Abstract
A sinusoidal modulation scheme is described for selective heteronuclear polarization transfer between two dilute spins in double cross polarization magic-angle-spinning nuclear magnetic resonance spectroscopy. During the second N → C cross polarization, the 13C RF amplitude is modulated sinusoidally while the 15N RF amplitude is tangent. This modulation induces an effective spin-lock field in two selective frequency bands in either side of the 13C RF carrier frequency, allowing for simultaneous polarization transfers from 15N to 13C in those two selective frequency bands. It is shown by experiments and simulations that this sinusoidal modulation allows one to selectively polarize from 15N to its covalently bonded 13Cα and 13C′ carbons in neighboring peptide planes simultaneously, which is useful for establishing the back-bone connectivity between two sequential residues in protein structural elucidation. The selectivity and efficiency were experimentally demonstrated on a uniformly 13C,15N-labeled β1 immunoglobulin binding domain of protein G (GB1).
Keywords: Double cross polarization (DCP), NCA/NCO, Solid-state MAS NMR, Heteronuclear polarization transfer
1. Introduction
In the past decade, solid-state magic-angle-spinning (MAS) NMR has rapidly emerged as a powerful technique for studying insoluble proteins such as membrane proteins, amyloid proteins, and supramolecules. Resonance assignment is an essential step in the structural determination of uniformly 15N and 13C labeled proteins. In this regard, sequential assignments have been commonly used to extract structural constraints [1–12] via through-spaced dipolar couplings in well designed multi-dimensional experiments based on heteronuclear [13,14] and homonuclear dipolar recoupling [15–25] techniques in solid-state MAS NMR. While the heteronuclear correlation between 13C and 15N is one of the most important experiments in such sequential assignments, due to the fact that each backbone nitrogen covalently bonds with the Cα and carbonyl (C′) carbons in neighboring peptide planes, thus providing useful through-bond links between two sequential residues.
The heteronuclear polarization transfer between 13C and 15N can be achieved by the so-called double cross polarization, i.e. DCP [13,26,27], in which 15N is first polarized from the abundant 1H spins and then transferred to 13C via a second cross polarization from 15N to 13C. Whereas the 1H–15N or 1H–13C CP are relatively mature, most of the issues regarding DCP are primarily focused on the second N → C CP involving polarization transfer, in terms of selectivity, efficiency, and the influence of RF instability, between two low-γ nuclear species [28–42]. For example, SPECIFIC CP [31], a DCP experiment with carefully adjusted offsets and RF amplitudes on both the 13C and 15N channels, leads to selective polarization transfer from 15N to one of its bonded 13Cs (either 13Cα or 13C′), known as the two-dimensional (2D) NCA and NCO experiments, respectively. With the two NCA and NCO spectra, the connectivity between the Cα[i] and C′ [i–1] in the adjacent peptide planes can thus be established through N[i]. Alternatively, in the CANCO experiments [43], the 13Cα[i] is first selectively polarized from 1H, and then sequentially transferred to 13C′ [i–1] through two-step selective polarization transfers (i.e. from 13Cα [i] → 15N[i] and then from 15N[i] → 13C′[i–1]). However, such three-dimensional (3D) experiments (if including the 15N chemical shift dimension) suffer from very low sensitivity due to the multistep polarization transfers among the dilute spins. The efficiency for each heteronuclear polarization transfer between 13C and 15N is around 30–40%. Therefore, there is only 10–15% polarization transfer efficiency from 13Cα[i] to 13C′[i–1] [43].
In this work, we propose a dual-band selective double cross polarization to simultaneously polarize the 13Cα[i] and 13C′[i–1] from 15N[i], which allows one to establish the backbone 13Cα[i]–15N[i]–13C′[i–1] connectivity in a single 2D 15N–13C correlation spectrum, rather than in the two NCA and NCO spectra or in a low-sensitive CANCO spectrum, provided that there is a sufficient resolution in the 15N dimension. In this new scheme, the RF amplitude of the 13C channel is modulated sinusoidally while that of the 15N channel is kept constant or tangent-modulated during the second N → C CP. This sinusoidal modulation [44,45] directly applies the RF amplitude onto the Cα and C′ chemical shift regions simultaneously, even if the carrier frequency is away from those regions. The modulation period needs not to be commensurate with the period of sample rotation. We refer to this new method as sine-modulated double cross polarization (smDCP). It will be shown by experiments and simulations that smDCP can be effectively used for the dual-band selective heteronuclear polarization transfer using low RF amplitudes, thus improving selectivity and relaxing the demand on 1H decoupling power. The advantages of this new scheme will be illustrated by using a uniformly 13C,15Nlabeled β1 immunoglobulin binding domain of protein G (GB1) in the following sections.
2. Materials and experiments
The 56-residue β1 immunoglobulin binding domain of protein G (GB1) was expressed in E. coli BL21 (DE3) and minimal media (1.0 g/L 15N NH4Cl, 2.0 g/L 13C glucose), induced with 1.0 mM isopropyl β-D-thiogalactoside (IPTG) for 4 h. The cell pellet was resuspended in PBS buffer (50 mM Phosphate, 150 mM NaCl, pH 8.0) and incubated at 80 °C for 5 min. After centrifugation at 10,000 rpm for 30 min, the supernatant was purified by ion exchange (AKTA purifier 10.0), gelfiltration (Sephadex 100) and following desalination (AKTA purifier 10.0) with phosphate buffer (pH 8.0). The precipitation of GB1 was incubated over night at 4 °C in precipitation solution of 33% isopropanol (IPA) and 67% 2- methylpentane-2,4-diol (MPD). The final microcrystals were centrifuged at 5000 rpm for 2 min and transferred into a 4 mm rotor.
