Abstract
Laccase production by Coriolopsis caperata RCK2011 under solid state fermentation was optimized following Taguchi design of experiment. An orthogonal array layout of L18 (21 × 37) was constructed using Qualitek-4 software with eight most influensive factors on laccase production. At individual level pH contributed higher influence, whereas, corn steep liquor (CSL) accounted for more than 50% of the severity index with biotin and KH2PO4 at the interactive level. The optimum conditions derived were; temperature 30°C, pH 5.0, wheat bran 5.0 g, inoculum size 0.5 ml (fungal cell mass = 0.015 g dry wt.), biotin 0.5% w/v, KH2PO4 0.013% w/v, CSL 0.1% v/v and 0.5 mM xylidine as an inducer. The validation experiments using optimized conditions confirmed an improvement in enzyme production by 58.01%. The laccase production to the level of 1623.55 Ugds−1 indicates that the fungus C. caperata RCK2011 has the commercial potential for laccase.
Laccase (EC 1.10.3.2; benzenediol: oxygen oxidoreductase), a multicopper enzyme belonging to the blue oxidases, was first detected in 1883 in the exudates of the Japanese lacquer tree, Rhus vernicifera1. Majority of the laccases have been reported from white rot fungi, however, they could be found in some other type of fungi, bacteria and insects2. These enzymes are characterized by their ability to catalyze one-electron oxidation of four reducing-substrate molecules concomitant with four-electron reduction of molecular oxygen to water. Currently the catalytic properties of laccases are being exploited and they have become industrially important enzymes because of their diverse applications: in pulp bleaching, detergents, adhesives, fibre functionalization, detoxification, denim bleaching, textile dye decolourization, baking, biosensors and in biofuel cells3,4.
Laccase production from microorganisms has been reported under both submerged fermentation (SmF) and solid state fermentation (SSF) conditions. SSF, whereby an insoluble substrate is fermented with sufficient but no free moisture, typically uses agricultural residues such as wheat bran, wheat straw, rice bran etc., and offers numerous economical and practical advantages over SmF. Laccase production under different fermentation conditions is influenced by physical and chemical environment of cultural conditions, such as quality and quantity of carbon and nitrogen source, pH, temperature, aeration, presence of inducers, vitamins, amino acids, phosphorus, metal ion etc.5,6,7. For effective laccase production, it is essential to optimize simultaneously the culture conditions and composition of media. Therefore, the optimum conditions for the laccase production should be investigated for a cost effective commercial production. In the conventional approach, an optimization process usually involves one factor at a time (OFAT). This procedure is time consuming, cumbersome, requires more experimental data sets and do not provide information about the mutual interactions of the parameters. In contrast, statistically planned experiments reduce the number of experiments by developing a specific design of experiments, which also minimize the error in determining the values for significant parameters. Therefore, statistical tools and related experimental design helps to gain more information about the optimization conditions. In this regards, normally the response surface methodology (RSM) has received much attention in scientific researches8.
Recently Taguchi method, another method for designing factorial experiments, has been used by some researchers9, possesses some advantages, such as much quantitative information can be extracted by only a few experiment trials and provides a systematic and efficient plan for performing experiments under the consideration of the interactive effects among the control factors10. Taguchi method is one of the powerful optimization techniques and requires half of the time than RSM11,12. Although, Taguchi method has gained much popularity in engineering applications, its utilization in the field of biotechnology has been somewhat limited. However, recent studies have confirmed its applicability to optimize biochemical processes. It was successfully employed to improve xylanase production by 227%13 and to increase L-asparaginase production by Staphylococcus isolate14. Taguchi method of orthogonal array (OA) design of experiment (DOE) is a simple statistical tool to obtain extensive parameter data from only a few experiments. It involves the study of any given system by a set of independent variables (factors) over a specific region of interest (levels)15,16. Unlike traditional DOE, which focuses on the average process performance characteristics, it concentrates on the effect of variation on the process characteristics17 and makes the product or process performance insensitive to variation by proper design of parameters. This approach also facilitates to identify the influence of individual factors, establish relationship between variables and operational conditions and finally establish the performance at the optimum levels obtained with a few well-defined experimental sets18. Analysis of variance (ANOVA) was applied to determine optimum configuration of optimized variables. This approach does not only help in saving considerable time and cost but also leads to a more fully developed process by providing systematic, simple and efficient methodology for the optimization of the near optimum design parameters with only a few well defined experimental sets19.
In the present study, the objective was to identify the best conditions for the production of extracellular laccase by Coriolopsis caperata RCK2011 under SSF by applying Taguchi DOE. The experiments were designed with eight factors including temperature, pH, wheat bran, inoculum size, corn steep liquor (CSL), KH2PO4, biotin and xylidine for laccase production at three levels with OA layout of L18 (21 × 37).
Results
Evaluation of bioprocess for laccase production
Optimization of culture conditions by preliminary studies indicated that medium temperature - 30°C, pH - 5.0, wheat bran - 5 g, inoclum size - 1 ml (0.015 g dry wt.), nitrogen source (NH4Cl - 0.15% N2 equivalent), MgSO4 - 0.1% (w/v), KH2PO4 - 0.05% (w/v), CSL - 0.2% (v/v), vitamins (biotin) - 0.5% (w/v) and inducer (xylidine) - 1.0 mM are critical fermentation factors for laccase production by C. caperata RCK2011 (results not shown). Under these optimized conditions the production of laccase was improved by 77% (from 211.45 Ugds−1 to 916.0 Ugds−1). The results suggested that optimization of each fermentation factor such as temperature, pH, carbon, nitrogen, vitamins, inducers and others are the prerequisite for laccase production at an industrial scale. However, the conventional method of optimization does not provide any information about interactive influence of the factors responsible for higher production, which is known to positively regulate the microbial metabolism20. Therefore, statistical optimization of SSF factors for maximizing laccase production by C. caperata RCK2011 was attempted using eight critical fermentation factors and their selected levels (Table 1). These factors and their levels were selected based on our preliminary experiments on laccase production by C. caperata RCK2011.
