabiting couples and other sexual partnerships, remains an important element of a comprehensive strategy both for identifying those living with HIV infection, and reducing the risk of HIV transmission in serodiscordant couples.
Acknowledgments
Financial Support: This work was supported by funding from the US National Institutes of Health (RO1: MH66767, AI23980, AI40951, AI51231, HD40125; the AIDS International Training and Research Program (AITRP) FIC D43 TW001042, and the Social & Behavioral Core of the Emory Center for AIDS Research P30 AI050409), and the International AIDS Vaccine Initiative.
Footnotes
Author contributions: Patrick S. Sullivan, DVM, PhD: analyzed data, drafted manuscript
Ulgen Fideli, MSPH, MHS, PA-C: conceived analysis, analyzed data
Kristin M. Wall, MS: analyzed data, drafted manuscript
Elwyn Chomba, MD: conceived study, oversaw data collection, reviewed manuscript and gave critical input
Cheswa Vwalika, MBBS, MPH: oversaw data collection, reviewed manuscript and gave critical input
William Kilembe, MD: oversaw data collection, reviewed manuscript and gave critical input
Amanda Tichacek, MPH: data management, reviewed manuscript and gave critical input
Nicole Luisi, MPH: data management, reviewed manuscript and gave critical input
Joseph Mulenga, MD MBA: oversaw data collection, reviewed manuscript and gave critical input
Debrah Boeras, PhD: supervised viral load and clade typing, reviewed manuscript and gave critical input
Eric Hunter, PhD: conceived study, developed clade typing tools specific to Clades C and A areas, reviewed manuscript and gave critical input
Susan Allen, MD, MPH: conceived study, oversaw data collection, reviewed manuscript and gave critical input
Reference List
- 1.Pilcher CD, Eron JJ, Jr., Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lifson AR, Allen S, Wolf W, Serufilira A, Kantarama G, Lindan CP. Classification of HIV infection and disease in women from Rwanda. Evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med. 1995;122:262–270. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lindan CP, Allen S, Serufilira A, Lifson AR, Van de Perre P, Chen-Rundle A. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- 4.Modjarrad K, Zulu I, Karita E, Kancheya N, Funkhouser E, Allen S. Predictors of HIV Serostatus among HIV Discordant Couples in Lusaka, Zambia and Female Antenatal Clinic Attendants in Kigali, Rwanda. AIDS Res Hum Retroviruses. 2005;21:5–12. doi: 10.1089/aid.2005.21.5. [DOI] [PubMed] [Google Scholar]
- 5.Peters PJ, Karita E, Kayitenkore K, Meinzen-Derr J, Kim DJ, Tichacek A. HIV-infected Rwandan women have a high frequency of long-term survival. AIDS. 2007;21(Suppl 6):S31–37. doi: 10.1097/01.aids.0000299408.52399.e1. [DOI] [PubMed] [Google Scholar]
- 6.Peters PJ, Zulu I, Kancheya NG, Lakhi S, Chomba E, Vwalika C. Modified Kigali combined staging predicts risk of mortality in HIV-infected adults in Lusaka, Zambia. AIDS Res Hum Retroviruses. 2008;24:919–924. doi: 10.1089/aid.2007.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Cormier E, Gilmour J, Price MA, Prentice HA, Song W. Human leukocyte antigen variants B*44 and B*57 are consistently favorable during two distinct phases of primary HIV-1 infection in sub-Saharan Africans with several viral subtypes. J Virol. 2011;85:8894–8902. doi: 10.1128/JVI.00439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J, Malhotra R, Song W, Brill I, Hu L, Farmer PK. Human leukocyte antigens and HIV type 1 viral load in early and chronic infection: predominance of evolving relationships. PLoS One. 2011;5:e9629. doi: 10.1371/journal.pone.0009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goepfert PA, Lumm W, Farmer P, Matthews P, Prendergast A, Carlson JM. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008;205:1009–1017. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 12.Novitsky V, Ndung'u T, Wang R, Bussmann H, Chonco F, Makhema J. Extended high viremics: a substantial fraction of individuals maintain high plasma viral RNA levels after acute HIV-1 subtype C infection. AIDS. 2011;25:1515–1522. doi: 10.1097/QAD.0b013e3283471eb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan D, Mahe C, Whitworth J. Absence of a recognizable seroconversion illness in Africans infected with HIV-1. Aids. 2001;15:1575–1576. doi: 10.1097/00002030-200108170-00016. [DOI] [PubMed] [Google Scholar]