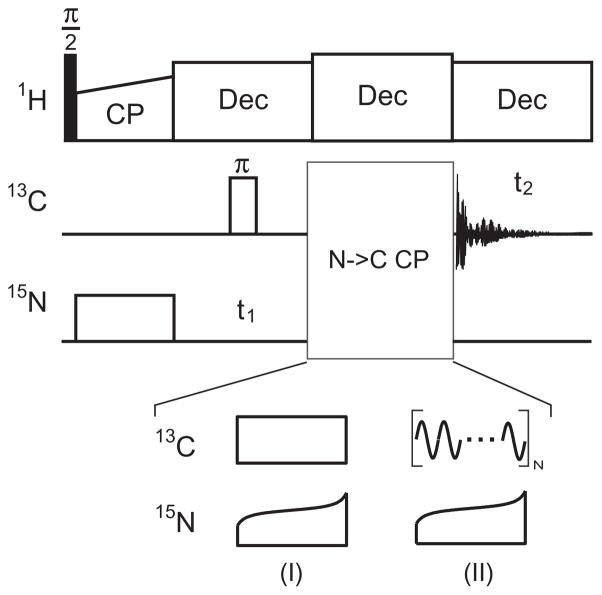
All the NMR experiments were performed on a wide-bore Varian 600 MHz NMR spectrometer of VNMRS system, equipped with a 4 mm triple-resonance T3-HXY MAS probe, at a temperature of 283 K (calibrated in separate experiments using the lead nitrate sample [46]). The sample spinning rate was set to 11 kHz ± 2 Hz. Fig. 1 shows the pulse sequences used in our experiments. The 1H π/2 pulse was 3.3 μs, while the 13C π pulse length of 7.2 μs was used for decoupling in the 15N chemical shift dimension (i.e., t1 dimension). During the first 1H–15N CP with a contact time of 1.2 ms, the 15N RF amplitude was about 37 kHz and the 1H RF-field was ramped from 90% to 110% of 57 kHz. The contact time for the second N → C CP was set to 5 ms, while the 15N and 13C RF amplitudes were varied depending upon the experimental schemes and will be described in their corresponding figures. The SPINAL-64 [47] sequence with 71 kHz RF amplitude and a 7.9 μs flip pulse was applied during both t1 and t2 dimension for 1H decoupling, while an 83 kHz CW decoupling was used during the 15N–13C contact. The 13C chemical shifts were referenced to adamantane at 40.48 ppm of the downfield signal [48] and the 15N reference was calculated based on the gyromagnetic ratio as recommended by IUPAC [49]. The RF amplitudes were calculated based on several measured amplitudes with the GB1 sample and the linearity of Varian linear amplifiers.
Fig. 1.
Pulse sequences used in 2D 15N–13C heteronuclear correlation experiments in solid-state MAS NMR. (I) The standard 15N–13C CP where the 15N RF amplitude is tangent but the 13C RF amplitude remains constant. (II) The sine modulated scheme where the 15N RF amplitude is tangent while the 13C RF amplitude is sinusoidally modulated at a given modulation frequency ωm.
Fig. 1(I) shows the standard N → C CP scheme where the 15N RF amplitude is tangent while the 13C RF amplitude remains constant, or vice versa, to enhance the CP efficiency [30,50]. For comparison, we use the rectangular 13C RF amplitude in our standard DCP experiments and refer it as ‘rectangular DCP’ in the following discussions. While in the smDCP scheme, the 15N RF amplitude is tangent while the 13C RF amplitude ω1C is sinusoidally modulated, as shown in Fig. 1(II):
| (1) |
where, and ωm represent the maximum RF amplitude and the modulation frequency, respectively. Mathematically, such a sine-wave modulation induces a spin-lock field in two frequency bands positioning at distances of ±ωm away from the carrier frequency, which allows for polarization transfers from 15N to 13C in these two frequency bands. While the effective RF amplitude applied on these two frequency bands is just about half of the maximum RF amplitude .
3. Results and discussion
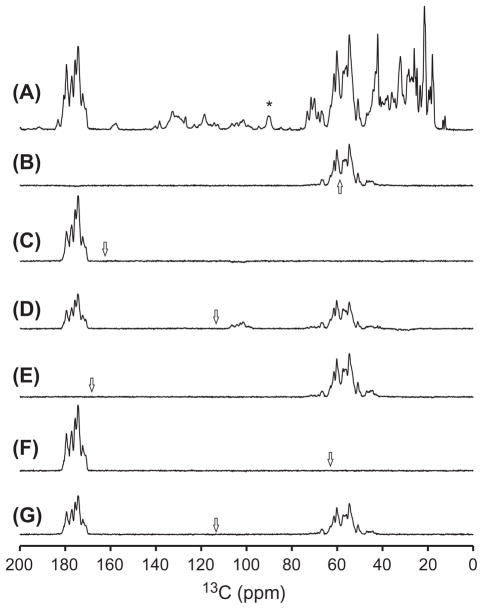
Fig. 2 shows the one-dimensional (1D) 13C spectra of the GB1 sample spinning at 11 kHz recorded under different N → C polarization conditions. With the direct CP from 1H to 13C, all 13C resonances were observed, as shown in Fig. 2A. Fig. 2B and C show the SPECIFIC CP spectra using the rectangular DCP scheme (e.g. Fig. 1I) with the selective polarization pathway from 15N to 13Cα and 13C′, respectively. In these experiments, the 13C carrier frequency was set closely to either Cα or C′ region and its RF amplitude was chosen to be low in order to achieve the required selectivity. The efficiency for these SPECIFIC CP experiments was ~50% as compared to the direct CP from 1H to 13C. If the 13C carrier frequency was set to the mid way between the Cα and C′ regions, the rectangular DCP experiment could also select both the 13Cα and 13C′ region simultaneously with a carefully adjusted RF amplitude [13,30,51], as shown in Fig. 2D. However, both simulations (not shown) and experiments indicate that higher 13C RF amplitude is needed for such a dual-band selectivity. Unfortunately, using the higher RF amplitude compromises the selectivity. As can be seen in Fig. 2D, small aliphatic carbon resonances (from 15 to 40 ppm), as well as the spinning sidebands at around 100 ppm, appear to be visible. Moreover, for the glycine 13Cα region (~42 ppm), the signal intensities seem to be much smaller than that in the SPECIFIC CP spectrum. The efficiency for this dual-band selectivity was ~34%, as compared to the spectrum in Fig. 2A.