Table 1. Selected culture conditions factors and assigned levels.
| Serial No. | Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| 1 | Temperature (°C) | 30 | 35 | - |
| 2 | pH | 4.5 | 5.0 | 5.5 |
| 3 | Wheat bran (g) | 3 | 5 | 7 |
| 4 | Inoculum (ml) | 0.5 | 1.0 | 1.5 |
| 5 | Biotin (% w/v) | 0.4 | 0.5 | 0.6 |
| 6 | KH2PO4 (% w/v) | 0.03 | 0.05 | 0.07 |
| 7 | Corn steep liquor (% v/v) | 0.1 | 0.2 | 0.3 |
| 8 | Xylidine (inducer) (mM) | 0.5 | 1.0 | 1.5 |
Statistical optimization of laccase production
Effect of temperature, pH, wheat bran, inoculum size, CSL, KH2PO4, biotin and xylidine were studied by Taguchi method, which is a fractional factorial experimental design L18 (21 × 37) OA (Table 2). The results of experiments revealed that the maximum production of laccase (1576.13 Ugds−1) occurred when experimental conditions were: temperature (30°C), pH (4.5), wheat bran (3 g), inoculum (0.5 ml), biotin - 0.4% (w/v), KH2PO4 - 0.03% (w/v), CSL - 0.1% (v/v) and xylidine - 0.5 mM. Whereas, minimum average production of laccase 49.23 Ugds−1 was observed at temperature (35°C), pH (5.5), wheat bran (3 g), inoculum (1.5 ml), biotin - 0.5% (w/v), KH2PO4 - 0.07% (w/v), CSL - 0.1% (v/v) and xylidine - 1.0 mM.
Table 2. L18 (21 × 37) orthogonal array of designed experiments.
| Column | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Laccase Activity (Ugds−1) ± S.D. |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1576.13 ± 4.75 |
| 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1330.46 ± 61.18 |
| 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 667.66 ± 5.57 |
| 4 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 1053.48 ± 48.88 |
| 5 | 1 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 1075.54 ± 10.79 |
| 6 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 830.08 ± 11.34 |
| 7 | 1 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 206.26 ± 9.62 |
| 8 | 1 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 759.86 ± 38.69 |
| 9 | 1 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 259.13 ± 1.06 |
| 10 | 2 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 310.35 ± 6.15 |
| 11 | 2 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 783.21 ± 21.62 |
| 12 | 2 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 524.93 ± 5.33 |
| 13 | 2 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 643.64 ± 18.12 |
| 14 | 2 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 730.47 ± 75.31 |
| 15 | 2 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 952.21 ± 26.91 |
| 16 | 2 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 49.23 ± 1.40 |
| 17 | 2 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 311.32 ± 9.92 |
| 18 | 2 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 213.46 ± 0.71 |
S.D. = Standard deviation.
Fermentation factors and their interactions on laccase production
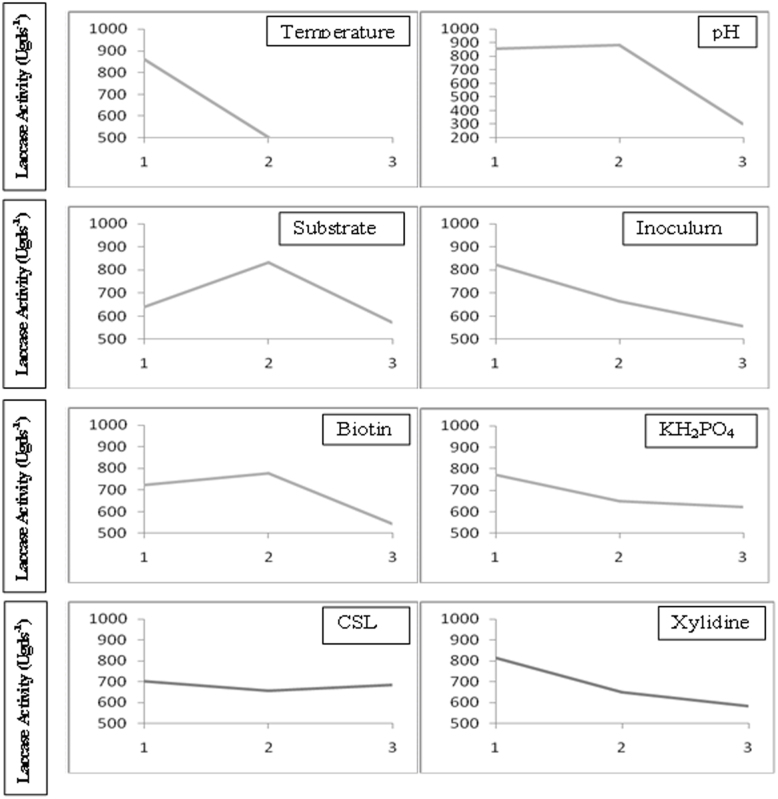
The main effects of the fermentation factors at the assigned levels on laccase production are depicted in Table 3. By the term “main effects”, the average of obtained results (as unit of enzyme, produced per g of dry substrate), in which each factor is at given level, is meant. Production levels were found to be very much dependent on the culture conditions. Higher laccase production 865.0 and 861.66 Ugds−1 were observed up to level 1 of pH and temperature, respectively and above these values the enzyme production repressed. Wheat bran caused higher laccase production 831.33 Ugds−1 with increase in concentration at level 2 but not at level 3. Inoculum, KH2PO4, CSL and xylidine had higher effects in level 1 on laccase production, whereas, increasing biotin concentration resulted in increase in laccase production 777.83 Ugds−1 up to level 2 and subsequent increase resulted in decrease in laccase production. The difference between the average value of each factor at level 2 and 1 (L2-L1) indicated the relative influence of the effect. The larger the difference the stronger the influence. The sign of the difference (+ or −) indicated whether the change from level 1 to level 2 or 3 increased or decreased the result. It can be seen from Table 3 that among the factors studied, wheat bran showed stronger influence (191.83 Ugds−1) compared to other factors followed by biotin (54.83 Ugds−1) and pH (15.5 Ugds−1) in laccase production. Figure 1 represents the influence of each individual factor on laccase production.
