Abstract
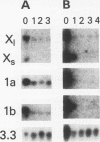
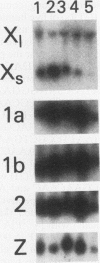
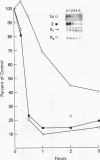
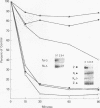
Histone H2A.X is a replication-independent histone H2A isoprotein species that is encoded by a transcript alternatively processed at the 3' end to yield two mRNAs: a 0.6-kb mRNA ending with the stem-loop structure characteristic of the mRNAs for replication-linked histone species, and a second, polyadenylated 1.6-kb mRNA ending about 1 kb further downstream (C. Mannironi, W. M. Bonner, and C. L. Hatch, Nucleic Acids Res. 17:9113-9126, 1989). Of the two, the 0.6-kb H2A.X stem-loop mRNA predominates in many cell lines, indicating that the presence of two types of mRNA may not completely account for the replication independence of H2A.X protein synthesis. The ambiguity is resolved by the finding that the level of the 0.6-kb H2A.X mRNA is only weakly downregulated during the inhibition of DNA replication and only weakly upregulated during the inhibition of protein synthesis, while the levels of other replication-linked mRNAs are strongly down- or upregulated under these two conditions. Analysis of the nuclear transcription rates of specific H2A genes showed that while the rates of transcription of replication-linked H2A genes decreased substantially during the inhibition of DNA synthesis and increased substantially during the inhibition of protein synthesis, the rate of H2A.X gene transcription decreased slightly under both conditions. These differences in transcriptional regulation between the H2A.X gene and other replication-linked histone genes are sufficient to account for the differences in regulation of their respective stem-loop mRNAs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Damjanov I., Simon D., Banting G. S., Carlin C., Dracopoli N. C., Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984 Feb;50(2):147–162. [PubMed] [Google Scholar]
- Bandyopadhyay R., Stein G., Stein J. Coordinate turnover of nuclear and cytoplasmic histone messenger RNA following inhibition of DNA replication in HeLa S3 cells. Biochemistry. 1987 May 19;26(10):2938–2944. doi: 10.1021/bi00384a040. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Wu R. S., Panusz H. T., Muneses C. Kinetics of accumulation and depletion of soluble newly synthesized histone in the reciprocal regulation of histone and DNA synthesis. Biochemistry. 1988 Aug 23;27(17):6542–6550. doi: 10.1021/bi00417a052. [DOI] [PubMed] [Google Scholar]
- Breitman T. R. Growth and differentiation of human myeloid leukemia cell line HL60. Methods Enzymol. 1990;190:118–130. doi: 10.1016/0076-6879(90)90016-t. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Lavery D. J. Pulse labeling of heterogeneous nuclear RNA in isolated nuclei. Methods Enzymol. 1989;180:82–96. doi: 10.1016/0076-6879(89)80094-1. [DOI] [PubMed] [Google Scholar]
- Dalton S., Robins A. J., Harvey R. P., Wells J. R. Transcription from the intron-containing chicken histone H2A.F gene is not S-phase regulated. Nucleic Acids Res. 1989 Feb 25;17(4):1745–1756. doi: 10.1093/nar/17.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S. G., Miller H., Brenner C. A., Nocente-McGrath C., Francis S., McIsaac R. Characterization of a cDNA clone coding for a sea urchin histone H2A variant related to the H2A.F/Z histone protein in vertebrates. Nucleic Acids Res. 1987 Jun 11;15(11):4629–4644. doi: 10.1093/nar/15.11.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Green G. R., Patel J. C., Hecht N. B., Poccia D. L. A complex pattern of H2A phosphorylation in the mouse testis. Exp Cell Res. 1991 Jul;195(1):8–12. doi: 10.1016/0014-4827(91)90493-e. [DOI] [PubMed] [Google Scholar]