Fig. 2.
1D 13C CP/MAS spectra of uniformly 13C,15N-labeled GB1 protein: (A) 13C spectrum directly polarized from 1H. The asterisk indicates the signal of a spacer used to provide a better sealing of the rotor. (B–G) Double cross polarized 13C spectra using different N → C polarization conditions, normalized with the spectrum in (A) to illustrate their polarization efficiency. The arrows in the spectra indicate the 13C carrier position in the experiments. The single-band selective SPECIFIC CP NCA (B) using ω1N = 9.0 kHz with 10% tangent ramp and ω1C = 3.0 kHz, and NCO (C) spectra using ω1N = 7.0 kHz with 15% tangent ramp and ω1C = 4.4 kHz. The dual-band rectangular DCP 13C spectrum (D) using ω1N = 7.0 kHz with 11% tangent ramp and ω1C = 16.0 kHz. The NCA (E) and NCO (F) spectra using the sine modulated 13C RF field with the amplitude of and ωm = 16.667 kHz and ω1N = 9.0 kHz with 10% and 17% tangent ramp, respectively. The dual-band selective 13C smDCP spectrum (G) using the sine modulated 13C RF field with the amplitude of and ωm = 8.889 kHz and ω1N = 9.0 kHz with 13% tangent ramp. The nominal RF amplitudes were calculated assuming a fine linearity of the Varian linear amplifier, which should have an accuracy of 0.5 kHz. In all experiments, 64 transients were used to accumulate the signals with a recycle delay of 3.5 s.
On the other hand, for the sine modulation scheme, even if the 13C carrier is set far away from the targeted region, the modulation effectively compensates the carrier offset, such that low RF amplitude can be used to achieve the needed selectivity. As shown in Fig. 2E and F, the 13C carrier frequency was set to ±16.667 kHz away from the Cα and C′ regions, respectively. With the sine modulation frequency of 16.667 kHz and a low 13C RF amplitude of 6.0 kHz, the Cα and C′ resonances were selectively polarized. As shown in the figures, the smDCP spectra are almost identical to their respective SPECIFIC CP spectra, with an efficiency of ~50% as compared to the spectrum in Fig. 2A. This implies that both frequency bands induced by the sine modulation in either side of the carrier frequency can be used for the efficient N → C polarization transfer. When the 13C carrier was set to the mid way between the Cα and C′ regions and the modulation frequency matched 8.889 kHz (half of the distance between the Cα and C′ regions), both the 13Cα and 13C′ regions were selected simultaneously with the efficiency of 37%, as shown in Fig. 2G. Apparently, the selectivity was comparable with that in the SPECIFIC NCO and NCA experiments, while the unwanted signals appearing in Fig. 2D was not observed in the spectrum, owing to the use of the low 13C RF amplitude.
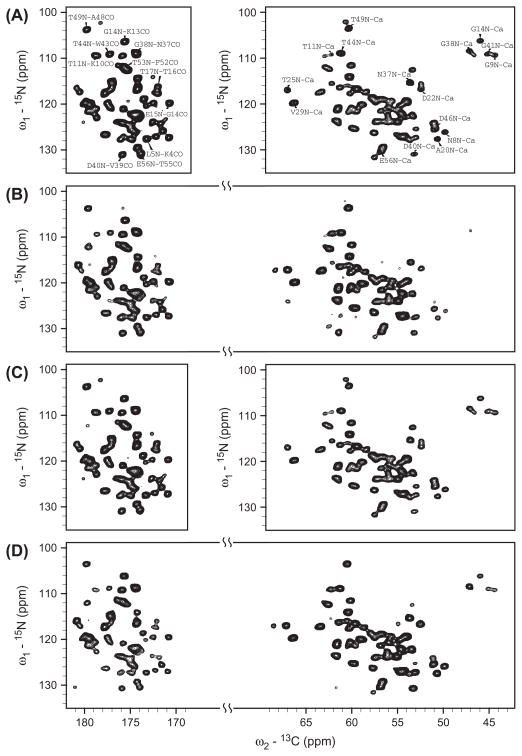
Fig. 3 shows the 2D 15N–13C heteronuclear correlation spectra of the uniformly 13C,15N-labeled GB1 under different polarization conditions. Fig. 3A shows the SPECIFIC NCO (left) and NCA (right) spectra recorded in the separate experiments where the 13C carrier frequency was set closely to the C′ and Cα regions, respectively. Since our sample was prepared at pH 8.0, different from pH 5.5 used in the GB1 sample preparation in the literature [52], the protein’s nano-crystalline form might be different. Thus, it is not surprising that the GB1 samples at different pHs gave rise to slightly different resonance patterns in the NCO and NCA spectra. However, their spectral resolution in both 13C and 15N dimensions are comparable [52], confirming that our sample was well prepared. It is worth noting that the slight difference in the resonance patterns resulting from the sample preparations should not affect any conclusion derived in this paper. Fig. 3A shows some partial assignments. Fortunately many resonances could be recognized based on the GB1 assignments as reported in the literature[53], despite the fact that there exist some additional peaks, such as the ones near the T49N-Cα and T49N-A48CO resonances. Fig. 3B shows the dual-band 15N–13C′/13Cα rectangular DCP spectrum using the rectangular 13C RF field during the N → C contact. Overall, the 15N–13C resonance patterns are similar to their respective SPECIFIC NCO and NCA spectra in Fig. 3A. However, some resonances such as the Gly 15N–13Cα resonances (G9N–Cα, G14N–Cα, G38N–Cα, and G41N–Cα) become very weak, while other resonances that did not appear in the SPECIFIC CP spectra started to show up in the spectrum (such as the peak in the left side of the T25N–Cα resonance), implying that the selectivity over the dual bandwidths is hardly uniform, which was also seen in 1D spectrum (e.g. Fig. 2D). It is worth noting that additional cross-peaks around (115 ppm, 103 ppm) and (141 ppm, 41 ppm) (not shown in the spectrum) should belong to the sidebands of 13C′ and 15N and to the folded-in aliphatic 13C and 15N in sidechains. Thus, the selectivity for the dual-band rectangular DCP is poor owing to the use of the required large 13C RF amplitude during the N → C contact.