Table 3. Main effects of selected factors.
| Serial No. | Factor | Level 1 | Level 2 | Level 3 | L2-L1 |
|---|---|---|---|---|---|
| 1 | Temperature | 861.666 | 501.666 | - | −360.001 |
| 2 | pH | 865.0 | 830.5 | 299.5 | 15.5 |
| 3 | Wheat bran | 639.5 | 831.333 | 574.166 | 191.832 |
| 4 | Inoculum | 822.333 | 665.166 | 557.5 | −157.167 |
| 5 | Biotin | 723 | 777.833 | 544.166 | 54.832 |
| 6 | KH2PO4 | 773.833 | 649.166 | 622 | −124.667 |
| 7 | Corn steep liquor | 702.166 | 656.5 | 686.333 | −45.667 |
| 8 | Xylidine (inducer) | 814.166 | 649 | 581.833 | −165.167 |
Figure 1. Impact of selected factor levels on laccase production by Coriolopsis caperata RCK2011 under solid state fermentation.
Understanding the interaction between two factors gives a better insight into the overall process analysis. A factor may interact with any or all of the other factors creating the possibility of presence of a large number of interactions. The severity index (SI) was evaluated from Taguchi DOE that represents the influence of two individual factors at various levels of interaction. Table 4 shows the interactions of severity index for different factors. In this table, the ‘Columns' represents the locations to which the interacting factors are assigned. Interaction SI presents 100% of SI for 90° angle between the lines (factors), while 0% SI for parallel lines. If the interaction between the factors is reverse, that can be shown by ‘Reserved column'. ‘Levels' indicated the level of factors desirable for the optimum conditions. The highest interaction SI 85.94% was observed in between biotin and CSL (at levels 1 and 1; reserved column 2) followed by KH2PO4 and CSL (at levels 1 and 1; 1st reserved column) with SI 72.45%. It was interesting to note that biotin and CSL with less impact factor showed higher SI in combination. On the contrary, the SI interaction between high impact factors temperature and wheat bran was 11.4%. While, the least interaction SI 3.17% was found when strong impact factor pH interacting with wheat bran. It was evident from these observations that the influence of individual factors on laccase production had varying effects while in combination; the enzyme production was quite independent of the individual influence. Figure 2 shows the variation in laccase activity at chosen levels. Supplementary Figure S1 depicts the contribution of selected factors on the laccase production at optimum performance. pH has shown maximum positive impact on the production of laccase in individual cases. The data indicates relative interactions of factors on the laccase production.
Table 4. Estimated interaction of severity index for different factors.
| Serial No. | Factors | Columns | SI (%) | Reserved column | Levels |
|---|---|---|---|---|---|
| 1 | Biotin × CSL | 5 × 7 | 85.94 | 2 | [1,1] |
| 2 | KH2PO4 × CSL | 6 × 7 | 72.45 | 1 | [1,1] |
| 3 | Biotin × KH2PO4 | 5 × 6 | 69.76 | 3 | [1,1] |
| 4 | CSL × xylidine | 7 × 8 | 69.16 | 15 | [1,1] |
| 5 | Wheat bran × inoculum | 3 × 4 | 68.08 | 7 | [1,1] |
| 6 | Inoculum × xylidine | 4 × 8 | 60.37 | 12 | [1,1] |
| 7 | Wheat bran × Biotin | 3 × 5 | 53.99 | 6 | [2,2] |
| 8 | KH2PO4 × xylidine | 6 × 8 | 53.2 | 14 | [1,1] |
| 9 | Wheat bran × xylidine | 3 × 8 | 51.82 | 11 | [2,2] |
| 10 | Wheat bran × KH2PO4 | 3 × 6 | 46.99 | 5 | [1,1] |
| 11 | Inoculum × Biotin | 4 × 5 | 46.1 | 1 | [1,1] |
| 12 | Inoculum × KH2PO4 | 4 × 6 | 40.6 | 2 | [1,1] |
| 13 | Wheat bran × CSL | 3 × 7 | 36.65 | 4 | [2,1] |
| 14 | Temperature × xylidine | 1 × 8 | 25.6 | 9 | [1,1] |
| 15 | Temperature × CSL | 1 × 7 | 25.32 | 6 | [1,1] |
| 16 | pH × biotin | 2 × 5 | 24.48 | 7 | [1,1] |
| 17 | Inoculum × CSL | 4 × 7 | 24.07 | 3 | [1,3] |
| 18 | Temperature × pH | 1 × 2 | 22.05 | 3 | [1,3] |
| 19 | pH × xylidine | 2 × 8 | 21.63 | 10 | [1,2] |
| 20 | pH × KH2PO4 | 2 × 6 | 20.86 | 4 | [1,1] |
| 21 | Temperature × biotin | 1 × 5 | 19.46 | 4 | [1,2] |
| 22 | pH × CSL | 2 × 7 | 12.19 | 5 | [1,1] |
| 23 | Temperature × wheat bran | 1 × 3 | 11.4 | 2 | [1,2] |
| 24 | Temperature × inoculum | 1 × 4 | 10.81 | 5 | [1,1] |
| 25 | Biotin × xylidine | 5 × 8 | 8.79 | 13 | [1,1] |
| 26 | Temperature × KH2PO4 | 1 × 6 | 7.79 | 7 | [1,1] |
| 27 | pH × inoculum | 2 × 4 | 5.61 | 6 | [1,1] |
| 28 | pH × wheat bran | 2 × 3 | 3.17 | 1 | [1,2] |
Figure 2. Relative influence of factors and interactions.
