Abstract
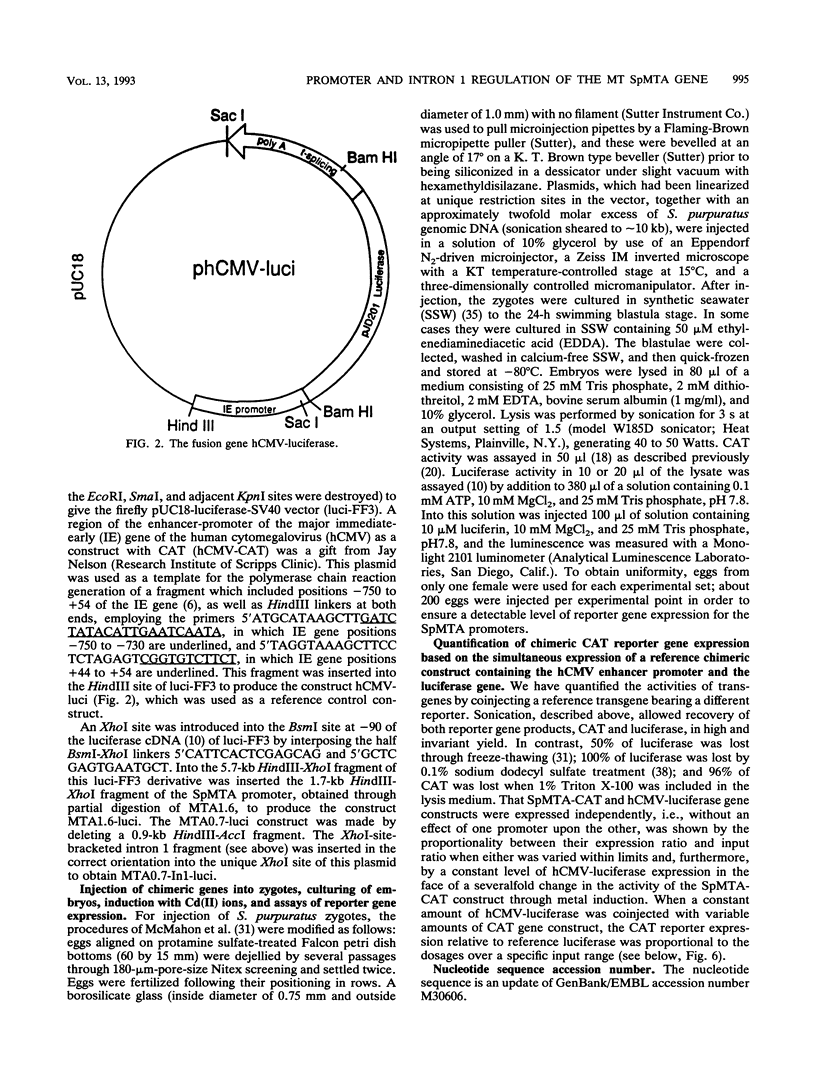
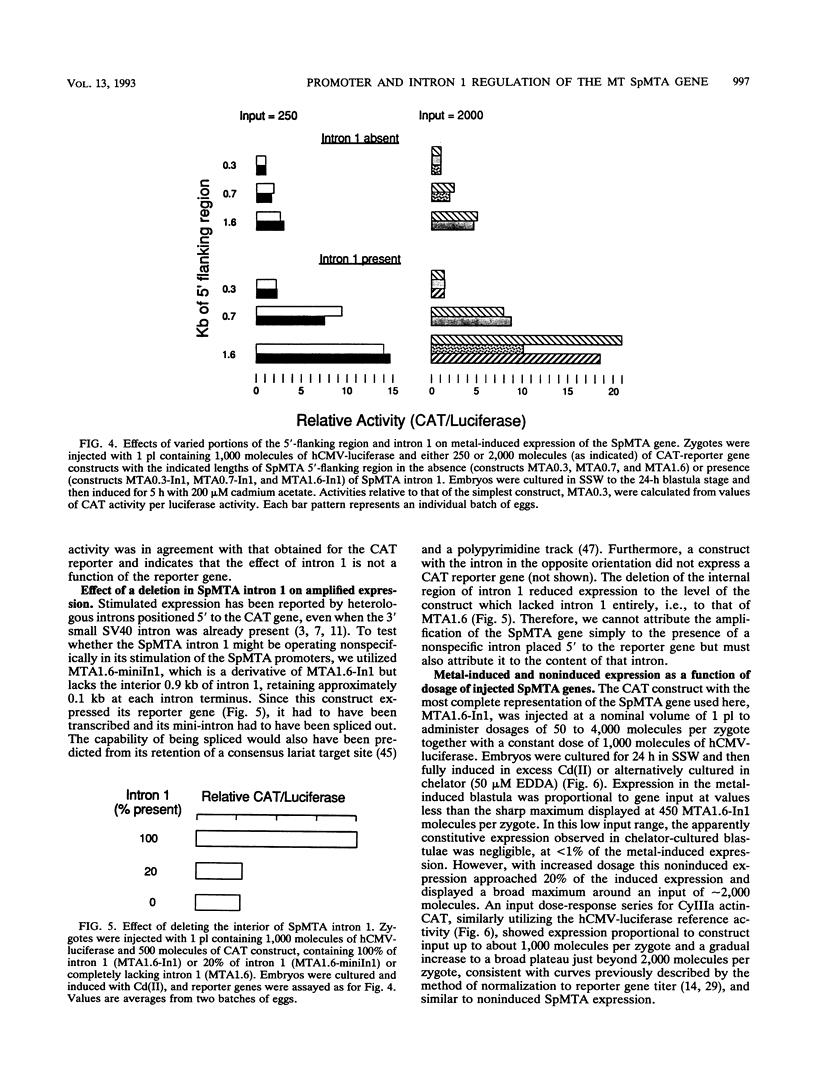
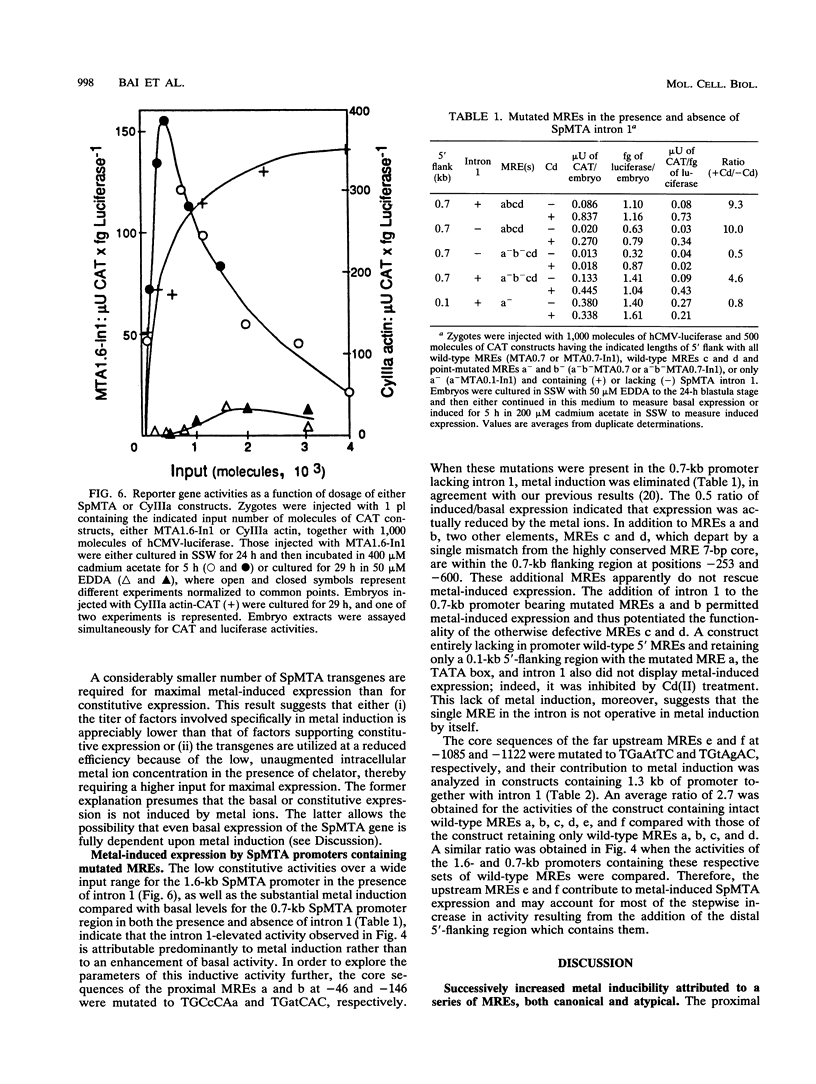
The SpMTA metallothionein gene of the sea urchin Strongylocentrotus purpuratus is regulated developmentally, histospecifically, and by heavy-metal induction. The sequenced 5' flank of the gene can be divided into proximal, middle, and distal regions, each containing a pair of metal response elements (MREs). Canonical 7-bp core sequences are present in all except the middle-region MREs c and d, which contain 1-bp mismatches. Metal-induced expression in transgenic blastulae was increased with each consecutive addition of the middle and distal regions to a chimeric reporter gene construct containing the proximal SpMTA promoter region. Reduced metal induction through point mutation of the distal MREs e and f indicated that the MREs themselves were largely responsible for the transcriptional increase. These activities were further enhanced by SpMTA intron 1, but not when a specific interior region of the intron had been deleted. The atypical MREs c and d did not support induction by themselves, i.e., when present alone with mutated proximal MREs a and b. However, in the presence of intron 1, they were able to substitute for the nullified MREs a and b in the promotion of metal-induced expression. This capability suggests, furthermore, that these atypical MREs, in addition to responding to an intron 1 region, participate cooperatively with the canonical proximal MREs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Blasquez V. C., Xu M., Moses S. C., Garrard W. T. Immunoglobulin kappa gene expression after stable integration. I. Role of the intronic MAR and enhancer in plasmacytoma cells. J Biol Chem. 1989 Dec 15;264(35):21183–21189. [PubMed] [Google Scholar]
- Bornstein P., Alfi D., Devarayalu S., Framson P., Li P. Characterization of the mouse thrombospondin gene and evaluation of the role of the first intron in human gene expression. J Biol Chem. 1990 Sep 25;265(27):16691–16698. [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988 Feb 5;263(4):1603–1606. [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Harless M. L., Wright D. A., Kellems R. E. Identification and characterization of transcriptional arrest sites in exon 1 of the human adenosine deaminase gene. Mol Cell Biol. 1990 Sep;10(9):4555–4564. doi: 10.1128/mcb.10.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Hamer D. H. Fine mapping of a mouse metallothionein gene metal response element. Mol Cell Biol. 1989 Mar;9(3):1376–1380. doi: 10.1128/mcb.9.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Introns in the 3'-untranslated region can inhibit chimeric CAT and beta-galactosidase gene expression. Gene. 1989 Dec 7;84(1):135–142. doi: 10.1016/0378-1119(89)90147-9. [DOI] [PubMed] [Google Scholar]