Fig. 3.
2D 15N–13C heteronuclear correlation spectra of uniformly 13C,15N-labeled GB1 protein using different N → C polarization conditions. (A) Single-band SPECIFIC 15N–13Cα (Right) and 15N–13C′ (Left) spectra recorded in two separate experiments with the same experimental parameters as in Fig. 2B and C, respectively. (B) Dual-band rectangular DCP 15N–13Cα/13C′ spectrum using the same parameters as in Fig. 2D. (C) Single-band selective 15N–13Cα (Right) and 15N–13C′ (Left) smDCP spectra recorded in two separate experiments with the same experimental conditions as in Fig. 2E and F, respectively. (D) Dual-band selective 15N–13Cα/13C′ smDCP spectrum using the same parameters as in Fig. 2G. The spectra were processed by nmrPipe [54] and plotted with Sparky [55]. In all experiments, 40 transients were used to accumulate the signals for each t1 increment with a recycle delay of 3.5 s.
Fig. 3C shows the single-band 15N–13C′ (left) and 15N–13Cα (right) smDCP spectra using the sine modulated 13C RF amplitude during the N → C contact. It can be clearly noted that these spectra are almost identical to those in Fig. 3A, except for the one appearing at (112 ppm and 179 ppm) (below the T11 N-K10CO resonance), again implying that the frequency bandwidths induced by the sine modulation in both sides of the carrier frequency can be used for selective N → C polarization transfer. Fig. 3D shows the dual-band selective 15N–13C′/13Cα smDCP spectrum when the two frequency bandwidths induced by the sine modulation fell into the C′ and Cα regions simultaneously. Clearly, the resonance patterns in both the 15N–13C′ and 15N–13Cα regions are almost the same as in the single-band selective 15N–13C′and 15N–13Cα spectra as shown in Fig. 3A and C, despite the fact that a few additional peaks, such as (130 ppm and 180 ppm), (130 ppm and 61 ppm) and (118 ppm and 68 ppm), start to appear or gain in intensity. However, the Gly resonances, which almost disappeared in Fig. 3B, were as intensive as in the single-band selective 15N–13C′ and 15N–13Cα spectra as shown in Fig. 3A and C. On the other hand, those unwanted resonances appearing at around (115 ppm, 103 ppm) and (141 ppm, 41 ppm) in Fig. 3B completely disappeared in the dual-band selective 15N–13C′/13Cα smDCP spectrum. Thus, it is concluded that this sine modulation scheme provides an excellent selectivity.
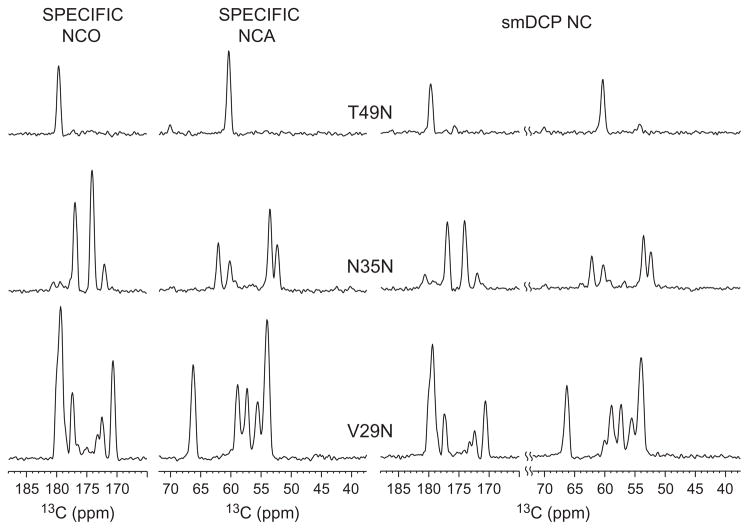
Fig. 4 shows the three 1D slices taken along T49N (Top), N37N (Middle), and V29N (Bottom) from the 2D SPECIFIC NCO/NCA spectra in Fig. 3A and from the smDCP NC spectrum in Fig. 3D, respectively. From their relative intensities, it can be noticed that the signal-to-noise (S/N) ratio for the dual-band selective smDCP is about 68–74% of that for the SPECIFIC NCO/NCA, which is consistent with the observation of the efficiency in the 1D spectra (c.f. Fig. 2). Therefore, the S/N for a single dual-banded smDCP spectrum (in the same overall time by doubling the number of scans) should be comparable to the two separate SPECIFIC NCO/NCA spectra. Our experimental result is beyond what is expected from the fact that the S/N for the dual-banded smDCP is considered to be half of that for SPECIFIC CP since the 15N magnetization is transferred to both carbon sites in the former scheme as compared to only one site in the latter scheme. However, one has to realize that such an expectation holds only when the 15N magnetization is completely transferred to the 13C and/or there exhibits the same spin dynamics during the N → C CP. Due to the fact that the C–N dipolar coupling is very small (~ a few kHz), the polarization transfer rate from N to C is rather slow. A few millisecond CP contact time (e.g. 5 ms in our experiments) can hardly transfer the 15N magnetization completely to 13C. On the other hand, the spin dynamics during the N → C CP may be different in these two schemes since they exhibit different spin systems during CP (N–C for SPECIFIC CP and C–N–C for the dual-banded smDCP). Such a difference might lead to different spin dynamics, as been observed in different 1H–S systems [56–58].
Fig. 4.
1D slices taken from the SPECIFIC NCO/NCA spectra in Fig. 3A and from the smDCP NC spectrum in Fig. 3D along T49 N (Top), N37N (Middle), and V29N (Bottom).