Analysis of variance (ANOVA)
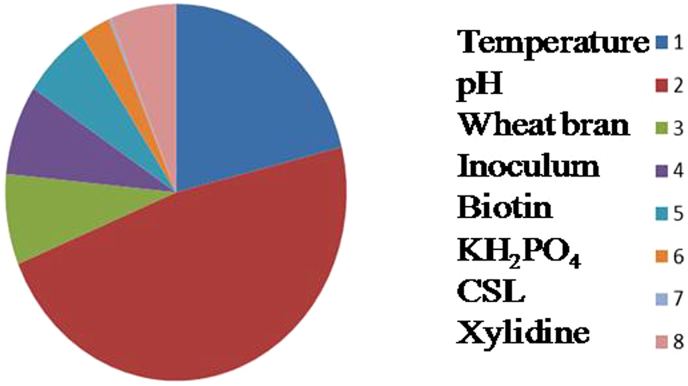
Understanding the impact of each individual factor is the key for a successful fermentation process. ANOVA was used to analyze the results of the OA experiment and to determine how much variation each factor has contributed (Table 5). From the calculated ratios (F) of all selected parameters, it was noticed that all factors and interactions considered in the experimental design were statistically significant at 95% confidence limit, indicating that nearly all the variability of experimental data can be explained in terms of significant effects. Statistical analysis of the laccase production data using above experimental designs revealed that among all selected factors pH contributed maximally (47%) on the overall enzyme production followed by the incubation temperature (21%). Wheat bran and inoculum concentration showed almost similar impact 7.76 and 7.70%, respectively on laccase production. Biotin and xylidine also showed impact of 6.41 and 6.21%, respectively. CSL and KH2PO4 showed the least impact (0.23 and 2.84%, respectively) at the individual level on overall production of laccase under the selected fermentation conditions, even though it has major impact on mass transfer of nutrients during microbial growth. This study revealed that overall 68.73% contribution was noticed with only two selected parameters (medium pH and incubation temperature) and rest 31.27% by other selected factors.
Table 5. Analysis of Variance (ANOVA).
| Serial No. | Factors | DOF (f) | Sums of squares (S) | Variance (V) | F-ratio (F) | Pure sum (S′) | Percentage P (%) |
|---|---|---|---|---|---|---|---|
| 1 | Temperature | 1 | 583200.223 | 583200.223 | 664004473.877 | 583200.223 | 21.119 |
| 2 | pH | 2 | 1315183 | 657591.5 | 13151830000 | 1315182.999 | 47.625 |
| 3 | Wheat bran | 2 | 214406.27 | 107203.135 | 2144062705.485 | 214406.27 | 7.764 |
| 4 | Inoculum | 2 | 212860.294 | 106430.147 | 2128602949.625 | 212860.294 | 7.708 |
| 5 | Biotin | 2 | 179176.276 | 89588.138 | 1791762762.858 | 179176.276 | 6.418 |
| 6 | KH2PO4 | 2 | 78666.302 | 39333.151 | 786663028.971 | 78666.302 | 2.843 |
| 7 | Corn steep liquor | 2 | 6452.337 | 3226.168 | 64523371.988 | 6452.337 | 0.233 |
| 8 | Xylidine (inducer) | 2 | 171540.39 | 85770.195 | 1715403900.553 | 171540.389 | 6.211 |
| Other/error | 2 | −0.001 | −0.001 | 0.004 | |||
| Total | 17 | 2761485.095 | 100.00 | ||||
Optimum conditions and validation of laccase production
The Taguchi DOE provided optimum culture conditions for each factor and their contribution for achieving maximum laccase production. The optimum conditions and their contribution are shown in Table 6. Based on this information, optimum laccase production could be obtained at 30°C with medium (pH 5.0) consisting of 0.5% (w/v) biotin, 0.013% (w/v) KH2PO4, 0.1% (v/v) CSL, 0.5 mM inducer (xylidine) and 5 g wheat bran in 5 days of fermentation using 0.5 ml of inoculum. The pH and fermentation temperature have been found to play a significant role in the enzyme production compared to other selected factors. The expected laccase production at optimum conditions was 1692.16 Ugds−1 with total contribution from all the factors being 1010.49 Ugds−1 with grand average performance of 681.66 Ugds−1. The observed 40% grand average performance of the fungal strain and 60% contribution of all fermentation factors revealed the potential of the fermentation factors concentration and their interaction for laccase production by the fungus, C. caperata RCK2011. It is evident from the observations (Supplementary Figure S2), that the optimized conditions enhanced laccase production of 59.71% i.e. from 681.66 to 1692.16 Ugds−1. Further to validate the proposed methodology, experiments were performed for laccase production by employing the optimized culture conditions. The validation resulted in the laccase production of 1623.55 Ugds−1 (expected response 1692.16 Ugds−1), thus proving the validity of the method.
