Abstract
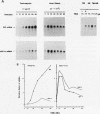
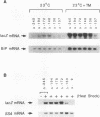
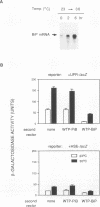
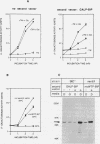
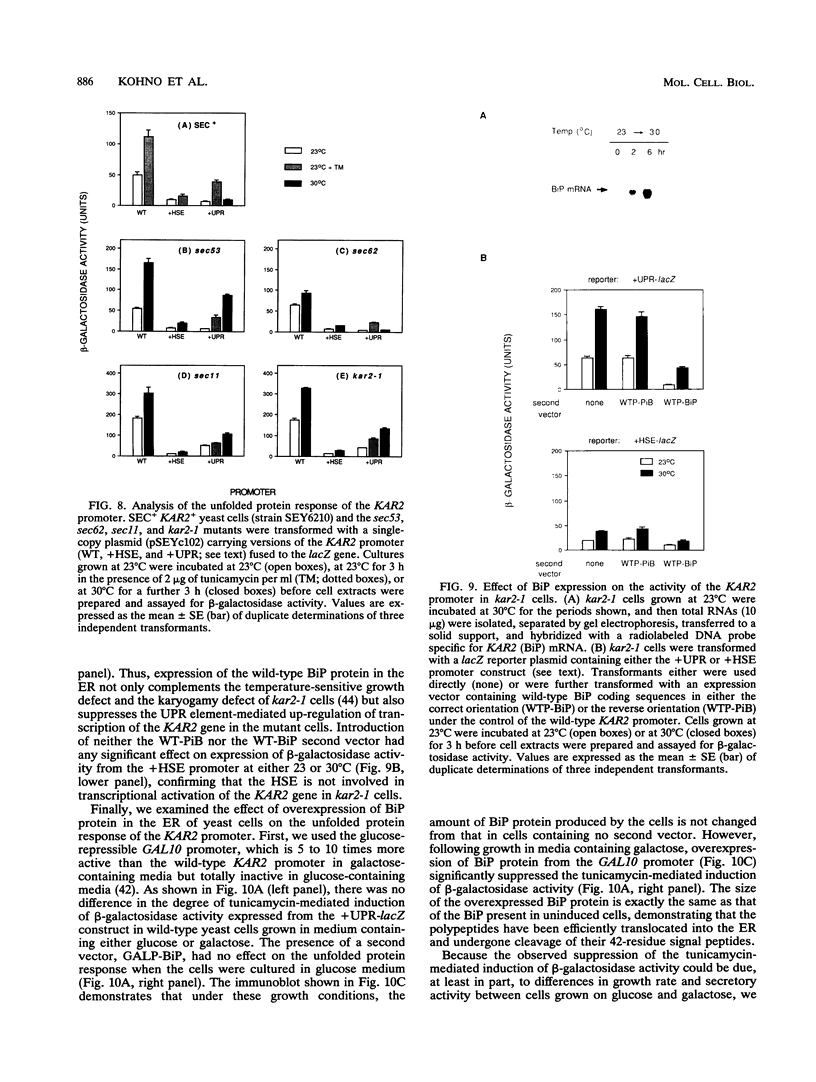
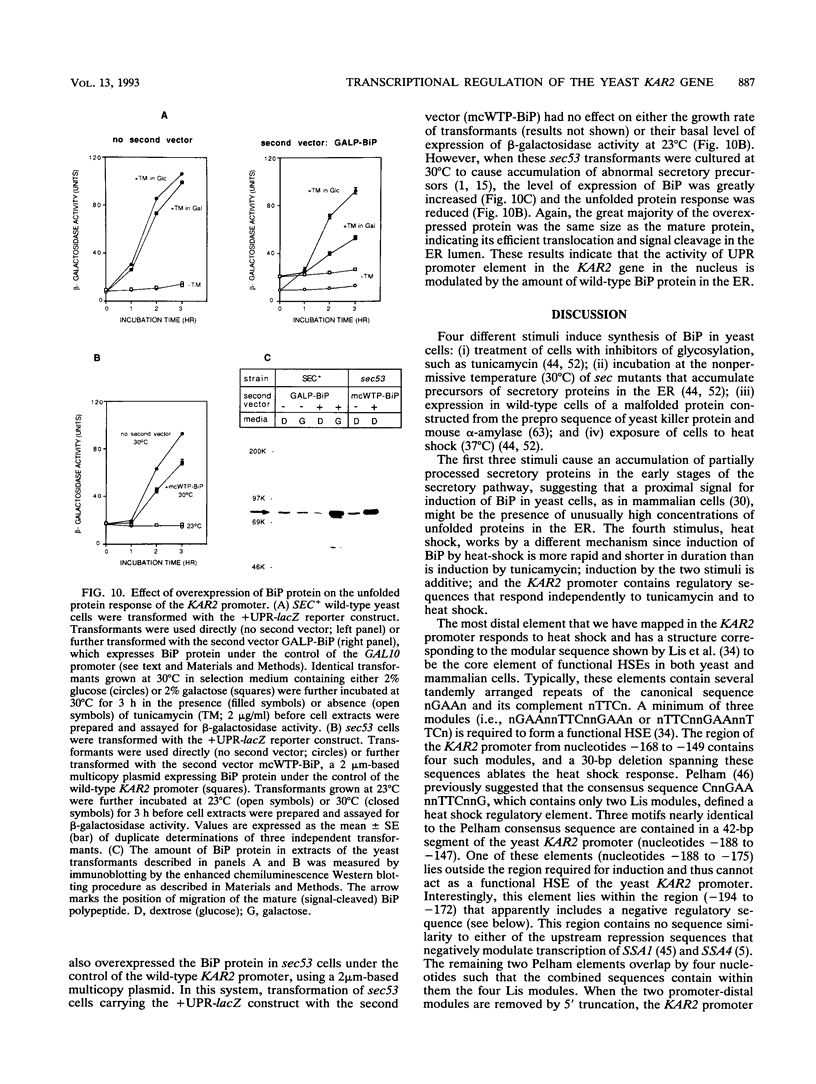
The endoplasmic reticulum (ER) of eukaryotic cells contains an abundant 78,000-Da protein (BiP) that is involved in the translocation, folding, and assembly of secretory and transmembrane proteins. In the yeast Saccharomyces cerevisiae, as in mammalian cells, BiP mRNA is synthesized at a high basal rate and is further induced by the presence of increased amounts of unfolded proteins in the ER. However, unlike mammalian BiP, yeast BiP is also induced severalfold by heat shock, albeit in a transient fashion. To identify the regulatory sequences that respond to these stimuli in the yeast KAR2 gene that encodes BiP, we have cloned a 1.3-kb segment of DNA from the region upstream of the sequences coding for BiP and fused it to a reporter gene, the Escherichia coli beta-galactosidase gene. Analysis of a series of progressive 5' truncations as well as internal deletions of the upstream sequence showed that the information required for accurate transcriptional regulation of the KAR2 gene in S. cerevisiae is contained within a approximately 230-bp XhoI-DraI fragment (nucleotides -245 to -9) and that this fragment contains at least two cis-acting elements, one (heat shock element [HSE]) responding to heat shock and the other (unfolded protein response element [UPR]) responding to the presence of unfolded proteins in the ER. The HSE and UPR elements are functionally independent of each other but work additively for maximum induction of the yeast KAR2 gene. Lying between these two elements is a GC-rich region that is similar in sequence to the consensus element for binding of the mammalian transcription factor Sp1 and that is involved in the basal expression of the KAR2 gene. Finally, we provide evidence suggesting that yeast cells monitor the concentration of free BiP in the ER and adjust the level of transcription of the KAR2 gene accordingly; this effect is mediated via the UPR element in the KAR2 promoter.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein M., Hoffmann W., Ammerer G., Schekman R. Characterization of a gene product (Sec53p) required for protein assembly in the yeast endoplasmic reticulum. J Cell Biol. 1985 Dec;101(6):2374–2382. doi: 10.1083/jcb.101.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J Cell Biol. 1991 Jun;113(5):1125–1132. doi: 10.1083/jcb.113.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Structure and regulation of the SSA4 HSP70 gene of Saccharomyces cerevisiae. J Biol Chem. 1990 Nov 5;265(31):18912–18921. [PubMed] [Google Scholar]