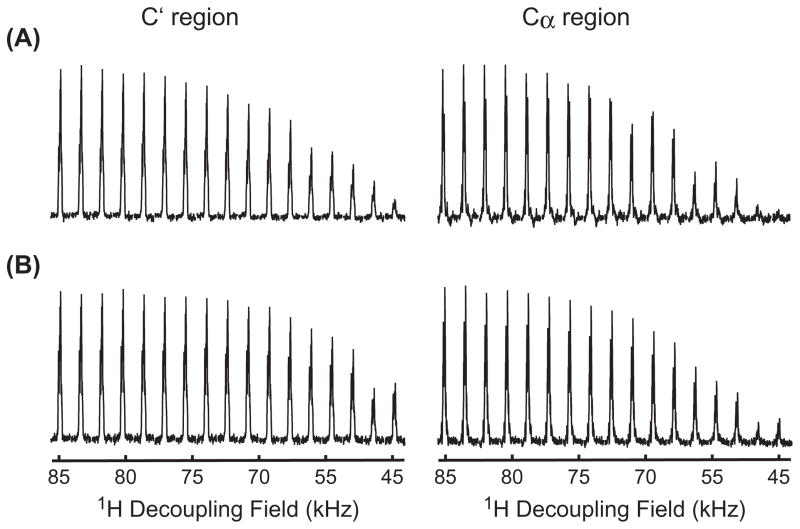
In the DCP experiments, low 13C RF amplitude in the N → C polarization transfer will relax the demand on the 1H decoupling field during the N → C contact. In our experiments, the maximum 13C RF amplitude used in the dual-band selective smDCP experiments (e.g. Fig. 2G) was about one-third of that used in the dual-band rectangular DCP experiments (e.g. Fig. 2D). Fig. 5 shows the dependence of the N → C polarization efficiency as a function of the applied 1H decoupling power during the N → C contact. As the 1H decoupling power decreasing from 85 to about 71 kHz, the polarization efficiency from N → Cα dropped to about 60% and 80% for the rectangular and sine modulated DCP schemes, respectively, while the efficiency from N → C′ decreased to ~75% and 90% in their respective spectra. It appears that the 1H decoupling power is more influential on the Cα carbons, probably owing to the strength of dipolar interactions between 1H and 13C as well as the difference between the applied 1H decoupling and the 13C RF amplitude during the N → C contact. A large difference between the 1H decoupling and 13C RF amplitude during the N → C contact is needed to provide sufficient mismatch between the 1H and 13C spins to avoid potential spin exchange between the two spins, especially when they possess a stronger dipolar coupling. Therefore, this might be the reason why the 15N → 13Cα polarization efficiency for the Gly residues was much worse in the dual-band rectangular DCP (e.g. Fig. 3B) than in the dual-band smDCP experiments (e.g. Fig. 3D). Indeed, the former experiment required five times higher 13C RF amplitude than the latter one under the same 1H decoupling power of 83 kHz during the N → C contact.
Fig. 5.
Dependence of the N → C polarization efficiency as a function of the 1H decoupling amplitude during the N → C contact time in different N → C polarization schemes. (A) The dual-band rectangular DCP using the parameters in Fig. 2D. (B) The dual-band smDCP using the parameters in Fig. 2G.
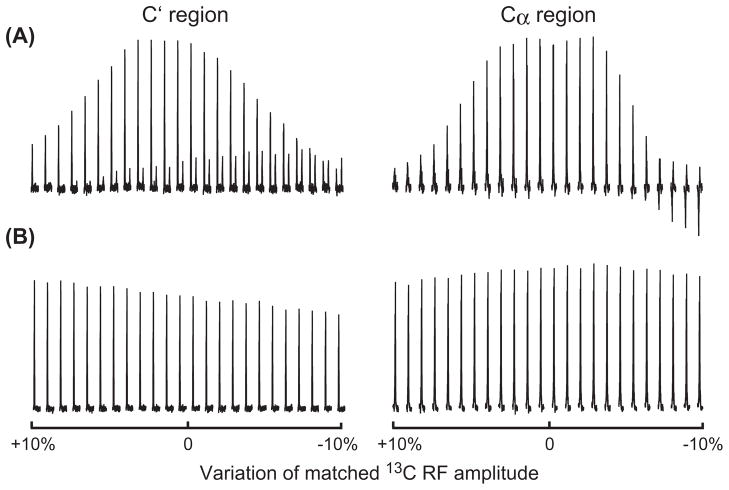
It had been reported that the parameters in the DCP experiments were sensitive to experimental settings, such as MAS speed, frequency offset and RF fields [33,38,39]. The influence of instability of 13C RF-field on the N → C polarization efficiency is also investigated for the dual-band polarization transfers, as shown in Fig. 6. It can be seen in Fig. 6A that the 15N → 13Cα and 15N → 13C′ polarization efficiencies for the rectangular DCP scheme suffer severely from the variation of 13C RF amplitude and decrease to about 30– 50% with about 10% variation. On the other hand, for the smDCP scheme the efficiency from 15N → 13Cα was just slightly affected by the 13C RF variation, while about 70% efficiency from 15N → 13C′ was preserved. Thus, there is an advantage to use this sine modulation scheme in long duration experiments.
Fig. 6.
Instability effect of 13C RF field on the N → C polarization efficiency in different schemes. The 13C RF amplitude was varied by ±10% of the optimized values (set to 0) in Fig. 2D and G.