- Forry-Schaudies S., Hughes S. H. The chicken tropomyosin 1 gene generates nine mRNAs by alternative splicing. J Biol Chem. 1991 Jul 25;266(21):13821–13827. [PubMed] [Google Scholar]
- Foss K., McClain W. H. Rapid site-specific mutagenesis in plasmids. Gene. 1987;59(2-3):285–290. doi: 10.1016/0378-1119(87)90336-2. [DOI] [PubMed] [Google Scholar]
- Franks R. R., Anderson R., Moore J. G., Hough-Evans B. R., Britten R. J., Davidson E. H. Competitive titration in living sea urchin embryos of regulatory factors required for expression of the CyIIIa actin gene. Development. 1990 Sep;110(1):31–40. doi: 10.1242/dev.110.1.31. [DOI] [PubMed] [Google Scholar]
- Frederickson R. M., Micheau M. R., Iwamoto A., Miyamoto N. G. 5' flanking and first intron sequences of the human beta-actin gene required for efficient promoter activity. Nucleic Acids Res. 1989 Jan 11;17(1):253–270. doi: 10.1093/nar/17.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Zhang W., Klein W. H. Repetitive DNA sequences linked to the sea urchin spec genes contain transcriptional enhancer-like elements. Dev Biol. 1990 May;139(1):186–196. doi: 10.1016/0012-1606(90)90287-s. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Harlow P., Watkins E., Thornton R. D., Nemer M. Structure of an ectodermally expressed sea urchin metallothionein gene and characterization of its metal-responsive region. Mol Cell Biol. 1989 Dec;9(12):5445–5455. doi: 10.1128/mcb.9.12.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Goto K., Okada T. S., Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987 Oct;1(8):818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Roscigno R. F., Mulligan G. J., Finn L. A., Weber K. S. Identification of two distinct intron elements involved in alternative splicing of beta-tropomyosin pre-mRNA. Genes Dev. 1990 Jan;4(1):98–110. doi: 10.1101/gad.4.1.98. [DOI] [PubMed] [Google Scholar]
- Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991 May 10;252(5007):833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karpinski B. A., Yang L. H., Cacheris P., Morle G. D., Leiden J. M. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol Cell Biol. 1989 Jun;9(6):2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A. Spatial and temporal control elements of the Drosophila engrailed gene. Genes Dev. 1990 Mar;4(3):433–443. doi: 10.1101/gad.4.3.433. [DOI] [PubMed] [Google Scholar]
