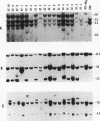
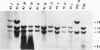
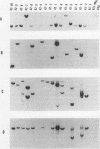
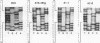Abstract
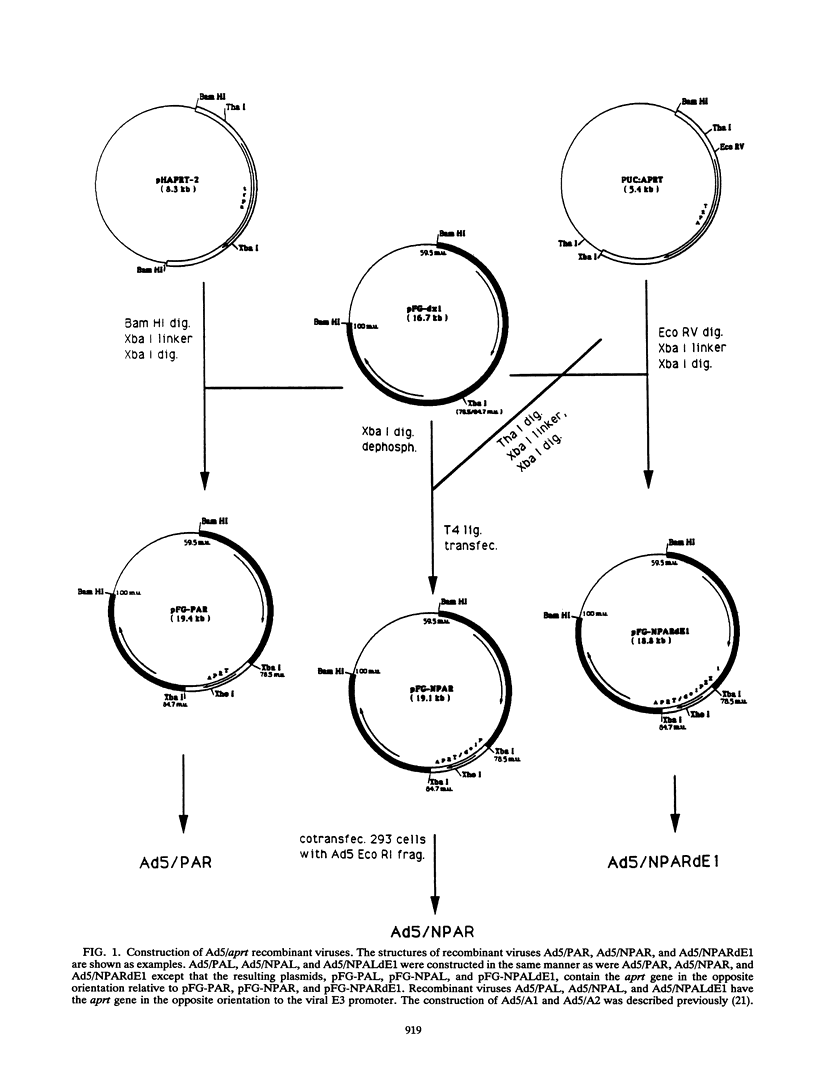
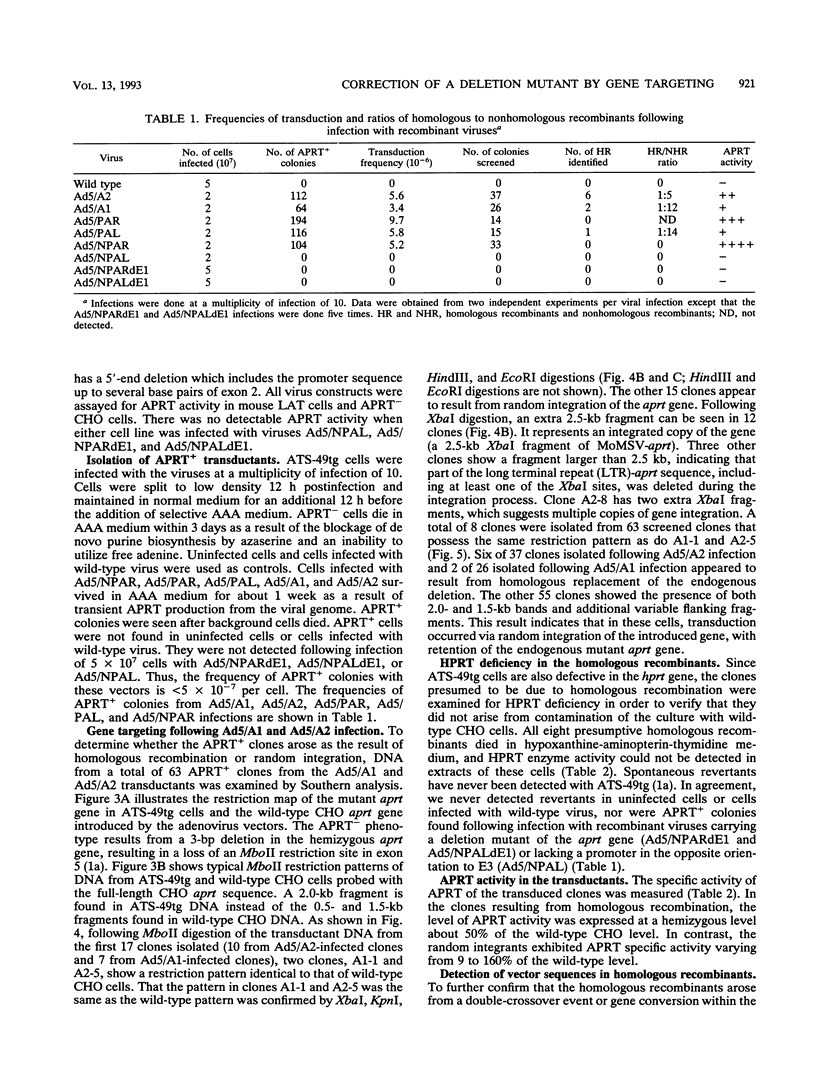
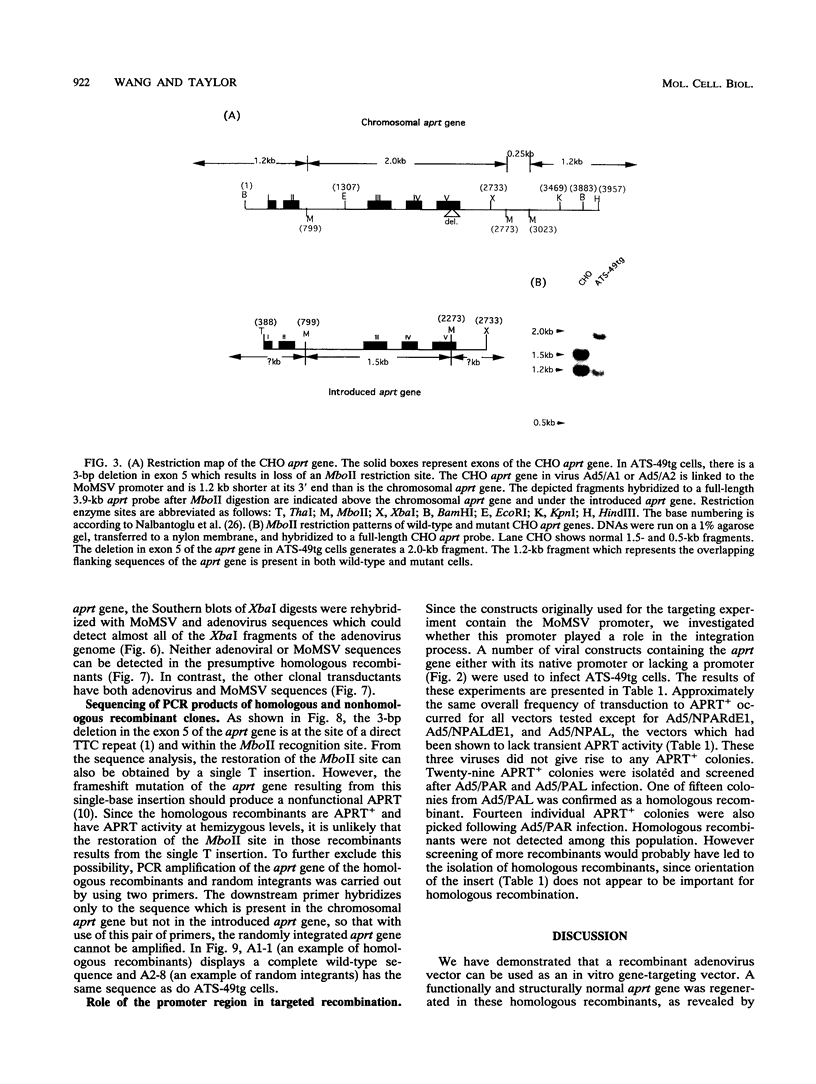
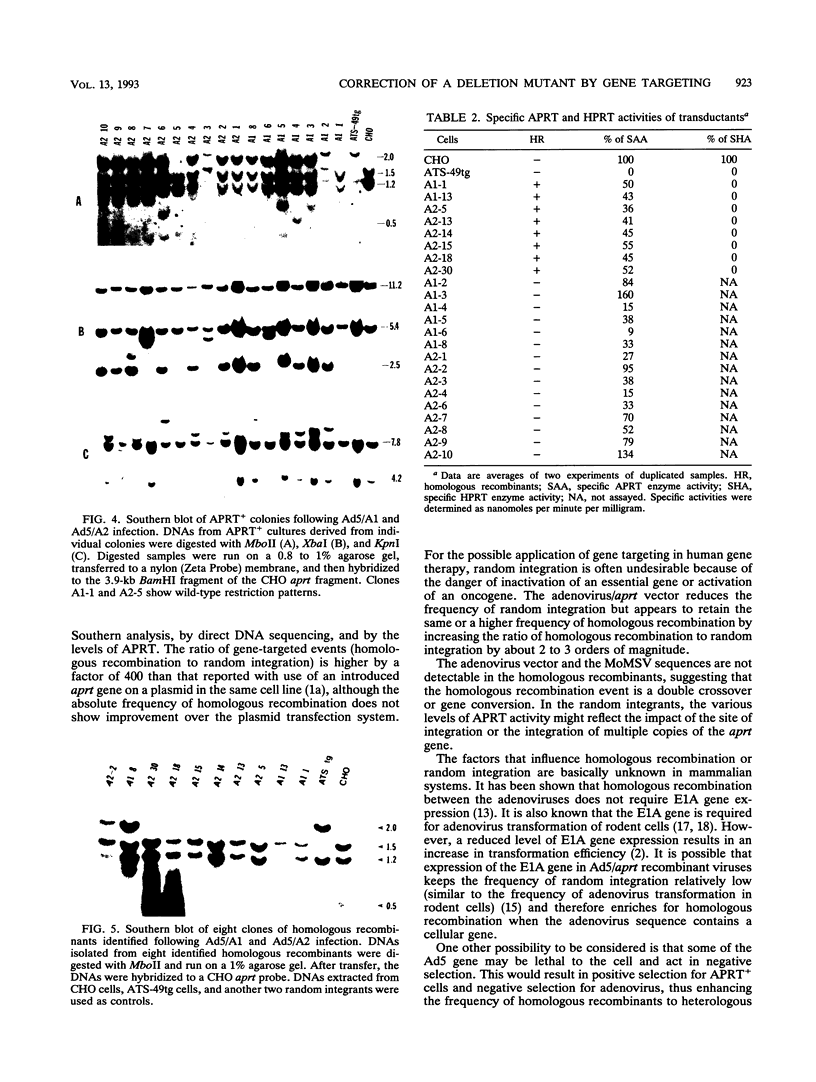
The usefulness of adenovirus type 5 as a vector for homologous recombination was examined in CHO cells by using the adenine phosphoribosyltransferase (aprt) gene. Infection of a hemizygous CHO APRT- cell line containing a 3-bp deletion in exon 5 of the aprt gene with a recombinant adenovirus containing the wild-type gene resulted in restoration of the APRT+ phenotype at a frequency of 10(-5) to 10(-6) per infected cell. A relatively high frequency (approximately 6 to 20%) of the transductants appears to result from a homologous recombination event. The mutation on the chromosomal aprt gene is corrected in the homologous recombinants, and APRT expression is restored to a normal hemizygous level. Neither adenovirus nor exogenous promoter sequences are detected in the homologous recombinants. The remaining transductants result from random integration of the aprt gene with the adenovirus sequence. A number of adenovirus vectors containing different promoter sequences linked to the hamster aprt gene were constructed. A possible role for the promoter region in the homologous recombination event was indicated by the lack of homologous recombination in constructs lacking an active promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Nairn R. S., Wilson J. H., Seidman M. M., Brotherman K. A., MacKinnon C., Scheerer J. B. Targeted homologous recombination at the endogenous adenine phosphoribosyltransferase locus in Chinese hamster cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4574–4578. doi: 10.1073/pnas.86.12.