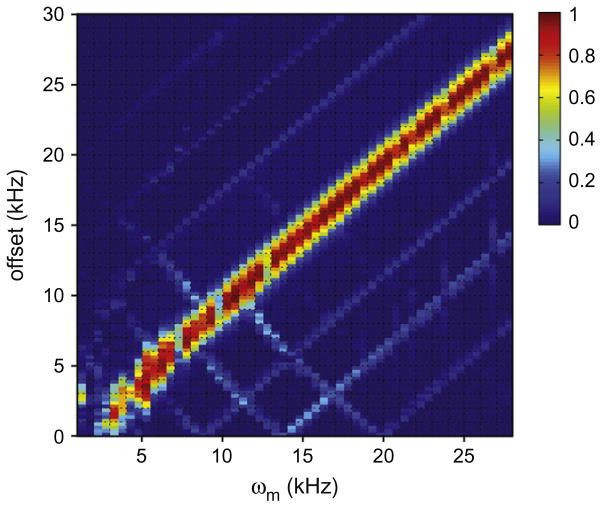
Fig. 7 shows the simulated selectivity for the sine modulation scheme (e.g. Fig. 1II) as a function of offset and modulation frequency ωm. In the simulations, the experimental parameters of ω1N = 9 kHz, , and the MAS rate of 11 kHz were used. Clearly, the frequency selective bandwidth (i.e. the offset range within which the polarization efficiency is above 60%) was about ωm ± 2 kHz, apparently independent of the spinning rate. In other words, the selective bandwidth is about ±2 kHz at distances of ±ωm away from the carrier frequency. This bandwidth is independent of spinning rate but depends on the RF amplitude used in the experiments. For a better selectivity, low-power RF amplitude is always preferable. Our simulations (not shown here) suggest that the dual-banded smDCP scheme should be effective at the magnetic field B0 up to 18.8 T with the spinning rate of 25 kHz. However, at very high fields where the chemical shift anisotropy (CSA) interactions become significant, there is a potential that the efficiency and selectivity could be compromised when using low-power RF amplitude which becomes unable to spin-lock the magnetization during the N → C CP. It has been demonstrated recently [60] that low-power symmetry-based band-selective sequences appear to be an effective approach for applications in high fields and fast sample spinning.
Fig. 7.
Simulated selectivity of the sine modulated scheme (e.g. Fig. 1II) as a function of offset and modulation frequency ωm. The SPINEVOLUTION [59] was used for the simulations. For simplicity, a constant 15N RF-field was utilized, and the spin system was 15N–13C, with a distance of rC–N = 1.448 Å. The chemical shift parameters for 13C were δiso = 0 ppm, δaniso = 24 ppm, η = 0.92; for 15N, δiso = 0 ppm, δaniso = 100 ppm, η = 0.8. In the simulations, ω1N = 9 kHz, , and the MAS rate of 11 kHz were used. The maximum intensity in this plot was normalized to 1.
4. Conclusion
We have demonstrated that sinusoidal modulation of the 13C RF amplitude during the second N → C cross polarization in double cross polarization (DCP) MAS NMR spectroscopy provides an effective spin-lock field in two selective frequency bands in either side of the 13C RF carrier frequency, allowing for simultaneous polarization transfers from 15N to 13C in those two selective frequency bands. When the two frequency bands fall into the C′ and Cα regions, the dual-band selective heteronuclear polarization transfer from 15N to both 13C′ and 13Cα can be achieved simultaneously in one experiment, rather than in two separate SPECIFIC NCO and NCA experiments or in a low-sensitive CANCO experiment, which is useful for establishing the backbone connectivity between two sequential residues in protein structural elucidation. It should be recognized that this information about the sequential Cα[i]–N[i]–C′[i–1] topology alone is not sufficient to obtain sequential resonance assignments. It has to be combined with other 2D or 3D experiments such as NCACX/NCOCX and/or 13C–13C correlation experiments. With the ability of selecting both the backbone Cα and C′ carbons, some elaborate 2D or 3D experiments could be designed to simplify crowded resonances typically observed in 13C–13C correlation spectra of biological samples being studied and to help assigning resonances, especially when combined with specific labeling approaches such as glycerol labeling strategy. For the dual-band selective polarization transfer, this new scheme greatly relaxes the demand on both 13C and 1H decoupling amplitudes. As compared to standard DCP experiments, this sine modulated scheme is also less sensitive to the instability of the 13C RF field. Moreover, such a modulation could also be applicable for dual-band selective heteronuclear polarization transfer between other spin pairs, as mentioned by Baldus et al. [31], such as 19F, 31P and even 1H in paramagnetic systems, with the use of a moderate RF amplitude when applied to the spin that has a large chemical shift distribution, especially under fast MAS condition [45].
Acknowledgments
This work was supported in part by the CAS/SAFEA International Partnership Program for Creative Research Teams and by Grants from the National Natural Science Foundation of China (21075133, 21173259, 20921004) and the National Basic Research Program of China (2009CB918600). Y.M. and R.F. are grateful for support from the National Institute of Health (NIH) Grant AI23007.
References
- 1.Takegoshi K, Nakamura S, Terao T. C-13–H-1 dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett. 2001;344:631–637. [Google Scholar]
- 2.Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature. 2002;420:98–102. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- 3.Jaroniec CP, Filip C, Griffin RG. 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon–nitrogen distances in uniformly C-13, N-15-labeled solids. J Am Chem Soc. 2002;124:10728–10742. doi: 10.1021/ja026385y. [DOI] [PubMed] [Google Scholar]
- 4.Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. Diluting abundant spins by isotope edited radio frequency field assisted diffusion. J Am Chem Soc. 2004;126:7196–7197. doi: 10.1021/ja047919t. [DOI] [PubMed] [Google Scholar]
- 5.Lewandowski JR, De Paepe G, Griffin RG. Proton assisted insensitive nuclei cross polarization. J Am Chem Soc. 2007;129:728–729. doi: 10.1021/ja0650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Paepe G, Lewandowski JR, Loquet A, Bockmann A, Griffin RG. Proton assisted recoupling and protein structure determination. J Chem Phys. 2008;129 doi: 10.1063/1.3036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholz I, Huber M, Manolikas T, Meier BH, Ernst M. MIRROR recoupling and its application to spin diffusion under fast magic-angle spinning. Chem Phys Lett. 2008;460:278–283. [Google Scholar]