- Böhni P. C., Deshaies R. J., Schekman R. W. SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol. 1988 Apr;106(4):1035–1042. doi: 10.1083/jcb.106.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. C., Erwin A. E., Lee A. S. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989 May;9(5):2153–2162. doi: 10.1128/mcb.9.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. C., Wooden S. K., Nakaki T., Kim Y. K., Lin A. Y., Kung L., Attenello J. W., Lee A. S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc Natl Acad Sci U S A. 1987 Feb;84(3):680–684. doi: 10.1073/pnas.84.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Kaufman R. J. Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 1992 Apr;11(4):1563–1571. doi: 10.1002/j.1460-2075.1992.tb05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Esmon P. C., Esmon B. E., Schauer I. E., Taylor A., Schekman R. Structure, assembly, and secretion of octameric invertase. J Biol Chem. 1987 Mar 25;262(9):4387–4394. [PubMed] [Google Scholar]
- Feldman R. I., Bernstein M., Schekman R. Product of SEC53 is required for folding and glycosylation of secretory proteins in the lumen of the yeast endoplasmic reticulum. J Biol Chem. 1987 Jul 5;262(19):9332–9339. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Graham K. S., Le A., Sifers R. N. Accumulation of the insoluble PiZ variant of human alpha 1-antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem. 1990 Nov 25;265(33):20463–20468. [PubMed] [Google Scholar]
- Graham T. R., Emr S. D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991 Jul;114(2):207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Lewis M. J., Semenza J., Dean N., Pelham H. R. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 1990 Mar;9(3):623–630. doi: 10.1002/j.1460-2075.1990.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L. M., Ting J., Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988 Oct;8(10):4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes F., Schekman R. The yeast SEC53 gene encodes phosphomannomutase. J Biol Chem. 1988 Jul 5;263(19):9155–9161. [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Doms R. W., Bole D. G., Helenius A., Rose J. K. Heavy chain binding protein recognizes incompletely disulfide-bonded forms of vesicular stomatitis virus G protein. J Biol Chem. 1990 Apr 25;265(12):6879–6883. [PubMed] [Google Scholar]
- Morgan W. D. Transcription factor Sp1 binds to and activates a human hsp70 gene promoter. Mol Cell Biol. 1989 Sep;9(9):4099–4104. doi: 10.1128/mcb.9.9.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Sant A., Kohno K., Normington K., Gething M. J., Sambrook J. F. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992 Jul;11(7):2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Nakanishi T., Kohno K., Ishiura M., Ohashi H., Uchida T. Complete nucleotide sequence and characterization of the 5'-flanking region of mammalian elongation factor 2 gene. J Biol Chem. 1988 May 5;263(13):6384–6391. [PubMed] [Google Scholar]