- Green G. R., Poccia D. L. Phosphorylation of sea urchin histone CS H2A. Dev Biol. 1989 Aug;134(2):413–419. doi: 10.1016/0012-1606(89)90113-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. E., Böhni R., Schneiderman M. H., Ramamurthy L., Schümperli D., Marzluff W. F. Regulation of histone mRNA in the unperturbed cell cycle: evidence suggesting control at two posttranscriptional steps. Mol Cell Biol. 1991 May;11(5):2416–2424. doi: 10.1128/mcb.11.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch C. L., Bonner W. M. Sequence of cDNAs for mammalian H2A.Z, an evolutionarily diverged but highly conserved basal histone H2A isoprotein species. Nucleic Acids Res. 1988 Feb 11;16(3):1113–1124. doi: 10.1093/nar/16.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch C. L., Bonner W. M. The human histone H2A.Z gene. Sequence and regulation. J Biol Chem. 1990 Sep 5;265(25):15211–15218. [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. The regulation of histone gene expression during the cell cycle. Biochim Biophys Acta. 1991 Mar 26;1088(3):327–339. doi: 10.1016/0167-4781(91)90122-3. [DOI] [PubMed] [Google Scholar]
- Henderson G. S., Conary J. T., Davidson J. M., Stewart S. J., House F. S., McCurley T. L. A reliable method for northern blot analysis using synthetic oligonucleotide probes. Biotechniques. 1991 Feb;10(2):190–197. [PubMed] [Google Scholar]
- Hurt M. M., Chodchoy N., Marzluff W. F. The mouse histone H2a.2 gene from chromosome 3. Nucleic Acids Res. 1989 Nov 11;17(21):8876–8876. doi: 10.1093/nar/17.21.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Steinbeisser H. DNA-dependent phosphorylation of histone H2A.X during nucleosome assembly in Xenopus laevis oocytes: involvement of protein phosphorylation in nucleosome spacing. EMBO J. 1991 Oct;10(10):3043–3050. doi: 10.1002/j.1460-2075.1991.tb07855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. J., Chodchoy N., Marzluff W. F., Skoultchi A. I. Coupling of replication type histone mRNA levels to DNA synthesis requires the stem-loop sequence at the 3' end of the mRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6189–6193. doi: 10.1073/pnas.84.17.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannironi C., Bonner W. M., Hatch C. L. H2A.X. a histone isoprotein with a conserved C-terminal sequence, is encoded by a novel mRNA with both DNA replication type and polyA 3' processing signals. Nucleic Acids Res. 1989 Nov 25;17(22):9113–9126. doi: 10.1093/nar/17.22.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Kato T., Morita T., Nozaki M., Kubota H., Yagi H., Matsushiro A. Polyadenylated and 3' processed mRNAs are transcribed from the mouse histone H2A.X gene. Nucleic Acids Res. 1991 May 11;19(9):2441–2447. doi: 10.1093/nar/19.9.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Bonner W. M. Specific alterations in the pattern of histone-3 synthesis during conversion of human leukemic cells to terminally differentiated cells in culture. Differentiation. 1984;28(2):186–190. doi: 10.1111/j.1432-0436.1984.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Ross J., Peltz S. W., Kobs G., Brewer G. Histone mRNA degradation in vivo: the first detectable step occurs at or near the 3' terminus. Mol Cell Biol. 1986 Dec;6(12):4362–4371. doi: 10.1128/mcb.6.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman S. E., Casano P. J., Pilch D. R., Marzluff W. F., Sittman D. B. Characterization of mouse H3.3-like histone genes. Gene. 1987;59(1):29–39. doi: 10.1016/0378-1119(87)90263-0. [DOI] [PubMed] [Google Scholar]
- Wells D., Kedes L. Structure of a human histone cDNA: evidence that basally expressed histone genes have intervening sequences and encode polyadenylylated mRNAs. Proc Natl Acad Sci U S A. 1985 May;82(9):2834–2838. doi: 10.1073/pnas.82.9.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2A, a heteromorphous family of eight protein species. Biochemistry. 1980 Jul 8;19(14):3238–3245. doi: 10.1021/bi00555a022. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Mechanism for differential sensitivity of the chromosome and growth cycles of mammalian cells to the rate of protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2959–2966. doi: 10.1128/mcb.5.11.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]
- Zhong R., Roeder R. G., Heintz N. The primary structure and expression of four cloned human histone genes. Nucleic Acids Res. 1983 Nov 11;11(21):7409–7425. doi: 10.1093/nar/11.21.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daal A., White E. M., Elgin S. C., Gorovsky M. A. Conservation of intron position indicates separation of major and variant H2As is an early event in the evolution of eukaryotes. J Mol Evol. 1990 May;30(5):449–455. doi: 10.1007/BF02101116. [DOI] [PubMed] [Google Scholar]