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami G. R., Babiss L. E. The efficiency of adenovirus transformation of rodent cells is inversely related to the rate of viral E1A gene expression. J Virol. 1990 Jul;64(7):3427–3436. doi: 10.1128/jvi.64.7.3427-3436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Berkner K. L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988 Jul-Aug;6(7):616–629. [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Campbell C. R., Keown W., Lowe L., Kirschling D., Kucherlapati R. Homologous recombination involving small single-stranded oligonucleotides in human cells. New Biol. 1989 Nov;1(2):223–227. [PubMed] [Google Scholar]
- Culver K., Cornetta K., Morgan R., Morecki S., Aebersold P., Kasid A., Lotze M., Rosenberg S. A., Anderson W. F., Blaese R. M. Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3155–3159. doi: 10.1073/pnas.88.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar R. L., Natarajan V., Vasudevachari M. B., Salzman N. P. Synthesis and processing of human immunodeficiency virus type 1 envelope proteins encoded by a recombinant human adenovirus. J Virol. 1989 Jan;63(1):129–136. doi: 10.1128/jvi.63.1.129-136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Eloit M., Gilardi-Hebenstreit P., Toma B., Perricaudet M. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J Gen Virol. 1990 Oct;71(Pt 10):2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- Epstein L. H., Young C. S. Adenovirus homologous recombination does not require expression of the immediate-early E1a gene. J Virol. 1991 Aug;65(8):4475–4479. doi: 10.1128/jvi.65.8.4475-4479.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Ensinger M. J., Kauffman R. S., Mayer A. J., Lundholm U. Cell transformation: a study of regulation with types 5 and 12 adenovirus temperature-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):419–426. doi: 10.1101/sqb.1974.039.01.054. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Grand R. J. The structure and functions of the adenovirus early region 1 proteins. Biochem J. 1987 Jan 1;241(1):25–38. doi: 10.1042/bj2410025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Ahmad Y., Graham F. L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J Virol. 1986 Jan;57(1):267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konan V., Sahota A., Graham F. L., Taylor M. W. Transduction of the CHO aprt gene into mouse L cells using an adeno-5/APRT recombinant virus. Somat Cell Mol Genet. 1991 Jul;17(4):359–368. doi: 10.1007/BF01233061. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Horwitz M. S. Chinese hamster ovary cells replicate adenovirus deoxyribonucleic acid. Mol Cell Biol. 1981 Mar;1(3):208–215. doi: 10.1128/mcb.1.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Miller B. W., Williams J. Cellular transformation by adenovirus type 5 is influenced by the viral DNA polymerase. J Virol. 1987 Nov;61(11):3630–3634. doi: 10.1128/jvi.61.11.3630-3634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce M. R., Micol J. L. PCR amplification of long DNA fragments. Nucleic Acids Res. 1992 Feb 11;20(3):623–623. doi: 10.1093/nar/20.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth S., Song K. Y., Ayares D., Wallace L., Moore P. D., Kucherlapati R. Transfection and homologous recombination involving single-stranded DNA substrates in mammalian cells and nuclear extracts. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5587–5591. doi: 10.1073/pnas.83.15.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W., Bradley W. E., Thompson L. H. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol Cell Biol. 1982 Sep;2(9):1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989 Mar 2;338(6210):87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Ellens D. J., Jansz H. S. Studies on the mechanism of replication of adenovirus DNA. II. The nature of single-stranded DNA in replicative intermediates. J Virol. 