Table 6. Optimum culture conditions and their contribution.
| Serial No. | Factors | Values | Level | Contribution |
|---|---|---|---|---|
| 1 | Temperature (°C) | 30 | 1 | 180 |
| 2 | pH | 5.0 | 2 | 198.833 |
| 3 | Wheat bran (g) | 5.0 | 2 | 149.666 |
| 4 | Inoculum (ml) | 0.5 | 1 | 140.666 |
| 5 | Biotin (% w/v) | 0.5 | 2 | 96.166 |
| 6 | KH2PO4 (% w/v) | 0.013 | 1 | 92.166 |
| 7 | Corn steep liquor (% v/v) | 0.1 | 1 | 20.5 |
| 8 | Xylidine (inducer) (mM) | 0.5 | 1 | 132.5 |
Total contributions from all factors = 1010.497.
Current grand average performance = 681.666.
Expected result at optimum conditions = 1692.163.
Discussion
Among statistical methods mostly Plakett-Burman design and RSM are preferred for optimizing biotechnological processes, could be because researchers are more familiar with them8,21,22. However, there are few reports showing usefulness of Taguchi methods in optimization of biotechnological process have appeared16. This method may be used quite easily and is less cumbersome. This method also has the ability to include physical factors along with the nutritional factors in the shortened fractional factorial designs. After primary studies of culture conditions, the laccase production was raised to 77% indicating the importance of fermentation factors in enzyme production. The enzyme secretion depends on the physiological, nutritional and biochemical nature of the microorganism employed, and even on the strain of the microorganism (s)6,23,24. The statistical optimization with the Taguchi L18 OA showed a significant variation in enzyme production ranging from 49.23 to 1576.13 Ugds−1 (Table 2). Similar variation of enzyme production with optimization experiments were noticed for laccase production by Pluerotus ostreatus 180420. Production levels were found to be very much dependent on the culture conditions (Table 3). pH and temperature effectively produced maximum laccase (865.0 and 861.66 Ugds−1) at level 1, whereas, wheat bran and biotin produced maximum laccase (831.33 and 777.83 Ugds−1) at level 2. It was observed that wheat bran showed stronger influence (191.83 Ugds−1) compared to other eight factors in the laccase production. It is reported that wheat bran is the good substrate for the growth and its soluble cellulose and hemicelluloses fractions served as carbon source which leads to a sufficient carbon and nitrogen ratio for efficient laccase production25. Wheat bran is used as a lignocellulosic source, which provides carbon and nitrogen source to the microbes. Wheat bran was reported as excellent growth substrates for laccase production by Coriolopsis unicolor26. The type and amount of the ligninolytic enzymes produced by the white rot fungi depends on the type of the plant material as substrate.
The interaction between biotin and CSL with less impact factor showed higher severity index (85.94%). CSL supplemented as organic N2 source in the medium has been reported to increase laccase production by P. ostreatus27. CSL is a complex substrate produced in the corn-processing industry and composed of peptides, sugars, lactic acids, vitamins and metallic ions28. CSL supplies nitrogen and carbon sources, and improves fungal growth during initial stages of colonization. The supplementation of biotin stimulated laccase production by C. bulleri. The increase in enzyme production could possibly be due to the alteration of membrane permeability, exocytosis, hyphal sheath desorption, enzyme stabilization, or even due to structural changes of the enzyme molecule itself5. The interaction between KH2PO4 and CSL produced second highest SI 72.45%. The elemental phosphorous is essential to living organisms because it is the part of the backbone of DNA, the carrier and transmitter of genetic information in cells. The optimum phosphorous content not only supported robust growth, but also helped in production of higher MnP, another lignin degrading enzyme, by Phanerochaete chrysosporium7. Moreover, CSL revealed varied interaction SI values with other factors such as xylidine (69.16%), wheat bran (36.65%), temperature (25.32%), inoculum (24.07%) and the least 12.19% with pH (strong impact factor).
Xylidine is the most widely reported inducer of laccase production29,30,31 and enhanced laccase specific production by 4 folds in Coriolopsis polyzona32. The aromatic compounds accelerated the enzyme production and shortened the time of peak activity appearance.
Laccase production increased with increasing inoculum concentration up to a critical value beyond which it decreased. Lower level of an inoculum may not be sufficient enough for initiating the growth of the organism, whereas, higher level may cause competitive inhibition33. However, the interaction among the strongest impact factor temperature and pH depicted SI 22.05%. Temperature is one of the most important factors affecting SSF and the optimal temperature generally lies in the range of 25–30°C34. The pH of the medium is also one of the most detrimental environmental factors affecting the mycelial growth, enzyme production and the transport of various components across the cell membrane35.