- 8.Weingarth M, Demco DE, Bodenhausen G, Tekely P. Improved magnetization transfer in solid-state NMR with fast magic angle spinning. Chem Phys Lett. 2009;469:342–348. [Google Scholar]
- 9.Scholz I, Meier BH, Ernst M. NMR polarization transfer by second-order resonant recoupling: RESORT. Chem Phys Lett. 2010;485:335–342. [Google Scholar]
- 10.Weingarth M, Bodenhausen G, Tekely P. Broadband magnetization transfer using moderate radio-frequency fields for NMR with very high static fields and spinning speeds. Chem Phys Lett. 2010;488:10–16. [Google Scholar]
- 11.Hou GJ, Yan S, Sun SJ, Han Y, Byeon IJL, Ahn J, Concel J, Samoson A, Gronenborn AM, Polenova T. Spin diffusion driven by R-symmetry sequences: applications to homonuclear correlation spectroscopy in MAS NMR of biological and organic solids. J Am Chem Soc. 2011;133:3943–3953. doi: 10.1021/ja108650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu BW, Lafon O, Trebosc J, Chen Q, Amoureux JP. Broad-band homonuclear correlations assisted by (1)H irradiation for bio-molecules in very high magnetic field at fast and ultra-fast MAS frequencies. J Magn Reson. 2011;212:320–329. doi: 10.1016/j.jmr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer J, McKay RA, Stejskal EO. Double-cross-polarization NMR of solids. J Magn Reson. 1979;34:443–447. [Google Scholar]
- 14.Hing AW, Vega S, Schaefer J. Transferred-echo double-resonance NMR. J Magn Reson. 1992;96:205–209. [Google Scholar]
- 15.Raleigh DP, Levitt MH, Griffin RG. Rotational resonance in solid-state NMR. Chem Phys Lett. 1988;146:71–76. [Google Scholar]
- 16.Tycko R, Dabbagh G. Measurement of nuclear magnetic dipole–dipole couplings in magic angle spinning NMR. Chem Phys Lett. 1990;173:461–465. [Google Scholar]
- 17.Bennett AE, Ok JH, Griffin RG, Vega S. Chemical shift correlation spectroscopy in rotating solids: radio frequency-driven dipolar recoupling and longitudinal exchange. J Chem Phys. 1992;96:8624–8627. [Google Scholar]
- 18.Tycko R, Smith SO. Symmetry principles in the design of pulse sequences for structural measurements in magic angle spinning nuclear-magnetic-resonance. J Chem Phys. 1993;98:932–943. [Google Scholar]
- 19.Nielsen NC, Bildsoe H, Jakobsen HJ, Levitt MH. Double-quantum homonuclear rotary resonance: efficient dipolar recovery in magic-angle- spinning nuclear-magnetic-resonance. J Chem Phys. 1994;101:1805–1812. [Google Scholar]
- 20.Gregory DM, Mitchell DJ, Stringer JA, Kiihne S, Shiels JC, Callahan J, Mehta MA, Drobny GP. Windowless dipolar recoupling: the detection of weak dipolar couplings between spin-1/2 nuclei with large chemical-shift anisotropies. Chem Phys Lett. 1995;246:654–663. [Google Scholar]
- 21.Lee YK, Kurur ND, Helmle M, Johannessen OG, Nielsen NC, Levitt MH. Efficient dipolar recoupling in the NMR of rotating solids: a sevenfold symmetrical radiofrequency pulse sequence. Chem Phys Lett. 1995;242:304–309. [Google Scholar]
- 22.Sun BQ, Costa PR, Kocisko D, Lansbury PT, Griffin RG. Internuclear distance measurements in solid state nuclear magnetic resonance: dipolar recoupling via rotor synchronized spin locking. J Chem Phys. 1995;102:702–707. [Google Scholar]
- 23.Hohwy M, Jakobsen HJ, Eden M, Levitt MH, Nielsen NC. Broadband dipolar recoupling in the nuclear magnetic resonance of rotating solids: a compensated C7 pulse sequence. J Chem Phys. 1998;108:2686–2694. [Google Scholar]
- 24.Verel R, Baldus M, Ernst M, Meier BH. A homonuclear spin-pair filter for solid-state NMR based on adiabatic-passage techniques. Chem Phys Lett. 1998;287:421–428. [Google Scholar]
- 25.Hohwy M, Rienstra CM, Jaroniec CP, Griffin RG. Fivefold symmetric homonuclear dipolar recoupling in rotating solids: application to double quantum spectroscopy. J Chem Phys. 1999;110:7983–7992. [Google Scholar]
- 26.Schaefer J, Stejskal EO, Garbow JR, McKay RA. Quantitative determination of the concentrations of C-13–N-15 chemical bonds by double cross-polarization NMR. J Magn Reson. 1984;59:150–156. [Google Scholar]
- 27.Stejskal EO, Schaefer J, McKay RA. Analysis of double cross-polarization rates in solid proteins. J Magn Reson. 1984;57:471–485. [Google Scholar]
- 28.Wu XL, Zilm KW. Cross-polarization with high-speed magic-angle-spinning. J Magn Reson, Ser A. 1993;104:154–165. [Google Scholar]
- 29.Sun BQ, Costa PR, Griffin RG. Heteronuclear polarization transfer by radiofrequency-driven dipolar recoupling under magic-angle-spinning. J Magn Reson, Ser A. 1995;112:191–198. [Google Scholar]
- 30.Baldus M, Geurts DG, Hediger S, Meier BH. Efficient N-15–C-13 polarization transfer by adiabatic-passage Hartmann–Hahn cross polarization. J Magn Reson, Ser A. 1996;118:140–144. [Google Scholar]
- 31.Baldus M, Petkova AT, Herzfeld J, Griffin RG. Cross polarization in the tilted frame: assignment and spectral simplification in heteronuclear spin systems. Mol Phys. 1998;95:1197–1207. [Google Scholar]
- 32.Brinkmann A, Levitt MH. Symmetry principles in the nuclear magnetic resonance of spinning solids: heteronuclear recoupling by generalized Hartmann–Hahn sequences. J Chem Phys. 2001;115:357–384. [Google Scholar]
- 33.Bjerring M, Nielsen NC. Solid-state NMR heteronuclear coherence transfer using phase and amplitude modulated rf irradiation at the Hartmann–Hahn sideband conditions. Chem Phys Lett. 2003;382:671–678. [Google Scholar]
- 34.Kehlet CT, Sivertsen AC, Bjerring M, Reiss TO, Khaneja N, Glaser SJ, Nielsen NC. Improving solid-state NMR dipolar recoupling by optimal control. J Am Chem Soc. 2004;126:10202–10203. doi: 10.1021/ja048786e. [DOI] [PubMed] [Google Scholar]