- Killen P. D., Burbelo P. D., Martin G. R., Yamada Y. Characterization of the promoter for the alpha 1 (IV) collagen gene. DNA sequences within the first intron enhance transcription. J Biol Chem. 1988 Sep 5;263(25):12310–12314. [PubMed] [Google Scholar]
- Livant D. L., Cutting A. E., Britten R. J., Davidson E. H. An in vivo titration of regulatory factors required for expression of a fusion gene in transgenic sea urchin embryos. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7607–7611. doi: 10.1073/pnas.85.20.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes N. F., Bushel P., Mendelsohn L., Wu J., Yen M. Y., Allan M. A short, highly repetitive element in intron -1 of the human c-Ha-ras gene acts as a block to transcriptional readthrough by a viral promoter. Mol Cell Biol. 1990 Sep;10(9):4990–4995. doi: 10.1128/mcb.10.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Flytzanis C. N., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985 Apr;108(2):420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Salser S. J., Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988 Apr;2(4):412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- Nemer M. An altered series of ectodermal gene expressions accompanying the reversible suspension of differentiation in the zinc-animalized sea urchin embryo. Dev Biol. 1986 Mar;114(1):214–224. doi: 10.1016/0012-1606(86)90397-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Thornton R. D., Stuebing E. W., Harlow P. Structure, spatial, and temporal expression of two sea urchin metallothionein genes, SpMTB1 and SpMTA. J Biol Chem. 1991 Apr 5;266(10):6586–6593. [PubMed] [Google Scholar]
- Nemer M., Travaglini E. C., Rondinelli E., D'Alonzo J. Developmental regulation, induction, and embryonic tissue specificity of sea urchin metallothionein gene expression. Dev Biol. 1984 Apr;102(2):471–482. doi: 10.1016/0012-1606(84)90212-4. [DOI] [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C. Primary differentiation and ectoderm-specific gene expression in the animalized sea urchin embryo. Dev Biol. 1985 Jun;109(2):418–427. doi: 10.1016/0012-1606(85)90468-3. [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Liu S. H., Leavitt J., Kedes L. Regulation of the human beta-actin promoter by upstream and intron domains. Nucleic Acids Res. 1989 Jan 25;17(2):601–615. doi: 10.1093/nar/17.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Bacterial beta-galactosidase as a marker of Rous sarcoma virus gene expression and replication. Mol Cell Biol. 1985 Feb;5(2):281–290. doi: 10.1128/mcb.5.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler S., Eismann E. R., Krämer H., Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990 Apr;9(4):973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Rippe R. A., Lorenzen S. I., Brenner D. A., Breindl M. Regulatory elements in the 5'-flanking region and the first intron contribute to transcriptional control of the mouse alpha 1 type I collagen gene. Mol Cell Biol. 1989 May;9(5):2224–2227. doi: 10.1128/mcb.9.5.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Stuart G. W., Palmiter R. D. Building a metal-responsive promoter with synthetic regulatory elements. Mol Cell Biol. 1985 Jun;5(6):1480–1489. doi: 10.1128/mcb.5.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Stuart G. W., Palmiter R. D. Metal regulatory elements of the mouse metallothionein-I gene. Experientia Suppl. 1987;52:407–414. doi: 10.1007/978-3-0348-6784-9_39. [DOI] [PubMed] [Google Scholar]
- Serfling E., Lübbe A., Dorsch-Häsler K., Schaffner W. Metal-dependent SV40 viruses containing inducible enhancers from the upstream region of metallothionein genes. EMBO J. 1985 Dec 30;4(13B):3851–3859. doi: 10.1002/j.1460-2075.1985.tb04157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Sherwood A. L., Bottenus R. E., Martzen M. R., Bornstein P. Structural and functional analysis of the first intron of the human alpha 2(I) collagen-encoding gene. Gene. 1990 May 14;89(2):239–244. doi: 10.1016/0378-1119(90)90011-f. [DOI] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Thiebaud P., Goodstein M., Calzone F. J., Thézé N., Britten R. J., Davidson E. H. Intersecting batteries of differentially expressed genes in the early sea urchin embryo. Genes Dev. 1990 Nov;4(11):1999–2010. doi: 10.1101/gad.4.11.1999. [DOI] [PubMed] [Google Scholar]
- Thiele D. J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek D. F., Smith C. W., Nadal-Ginard B. The rat alpha-tropomyosin gene generates a minimum of six different mRNAs coding for striated, smooth, and nonmuscle isoforms by alternative splicing. Mol Cell Biol. 1988 Feb;8(2):679–694. doi: 10.1128/mcb.8.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Nemer M. Metallothionein genes MTa and MTb expressed under distinct quantitative and tissue-specific regulation in sea urchin embryos. Mol Cell Biol. 1987 Jan;7(1):48–58. doi: 10.1128/mcb.7.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]