ANOVA analysis presented that pH contributed maximum impact (47%) followed by temperature, wheat bran, inoculum, biotin and xylidine (Table 5). While KH2PO4 and CSL showed the least impact factor 2.84 and 0.23%, respectively. The observed percent effect of the CSL and KH2PO4 on overall laccase production in the present investigation should be viewed with caution when compared to that of SSF with a defined medium composition. This is mainly due to the effect of a solid substrate (wheat bran) that provides support for microbial growth and also its role as carbon, nitrogen, vitamins, and proteins sources. The higher production with wheat bran is consistent with earlier reports on SSF using wheat bran as substrate36,37,38.
The present study revealed that medium pH and incubation temperature contributed 68.73% and rest 31.27% by other selected factors (Table 5). It clearly indicated that physical factor - pH and temperature are vital factors for laccase production by C. caperata RCK2011 under SSF. The validation of optimum conditions derived from Taguchi optimization process showed an increase of 58.01% in laccase production (Supplementary Figure 2). Similarly, Taguchi DOE methodology has been applied for optimizing laccase production in P. ostreatus 180420 and P. ostreatus IMI 39554539 under submerged fermentation.
In conclusion, the application of Taguchi DOE helped in reaching the optimal solutions and in critically analyzing the interactive effects of most influential parameters on laccase production by C. caperata RCK2011 for better understanding the bioprocess for improving the enzyme productivity.
Methods
White-rot fungus
Coriolopsis caperata RCK2011 a fungus isolated from the Aravali range forest in University of Delhi South Campus (UDSC), New Delhi, India, was grown and maintained on Malt Extract Agar (MEA) containing (g/L): Malt extract 20.0, KH2PO4 0.5, MgSO4.7H2O 0.5, Ca (NO3)2.4H2O 0.5, Agar 20.0 (pH 5.0) at 30°C5,40 and stored at 4°C.
Each 250 ml Erlenmeyer flask containing 50 ml of Malt Extract Broth (MEB) consisted of (g/L): Malt extract 20.0, KH2PO4 0.5, MgSO4.7H2O 0.5, Ca (NO3)2.4H2O 0.5 (pH 5.0) was inoculated with four mycelial discs (8.0 mm diameter each) and incubated at 30°C under static cultivation conditions for 7 days. The fungal mats thus obtained were homogenized using pestle and mortar under sterile conditions and were used as inoculum in solid state fermentation experiments.
Taguchi methodology
Optimization methodology adopted in this study was divided into four phases viz., planning, experimentation, software analysis, and validation of results. Each phase has separate objective and is interconnected in sequence to achieve the overall optimization process.
Design of experiments (phase I)
Taguchi DOE was used to set up the critical fermentation factors such as temperature, pH, wheat bran, inoculum size, CSL, KH2PO4, biotin, xylidine (an inducer) that have a significant influence on laccase production. The copper is generally used to enhance laccase production as it regulates the laccase gene transcription in the gene fermentation of the fungi. During optimization for laccase production following one factor at a time method, copper increased the laccase production by 17%, while xylidine induced laccase production by 38%. Therefore, xylidine was selected instead copper as an inducing factor. In the next step, matrix was designed with the appropriate OA for the selected factors and their levels. In the present study, OA L18 (which indicated 18 experimental trials) was selected for above controlled factors with three levels of factor variation (Table 1). All these factors were assigned with three levels, except incubation temperature which was assigned with two levels with a layout of L18 (21 × 37).
Solid state fermentation (phase II)
Solid state fermentation (SSF) experiments were performed for laccase production with C. caperata RCK2011 employing the selected 18 experimental trails (Table 2) in combination with eight factors at selected levels (Table 1). Prior to use, wheat bran was washed and dried in an oven (50°C; 24 h). All experiments were conducted in 250 ml Erlenmeyer flasks, each containing wheat bran (3, 5 or 7 g) with relative moisture content (in 1:3 ratio) containing NH4Cl (0.15% N2 equivalent), MgSO4 (0.1% w/v) and varying amount of KH2PO4 (0.03, 0.05 or 0.07% w/v), CSL (0.1, 0.2 or 0.3% v/v), dissolved in distilled water, the pH of mineral salt solution was adjusted to 4.5, 5.0 or 5.5 using 0.1 N NaOH or 0.1 N HCl. Flasks were sterilized by autoclaving at 121°C (15 psi) for 15 min. The flasks were inoculated with selected levels of homogenized inoculum 0.5, 1.0 or 1.5 ml (0.0075, 0.015 or 0.0225 g dry wt.) and incubated at different temperatures (30 or 35°C) in an incubator for 5 days. After 48 hrs of inoculation, the flasks were supplemented with filter sterilized biotin (0.4, 0.5 or 0.6% w/v) and xylidine (0.5, 1.0 or 1.5 mM).The entire contents of the flask were used for enzyme extraction and estimation. The laccase activities presented in this study are the average of three individual determinations.
Analysis of experimental data and prediction of performance (phase III)
The influence of individual factor on laccase production and their performance at optimum conditions using Taguchi approach were analyzed by Qualitek-4 software (Nutek Inc., MI, USA). The software is equipped to use from L-4 to L-64 arrays along with selected 2-63 factors and their 2–4 levels. The automatic design option allows Qualitek-4 to select the array used and assign factors to the appropriate columns.
Validation (phase IV)
In order to validate the optimized methodology, the SSF fermentation experiments were performed in triplicates for laccase production using the optimized culture conditions.