- 35.Khaneja N. Sensitivity enhanced recoupling experiments in solid-state NMR by gamma preparation. J Magn Reson. 2006;183:242–251. doi: 10.1016/j.jmr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Khaneja N, Kehlet C, Glaser SJ, Nielsen NC. Composite dipolar recoupling: anisotropy compensated coherence transfer in solid-state nuclear magnetic resonance. J Chem Phys. 2006;124:114503. doi: 10.1063/1.2179431. [DOI] [PubMed] [Google Scholar]
- 37.Kehlet C, Bjerring M, Sivertsen AC, Kristensen T, Enghild JJ, Glaser SJ, Khaneja N, Nielsen NC. Optimal control based NCO and NCA experiments for spectral assignment in biological solid-state NMR spectroscopy. J Magn Reson. 2007;188:216–230. doi: 10.1016/j.jmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Yang JH, Chou FC, Tzou DLM. Coherence selection in double CP MAS NMR spectroscopy. J Magn Reson. 2008;195:116–120. doi: 10.1016/j.jmr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Lin J, Bayro MJ, Griffin RG, Khaneja N. Dipolar recoupling in solid state NMR by phase alternating pulse sequences. J Magn Reson. 2009;197:145–152. doi: 10.1016/j.jmr.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjerring M, Nielsen AB, Tosner Z, Nielsen NC. Broadband heteronuclear dipolar recoupling without (1)H decoupling in solid-state NMR using simple cross-polarization methods. Chem Phys Lett. 2010;494:326–330. [Google Scholar]
- 41.Nielsen AB, Straaso LA, Nieuwkoop AJ, Rienstra CM, Bjerring M, Nielsen NC. Broadband heteronuclear solid-state NMR experiments by exponentially modulated dipolar recoupling without decoupling. J Phys Chem Lett. 2010;1:1952–1956. doi: 10.1021/jz100564j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Griffin RG, Khaneja N. Recoupling in solid state NMR using gamma prepared states and phase matching. J Magn Reson. 2011;212:402–411. doi: 10.1016/j.jmr.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Berthold DA, Frericks HL, Gennis RB, Rienstra CM. Partial C-13 and N-15 chemical-shift assignments of the disulfide-bond-forming enzyme DsbB by 3D magic-angle spinning NMR spectroscopy. ChemBioChem. 2007;8:434–442. doi: 10.1002/cbic.200600484. [DOI] [PubMed] [Google Scholar]
- 44.Artemov D, Bhujwalla ZM, Glickson JD. Band-selective heteronuclear cross polarization in liquids. J Magn Reson, Ser B. 1995;107:286–288. doi: 10.1006/jmrb.1995.1091. [DOI] [PubMed] [Google Scholar]
- 45.Demers JP, Vijayan V, Becker S, Lange A. Tailored low-power cross-polarization under fast magic-angle spinning. J Magn Reson. 2010;205:216–223. doi: 10.1016/j.jmr.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Neue G, Dybowski C. Determining temperature in a magic-angle spinning probe using the temperature dependence of the isotropic chemical shift of lead nitrate. Solid State Nucl Magn Reson. 1997;7:333–336. doi: 10.1016/s0926-2040(96)01291-x. [DOI] [PubMed] [Google Scholar]
- 47.Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 48.Morcombe CR, Zilm KW. Chemical shift referencing in MAS solid state NMR. J Magn Reson. 2003;162:479–486. doi: 10.1016/s1090-7807(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 49.Harris RK, Becker ED, De Menezes SMC, Goodfellow R, Granger P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts – (IUPAC recommendations, 2001) Pure Appl Chem. 2001;73:1795–1818. doi: 10.1006/snmr.2002.0063. [DOI] [PubMed] [Google Scholar]
- 50.Hediger S, Meier BH, Ernst RR. Adiabatic passage Hartmann–Hahn cross-polarization in NMR under magic-angle sample-spinning. Chem Phys Lett. 1995;240:449–456. [Google Scholar]
- 51.Rienstra CM, Hohwy M, Hong M, Griffin RG. 2D and 3D N-15–C-13–C-13 NMR chemical shift correlation spectroscopy of solids: assignment of MAS spectra of peptides. J Am Chem Soc. 2000;122:10979–10990. [Google Scholar]
- 52.Franks WT, Kloepper KD, Wylie BJ, Rienstra CM. Four-dimensional heteronuclear correlation experiments for chemical shift assignment of solid proteins. J Biomol NMR. 2007;39:107–131. doi: 10.1007/s10858-007-9179-1. [DOI] [PubMed] [Google Scholar]
- 53.Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of the beta 1 immunoglobulin binding domain of protein G (GB1): N-15 and C-13 chemical shift assignments and conformational analysis. J Am Chem Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]
- 54.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPIPE: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 55.Goddard TD, Kneller DG. Sparky 3. University of California; San Francisco: [Google Scholar]
- 56.Mehring M. Principles of High Resolution NMR in Solids. 2. Springer-Verlag; New York: 1983. [Google Scholar]
- 57.Alemany LB, Grant DM, Alger TD, Pugmire RJ. Cross polarization and magic angle sample spinning NMR spectra of model organic compounds. 3. Effect of the 13C–1H dipolar interaction on cross polarization and carbon-proton dephasing. J Am Chem Soc. 1983;105:6697–6704. [Google Scholar]
- 58.Fu R, Hu J, Cross TA. Towards quantitative measurements in solid-state CPMAS NMR: a Lee–Goldburg frequency modulated cross polarization scheme. J Magn Reson. 2004;168:8–17. doi: 10.1016/j.jmr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Veshtort M, Griffin RG. SPINEVOLUTION: a powerful tool for the simulation of solid and liquid state NMR experiments. J Magn Reson. 2006;178:248–282. doi: 10.1016/j.jmr.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Herbst C, Herbst J, Leppert J, Ohlenschlaeger O, Gorlach M, Ramachandran R. Chemical shift correlation at high MAS frequencies employing low-power symmetry-based mixing schemes. J Biomol NMR. 2011;50:277–284. doi: 10.1007/s10858-011-9516-2. [DOI] [PubMed] [Google Scholar]