- 14.Novitsky V, Wang R, Kebaabetswe L, Greenwald J, Rossenkhan R, Moyo S. Better control of early viral replication is associated with slower rate of elicited antiviral antibodies in the detuned enzyme immunoassay during primary HIV-1C infection. J Acquir Immune Defic Syndr. 2009;52:265–272. doi: 10.1097/QAI.0b013e3181ab6ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaslow RA, Phair JP, Friedman HB, Lyter D, Solomon RE, Dudley J. Infection with the human immunodeficiency virus: clinical manifestations and their relationship to immune deficiency. A report from the Multicenter AIDS Cohort Study. Annals of Internal Medicine. 1987;107:474–480. doi: 10.7326/0003-4819-107-4-474. [DOI] [PubMed] [Google Scholar]
- 16.Chomba E, Allen S, Kanweka W, Tichacek A, Cox G, Shutes E. Evolution of couples' voluntary counseling and testing for HIV in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:108–115. doi: 10.1097/QAI.0b013e31815b2d67. [DOI] [PubMed] [Google Scholar]
- 17.Kempf MC, Allen S, Zulu I, Kancheya N, Stephenson R, Brill I. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:116–125. doi: 10.1097/QAI.0b013e31815d2f3f. [DOI] [PubMed] [Google Scholar]
- 18.McKenna SL, Muyinda GK, Roth D, Mwali M, Ng'andu N, Myrick A. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11:S103–110. [PubMed] [Google Scholar]
- 19.Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Trask S, Fideli U. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 20.Boeras DI, Luisi N, Karita E, McKinney S, Sharkey T, Keeling M. Indeterminate and discrepant rapid HIV test results in couples' HIV testing and counselling centres in Africa. J Int AIDS Soc. 2011;14:18. doi: 10.1186/1758-2652-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. Aids. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Self WH. Acute HIV infection: diagnosis and management in the emergency department. Emerg Med Clin North Am. 2010;28:381–392. doi: 10.1016/j.emc.2010.01.002. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 23.Tindall B, Barker S, Donovan B, Barnes T, Roberts J, Kronenberg C. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945–949. [PubMed] [Google Scholar]
- 24.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hecht FM, Busch MP, Rawal B, Webb M, Rosenberg E, Swanson M. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. Aids. 2002;16:1119–1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 26.Daar ES, Little S, Pitt J, Santangelo J, Ho P, Harawa N. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134:25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 27.Gaines H, von Sydow M, Pehrson PO, Lundbegh P. Clinical picture of primary HIV infection presenting as a glandular-fever-like illness. BMJ. 1988;297:1363–1368. doi: 10.1136/bmj.297.6660.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trask SA, Derdeyn CA, Fideli U, Chen Y, Meleth S, Kasolo F. Molecular epidemiology of human immunodeficiency virus type 1 transmission in a heterosexual cohort of discordant couples in Zambia. Journal of virology. 2002;76:397–405. doi: 10.1128/JVI.76.1.397-405.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burchell AN, Calzavara L, Ramuscak N, Myers T, Major C, Rachlis A. Symptomatic primary HIV infection or risk experiences? Circumstances surrounding HIV testing and diagnosis among recent seroconverters. Int J STD AIDS. 2003;14:601–608. doi: 10.1258/095646203322301059. [DOI] [PubMed] [Google Scholar]
- 30.Sanders EJ, Wahome E, Mwangome M, Thiong'o AN, Okuku HS, Price MA. Most adults seek urgent healthcare when acquiring HIV-1 and are frequently treated for malaria in coastal Kenya. AIDS. 2011;25:1219–1224. doi: 10.1097/QAD.0b013e3283474ed5. [DOI] [PubMed] [Google Scholar]
- 31.Simooya OO, Mwendapole RM, Siziya S, Fleming AF. Relation between falciparum malaria and HIV seropositivity in Ndola, Zambia. BMJ. 1988;297:30–31. doi: 10.1136/bmj.297.6640.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen S, Lindan C, Serufilira A, Van de Perre P, Rundle AC, Nsengumuremyi F. Human immunodeficiency virus infection in urban Rwanda. Demographic and behavioral correlates in a representative sample of childbearing women. JAMA. 1991;266:1657–1663. [PubMed] [Google Scholar]
- 33.Thigpen MC, Filler SJ, Kazembe PN, Parise ME, Macheso A, Campbell CH. Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. The American journal of tropical medicine and hygiene. 2011;84:379–385. doi: 10.4269/ajtmh.2011.10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fideli U. The Rwanda/Zambia HIV Research Group. Clinical and laboratory manifestations of acute HIV infection in Zambia. IAS Conference on HIV Pathogenesis and Treatment. 2003;8(Suppl.1) abstract no. 440. [Google Scholar]
- 35.Cooper DA, Gold J, Maclean P, Donovan B, Finlayson R, Barnes TG. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985;1:537–540. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- 36.Chu C, Selwyn PA. Diagnosis and initial management of acute HIV infection. Am Fam Physician. 2010;81:1239–1244. [PubMed] [Google Scholar]
- 37.Casimir GJ, Mulier S, Hanssens L, Zylberberg K, Duchateau J. Gender differences in inflammatory markers in children. Shock. 2010;33:258–262. doi: 10.1097/SHK.0b013e3181b2b36b. [DOI] [PubMed] [Google Scholar]
- 38.Steinvil A, Shapira I, Arbel Y, Justo D, Berliner S, Rogowski O. Determinants of the erythrocyte sedimentation rate in the era of microinflammation: excluding subjects with elevated C-reactive protein levels. Am J Clin Pathol. 2008;129:486–491. doi: 10.1309/U04E2YFJRR6JQQTK. [DOI] [PubMed] [Google Scholar]
- 39.Powers KA, Miller WC, Pilcher CD, Mapanje C, Martinson FE, Fiscus SA. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. Aids. 2007;21:2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavreys L, Baeten JM, Chohan V, McClelland RS, Hassan WM, Richardson BA. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–1339. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 41.Kelley CF, Barbour JD, Hecht FM. The Relation Between Symptoms, Viral Load, and Viral Load Set Point in Primary HIV Infection. Journal of Acquired Immune Deficiency Syndromes. 2007;45:445–448. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 42.Lavreys L, Baeten JM, Overbaugh J, Panteleeff DD, Chohan BH, Richardson BA. Virus load during primary Human Immunodeficiency Virus (HIV) type 1 infection is related to the severity of acute HIV illness in Kenyan women. Clin Infect Dis. 2002;35:77–81. doi: 10.1086/340862. [DOI] [PubMed] [Google Scholar]
- 43.Vanhems P, Lambert J, Cooper DA, Perrin L, Carr A, Hirschel B. Severity and prognosis of acute human immunodeficiency virus type 1 illness: a dose-response relationship. Clin Infect Dis. 1998;26:323–329. doi: 10.1086/516289. [DOI] [PubMed] [Google Scholar]
- 44.Tyrer F, Walker AS, Gillett J, Porter K. The relationship between HIV seroconversion illness, HIV test interval and time to AIDS in a seroconverter cohort. Epidemiol Infect. 2003;131:1117–1123. doi: 10.1017/s0950268803001377. [DOI] [PMC free article] [PubMed] [Google Scholar]