Extraction and estimation of laccase
The fermented wheat bran from each flask was suspended in citrate phosphate buffer (100 mM, pH 5.0) in 1:10 ratio (w/v) and shaken gently for 45 min. The extrudates were squeezed through muslin cloth for maximizing the enzyme extraction and centrifuged at 6708 g for 20 min at 4°C. The enzyme solution thus obtained was assayed for laccase activity. Laccase (EC 1.10.3.2) activity was measured using Guaiacol as a substrate29,41,42. The reaction mixture (1 ml) contained 0.1 ml of the enzyme extract and 0.9 ml Guaiacol (10 mM) prepared in 100 mM citrate phosphate buffer (pH 5.0). The molar extinction coefficient of tetraguaiacol at 470 nm (26.6 × 103 M−1cm−1) was used for activity calculation43. One unit of laccase was defined as the amount of the enzyme required to transform 1 μmol guaiacol/min. The laccase activity is expressed as unit per gram of dry substrate (Ugds−1).
Author Contributions
R.C.K. conceptualized the study. R.S.R. designed the statistical experiments. P.N. performed experiments, R.C.K. and P.N. analyzed the results. R.C.K., P.N. and R.S.R. prepared the manuscript.
Supplementary Material
supplementary file
Acknowledgments
The authors express their sincere gratitude to Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi, India for financial assistance. Preeti Nandal also acknowledges University of Delhi for providing scholarship. Sreenivas would like to thank WEFO, UK (80561) for the financial support.
References
- Yoshida H. Chemistry of lacquer (Urushi). J. Chem. Soc. 96, 472–486 (1883). [Google Scholar]
- Valderrama B., Oliver P., Medrano-Soto A. & Vazquez-Duhalt R. Evolutionary and structural diversity of fungal laccases. Antonie van Leeuwenhoek 84, 289–299 (2003). [DOI] [PubMed] [Google Scholar]
- Mano N., Mao F. & Heller A. Characteristics of a miniature compartment-less glucose-O2 biofuel cell and its operation in a living plant. J. Am. Chem. Soc. 125, 6588–6594 (2003). [DOI] [PubMed] [Google Scholar]
- Couto S. R. & Toca H. J. L. Industrial and biotechnological applications of laccases: a review. Biotechnol. Adv. 24, 500–513 (2006). [DOI] [PubMed] [Google Scholar]
- Dhawan S. & Kuhad R. C. Effects of amino acids and vitamins on laccase production by the bird's nest fungus Cyathus bulleri. Bioresour. Technol. 84, 35–38 (2002). [DOI] [PubMed] [Google Scholar]
- Gnanamani A., Jayaprakashvel M., Arulmani M. & Sadulla S. Effect of inducers and culturing processes on laccase synthesis in Phanerochaete chrysosporium NCIM 1197 and the constitutive expression of laccase isozymes. Enzyme Microbial. Technol. 38, 1017–1021 (2006). [Google Scholar]
- Liang H., Gao D. W. & Zeng Y. G. Effects of phosphorous concentration on the growth and enzyme production of Phanerochaete chrysosporium. Bioresour. Technol. 107, 535–538 (2012). [DOI] [PubMed] [Google Scholar]
- Halland P. D. Experiment design in biotechnology. Marcel Dekker Inc., New York (1989). [Google Scholar]
- Roy R. K. Design of Experiments Using the Taguchi Approach: 16 Steps to Product and Process Improvement, 1st edn. John Wiley & Sons, New York (2001). [Google Scholar]
- Houng J. Y., Liao J. H., Wu J. Y., Shen S. C. & Hsu H. F. Enhancement of asymmetric bioreduction of ethyl 4-chloro acetoacetate by the design of composition of culture medium and reaction conditions. Process Biochem. 42, 1–7 (2006). [Google Scholar]
- Benyounis K. Y. & Olabi A. G. Optimization of different welding processes using statistical and numerical approaches - A reference guide. Adv. Eng. Soft. 39, 483–496 (2008). [Google Scholar]
- Aggarwal A., Singh H., Kumar P. & Singh M. Optimizing power consumption for CNC turned parts using response surface methodology and Taguchi's technique - A comparative analysis. J. Material. Pro. Technol. 200, 373–384 (2008). [Google Scholar]
- Lakshmi G. S., Rao C. S., Rao S., Hobbs P. J. & Prakasham R. S. Enhanced production of xylanase by a newly isolated Aspergillus terreus under solid state fermentation using palm industrial waste: a statistical optimization. Biochem. Eng. J. 48, 51–57 (2009). [Google Scholar]
- Prakasham R. S., Rao C. S., Rao R. S. & Sarma P. N. L-Asparaginase production by isolated Staphylococcus sp.-6A: design of experiment considering interaction effect for process parameters optimization. J. Appl. Microbiol. 102, 1382–1391 (2007). [DOI] [PubMed] [Google Scholar]
- Taguchi G. Introduction to quality engineering. UNIPUB/Kraus International, White Plains (1986). [Google Scholar]
- Rao R. S., Kumar C. G., Prakasham R. S. & Hobbs P. J. The Taguchi methodology as a statistical tool for biotechnological applications: a critical appraisal. Biotechnol. J. 3, 510–523 (2008). [DOI] [PubMed] [Google Scholar]
- Ross P. J. Taguchi Techniques for Quality Engineering, 2nd edn. McGraw-Hill, New York (1996). [Google Scholar]