- Ng D. T., Randall R. E., Lamb R. A. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989 Dec;109(6 Pt 2):3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D. T., Watowich S. S., Lamb R. A. Analysis in vivo of GRP78-BiP/substrate interactions and their role in induction of the GRP78-BiP gene. Mol Biol Cell. 1992 Feb;3(2):143–155. doi: 10.1091/mbc.3.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. H., Law D. T., Williams D. B. Binding protein BiP is required for translocation of secretory proteins into the endoplasmic reticulum in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1565–1569. doi: 10.1073/pnas.88.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Williams D. B., Moran L. A. An essential member of the HSP70 gene family of Saccharomyces cerevisiae is homologous to immunoglobulin heavy chain binding protein. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1159–1163. doi: 10.1073/pnas.87.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Park H. O., Craig E. A. Positive and negative regulation of basal expression of a yeast HSP70 gene. Mol Cell Biol. 1989 May;9(5):2025–2033. doi: 10.1128/mcb.9.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989 Nov;1(1):135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaina J., Conde J. Genes involved in the control of nuclear fusion during the sexual cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1982;186(2):253–258. doi: 10.1007/BF00331858. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Resendez E., Jr, Wooden S. K., Lee A. S. Identification of highly conserved regulatory domains and protein-binding sites in the promoters of the rat and human genes encoding the stress-inducible 78-kilodalton glucose-regulated protein. Mol Cell Biol. 1988 Oct;8(10):4579–4584. doi: 10.1128/mcb.8.10.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. Transcriptional regulation of an hsp70 heat shock gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 May;7(5):1906–1916. doi: 10.1128/mcb.7.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana C., Stevens T. H. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol Cell Biol. 1992 Oct;12(10):4601–4611. doi: 10.1128/mcb.12.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J., Lee A. S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988 May;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Kawamura A., Kohno K. Purification and characterization of BiP/Kar2 protein from Saccharomyces cerevisiae. J Biol Chem. 1992 Sep 5;267(25):17553–17559. [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooden S. K., Li L. J., Navarro D., Qadri I., Pereira L., Lee A. S. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol Cell Biol. 1991 Nov;11(11):5612–5623. doi: 10.1128/mcb.11.11.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]