1973 Nov;12(5):1131–1138. doi: 10.1128/jvi.12.5.1131-1138.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Viral DNA synthesis in isolated nuclei from adenovirus-infected KB cells. FEBS Lett. 1972 Mar;21(1):7–10. doi: 10.1016/0014-5793(72)80149-2. [DOI] [PubMed] [Google Scholar]
- Tang D. C., Taylor M. W. Transcriptional activation of the adenine phosphoribosyltransferase promoter by an upstream butyrate-induced Moloney murine sarcoma virus enhancer-promoter element. J Virol. 1990 Jun;64(6):2907–2911. doi: 10.1128/jvi.64.6.2907-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Grossman M., Wu C. H., Chowdhury N. R., Wu G. Y., Chowdhury J. R. Hepatocyte-directed gene transfer in vivo leads to transient improvement of hypercholesterolemia in low density lipoprotein receptor-deficient rabbits. J Biol Chem. 1992 Jan 15;267(2):963–967. [PubMed] [Google Scholar]
- Ye W. W., Mason B. B., Chengalvala M., Cheng S. M., Zandle G., Lubeck M. D., Lee S. G., Mizutani S., Davis A. R., Hung P. P. Co-expression of hepatitis B virus antigens by a non-defective adenovirus vaccine vector. Arch Virol. 1991;118(1-2):11–27. doi: 10.1007/BF01311300. [DOI] [PubMed] [Google Scholar]
- Yeivin A., Tang D. C., Taylor M. W. Sodium butyrate selectively induces transcription of promoters adjacent to the MoMSV viral enhancer. Gene. 1992 Jul 15;116(2):159–164. doi: 10.1016/0378-1119(92)90511-m. [DOI] [PubMed] [Google Scholar]
- Zheng H., Wilson J. H. Gene targeting in normal and amplified cell lines. Nature. 1990 Mar 8;344(6262):170–173. doi: 10.1038/344170a0. [DOI] [PubMed] [Google Scholar]
- de Boer J. G., Glickman B. W. Mutational analysis of the structure and function of the adenine phosphoribosyltransferase enzyme of Chinese hamster. J Mol Biol. 1991 Sep 5;221(1):163–174. doi: 10.1016/0022-2836(91)80212-d. [DOI] [PubMed] [Google Scholar]