- Joseph J. & Piganatiells J. R. An overview of the strategy and tactics of Taguchi. IIE Trans. 20, 247–253 (1988). [Google Scholar]
- Phadke M. S. & Dehnad K. Optimization of product and process design for quality and cost. Qual. Reliab. Eng. Int. 4, 159–169 (1988). [Google Scholar]
- Prasad K. K., Mohan S. V., Rao R. S., Pati B. R. & Sarma P. N. Laccase production by Pleurotus ostreatus 1804: Optimization of submerged culture conditions by Taguchi DOE methodology. Biochem. Eng. J. 24, 17–26 (2005). [Google Scholar]
- Souza M. C. O., Roberto I. C. & Milagres A. M. F. Solid-state fermentation for xylanase production by Thermoascus aurantiacus using response surface methodology. Appl. Microbiol. Biotechnol. 52, 768–772 (1999). [Google Scholar]
- Park Y. S., Kang S. W., Lee J. S., Hong S. I. & Kim S. W. Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Appl. Microbiol. Biotechnol. 58, 761–766 (2002). [DOI] [PubMed] [Google Scholar]
- Lorenzo M., Moldes D. & Sanroman M. A. Effect of heavy metals on the production of several laccase isoenzymes by Trametes versicolor and on their ability to decolourise dyes. Chemosph. 63, 912–917 (2006). [DOI] [PubMed] [Google Scholar]
- Levin L., Melignani E. & Ramos A. M. Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour. Technol. 101, 4554–4563 (2010). [DOI] [PubMed] [Google Scholar]
- Schlosser D., Grey R. & Fritsche W. Patterns of ligninolytic enzymes in T. versicolor. Distribution of extra and intracellular enzyme activities during cultivation on glucose, wheat straw and beech wood. Appl. Microbiol. Biotechnol. 47, 412–418 (1997). [Google Scholar]
- Elisashvili V. & Kachlishvili E. Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol. 144, 37–42 (2009). [DOI] [PubMed] [Google Scholar]
- Mikiashvili N., Wasser S. P., Nevo E. & Elisashvili V. Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. W. J. Microbiol. Biotechnol. 22, 999–1002 (2006). [Google Scholar]
- Akhtar M., Blanchette R. A., Myers G. & Kirk K. An overview of biomechanical pulping research. In: Young, R. A., Akhtar, M., editors. Environmentally friendly technologies for pulp and paper industry. NY: John Wiley and Sons; p. 309–383 (1998). [Google Scholar]
- Vasdev K. & Kuhad R. C. Induction of laccase production in C. bulleri under shaking and static culture conditions. Folia Microbiol. 39 (4), 326–330 (1994). [Google Scholar]
- Eggert C., Temp U. & Eriksson K. E. L. The ligninolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 62, 1151–1158 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar M. S. & Lele S. S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem. 41, 581–588 (2006). [Google Scholar]
- Jaouani A., Tabka M. G. & Penninckx M. J. Lignin modifying enzymes of Coriolopsis polyzona and their role in olive oil mill wastewaters decolourization. Chemosp. 62, 1421–1430 (2006). [DOI] [PubMed] [Google Scholar]
- Mazumder S., Basu S. K. & Mukherjee M. Laccase production in solid-state and submerged fermentation by Pleurotus ostreatus. Eng. Life Sci. 9(1), 45–52 (2009). [Google Scholar]
- Dix N. J. & Webster J. Fungal ecology. Chapman and Hall, London, U.K (1995). [Google Scholar]
- Kapoor M., Nair L. M. & Kuhad R. C. Cost effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem. Eng. J. 38, 88–97 (2008). [Google Scholar]
- Machuca A., Aoyama H. & Duran N. Production and characterization of thermostable phenol oxidases of the ascomycete Thermoascus aurantiacus. Appl. Biochem. Biotechnol. 27, 217–223 (1998). [Google Scholar]
- Kuhad R. C., Kapoor M. & Chaudhary K. Production of xylanase from Streptomyces sp. M-83 using cost-effective substrates and its application in improving digestibility of monogastric animal feed. Ind. J. Microbiol. 46 (2), 109–119 (2006). [Google Scholar]
- Gupta S., Kapoor M., Sharma K. K., Nair L. M. & Kuhad R. C. Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour. Technol. 99, 937–945 (2008). [DOI] [PubMed] [Google Scholar]
- Periasamy R. & Palvannan T. Optimization of laccase production by Pleurotus ostreatus IMI 395545 using the Taguchi DOE methodology. J. Basic Microbiol. 50, 548–556 (2010). [DOI] [PubMed] [Google Scholar]
- Vasdev K., Dhawan S., Kapoor R. K. & Kuhad R. C. Biochemical characterization and molecular evidence of a laccase from the bird's nest fungus Cythus bulleri. Fun. Gen. Biol. 42, 684–693 (2005). [DOI] [PubMed] [Google Scholar]
- Dhawan S. & Kuhad R. C. Ethidium bromide stimulated hyper laccase production from bird's nest fungus Cyathus bulleri. Lett. Appl. Microbiol. 36, 11–13 (2004). [DOI] [PubMed] [Google Scholar]
- Diwaniyan S., Kharb D., Raghukumar C. & Kuhad R. C. Decolorization of Synthetic Dyes and Textile Effluents by Basidiomycetous Fungi. Water Air Soil Pollut. 210, 409–419 (2010). [Google Scholar]
- Diaz J., Bernal A., Pomar F. & Merino F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 161, 179–188 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary file