Abstract
Presynaptic Ca2+ influx pathways, cytoplasmic Ca2+ buffering proteins and Ca2+ extrusion processes undergo considerable change during the first postnatal month in rodent neurons. These changes may be critical in establishing short-term plasticity at maturing presynaptic terminals where neurotransmitter release is directly dependent on the dynamics of cytoplasmic residual Ca2+ ([Ca2+]res). In particular, the robust paired-pulse facilitation characteristic of adult neurons is almost entirely lacking in newborns. To examine developmental changes in processes controlling [Ca2+]res, we measured the timecourse of [Ca2+]res decay in presynaptic terminals of Schaffer collateral to CA1 synapses in acute hippocampal slices following single and paired orthodromic stimuli in the stratum radiatum. Developmental changes were observed in both the rise time and slow exponential decay components of the response to single stimuli such that this decay was larger and faster in the adult. Furthermore, we observed a greater caffeine-sensitive basal Ca2+ store, which was differentially affected when active uptake into the endoplasmic reticulum was blocked, in the presynaptic fields of the Schaffer collateral to CA1 terminals of P6 and younger mice when compared to adults. These transitions in [Ca2+]res dynamics occurred gradually over the first weeks of postnatal life and correlated with changes in short-term plasticity.
Keywords: calcium-sensitive dye, hippocampus, kinetics, SERCA
Introduction
Marked developmental changes occur in the rodent hippocampus during the first 3 weeks after birth, with consequences that affect a wide range of processes extending from the expression and regulation of proteins to the determination of cell morphology and phenotype (Dumas, 2005; Danglot et al., 2006). This early postnatal period of rodent brain development correlates with a parallel developmental period in humans during which the onset of many neurological disorders including obsessive–compulsive disorder, attention-deficit hyperactivity disorder and schizophrenia are believed to occur (Anderson, 2002; Dumas, 2005).
Paired-pulse facilitation (PPF) is a measure of short-term plasticity in which the efficacy of the second of paired synaptic responses is increased in amplitude over that of the first response. It is generally accepted that PPF results from presynaptic residual Ca2+ ([Ca2+]res) and its downstream effects (Katz & Miledi, 1968; Wu & Saggau, 1994; Cabezas & Buno, 2006; Schiess et al., 2006) and this suggests that changes in [Ca2+]res clearance after stimulation should play an important role in the kinetics of PPF. PPF in the Schaffer collateral to CA1 pyramidal neuron (SC-CA1) synapse exhibits significant change during the early postnatal period of development, during which time there is an increase in the magnitude of PPF and a predicted change in [Ca2+]res regulation (Wasling et al., 2004). Thus the initiation of PPF can provide a criterion that distinguishes the transition between immature and mature synaptic transmission.
Significant changes in the kinetics of presynaptic Ca2+ dynamics at SC-CA1 synapses during early postnatal periods are likely to be crucial to the maturation of synaptic plasticity and brain function; however, little is known about the development of presynaptic components of synaptic plasticity at this synapse (Hanse et al., 2009). In adult rodents, the presynaptic [Ca2+]res clearance in SC-CA1 terminals has been accurately described as a double exponential process (Schiess et al., 2006), which suggests contributions from more than one underlying mechanism. These mechanisms could include buffering, diffusion, uptake into or release from internal stores, and extrusion (Neher & Augustine, 1992; Koester & Sakmann, 2000; Emptage et al., 2001; Carter et al., 2002; Cabezas & Buno, 2006). The [Ca2+]res clearance in SC-CA1 presynaptic terminals of immature animals may be even more complex. While [Ca2+]res clearance is only minimally sensitive to Ca2+ buffer saturation in rodent SC-CA1 terminals, immature terminals are somewhat more sensitive than are adult terminals (Blatow et al., 2003). This small change in buffer saturation, however, does not appear to account for the large developmental change in short-term synaptic plasticity (Dekay et al., 2006). One attractive alternative possibility, which could contribute significantly to [Ca2+]res kinetics but has not been analyzed in this synapse, is developmental changes in the process of Ca2+ uptake into, and release from, internal stores. In this study, we have addressed this question and we report modifications in [Ca2+]res clearance kinetic processes that correlate well with developmental changes in short-term synaptic plasticity.
Materials and methods
Slice preparation
Experiments were performed in coronal hippocampal slices prepared from C57/Bl6 mice at ages described in each experiment; adults were used between 60 and 120 days, average age 86 days. Animals were deeply anaesthetized by i.p. injection of 250 mg kg−1 ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA), brains were rapidly removed, and slices were cut at 300–400 μm with a vibroslicer (Pelco 101, St Louis, MO, USA) in an ice bath with a cutting solution containing (in mM): sucrose, 220; KCl, 3; NaH2PO4, 1.2; NaHCO3, 26; MgSO4, 12; CaCl2, 0.2; glucose, 10; and ketamine, 0.01 mg mL−1; equilibrated with 95%O2–5%CO2. Slices were then transferred to a bath containing artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2.5 CaCl2 and 10 glucose equilibrated with 95%O2–5%CO2 at 30°C for 1 h and then maintained at room temperature until transfer to a temperature-controlled recording chamber (Warner Instruments, Hamden, CT, USA or Scientific Systems Design, Mercerville, NJ, USA), which was maintained at 32°C and continuously perfused at 2 ml min−1 with ACSF saturated with 95%O2–5%CO2. All experiments were approved by the Institutional Animal Care and Use Committee at the University of New Mexico Health Sciences Center and conformed with NIH guidelines.
Presynaptic Ca2+ imaging
Presynaptic fibers were filled with the Ca2+ fluorophore, Mg Green AM or Fura2 AM (Molecular Probes, Eugene, OR, USA) using an established technique that measures a spatial and temporal average of [Ca2+] in the presynaptic terminal (Regehr & Tank, 1991; Wu & Saggau, 1994; Atluri & Regehr, 1996; Sinha et al., 1997; Kamiya & Ozawa, 1999). This technique provides simultaneous measurements of presynapic calcium and postsynaptic fEPSP in localized populations of synaptic contacts. In a small number of instances either the presynaptic or postsynaptic recording was not successful, but the data from the successful component were still included. To minimize the effect of exogenous buffers we used Mg Green, with a Ca2+ binding KD = 6 μM, for experiments in which we measured the timecourse of ΔF/F0 decay (Regehr & Atluri, 1995; Atluri & Regehr, 1996); however, in some experiments where the rapid decay timecourse was not being assessed, we improved the sensitivity of the ΔF/F0 measurements by using the higher affinity fluorophore Fura2. Briefly, an ejection electrode (tip diameter 5–10 μm) containing the fluorophore (0.9mM Mg Green AM, 10% DMSO, 1% pluronic acid in ACSF or 1 mM Fura2 AM, 10% DMSO, 1% pluronic acid in ACSF) was lowered into the fiber pathway between the stimulating electrode and the presynaptic terminal field to be investigated. While observing the emission image following excitation (490 nm Mg Green AM or 350 nm Fura2 AM) an air pressure pulse was applied with a syringe to the ejection electrode until a small bright spot (≈1 μL) was observed in the fiber pathway.
The slice was then maintained with a 2 mL min−1 flow of oxygenated ACSF at 32°C for 1 h to allow intracellular diffusion of the dye to the presynaptic imaging site 500 μm away from the ejection site. The excitation light was then reduced to a 100- to 200-μm-diameter spot with a diaphragm in the epi-illumination path, and the emitted light was measured with a photomultiplier tube. This spot included or was immediately adjacent to the area electrically summed by the field potential recording. A single stimulus or pairs of stimuli were delivered orthodromically at 0.05 or 0.067 Hz by a Master 8 pulse generator (AMPI Instruments, Jerusalem, Israel) under control of the imaging system (TILL Photonics, Pleasanton, CA, USA). For Mg Green studies, fluorescence responses are reported as the ratio of the change in fluorescence to the pre-stimulus fluorescence (ΔF/F0). For Fura-2 studies, we first determined the 350–380 nm fluorescent ratio and reported the response as the ratio of the change in this ratio to the pre-stimulus ratio (ΔR/R0). The ΔF/F0 and ΔR/R0 signals were corrected for bleaching by subtraction of a linear baseline slope and were inverted so that increasing presynaptic [Ca2+]i produced an upward deflection. To diminish noise inherent with the low-affinity Ca2+ indicator, it was necessary to average five fluorescence responses and to filter the photomultiplier tube signal at 1 kHz.
We used two tests to demonstrate that the measured ΔF/F0 signal was consistent with a [Ca2+] response predominately from the presynaptic Schaffer collateral axons and axon terminals (Wu & Saggau, 1994; Atluri & Regehr, 1996; Sinha et al., 1997; Kamiya & Ozawa, 1999). First, in the presence of 10 μM CNQX, 25 μM D-AP5 and 20 μM bicuculline, the fEPSP was blocked while the presynaptic fiber volley and ΔF/F0 signal were left unchanged; the ΔF/F0 signal was thus not a postsynaptic response. Second, subsequent addition of 600 nM TTX blocked both the presynaptic fiber volley and the ΔF/F0 signal, arguing against direct stimulation of inadvertently filled postsynaptic dendrites.
Field potential recordings
We used standard electrophysiological techniques for slice field excitatory postsynaptic potential (fEPSP) recordings in the SC-CA1 pyramidal neuron synapse in the stratum pyramidale in hippocampal slices (Schiess et al., 2006). Briefly, fEPSPs were recorded with an Axoclamp 2B or Multiclamp 700B amplifier (both from Axon Instruments, Union City, CA, USA) and a Digidata 1322A interface using PCLAMP 9.2 or 10 software (Axon Instruments) for experimental control and data analysis. fEPSPs were digitized at 20 kHz and filtered at 2 kHz. Presynaptic constant-current pulses (150 μs duration) were applied to Schaffer collateral fibers with an Iso-Flex constant current stimulator (API Instruments, Jerusalem, Israel) through a concentric bipolar electrode (FHC, Bowdoinham, ME, USA), at a current which was adjusted to produce 40–60% of the maximum fEPSP amplitude. The paired-pulse ratio (PPR) of the fEPSP signal was calculated as the ratio of the slope of the second fEPSP at a 50-ms interpulse interval to that of the initial fEPSP. The relationship between [Ca2+]res and PPR was determined using concurrent ΔF/F0 and fEPSP recordings of paired pulses at interpulse intervals of 50, 100, 150, 200, 300 and 500 ms. The [Ca2+]res was calculated by measuring the amplitude of the ΔF/F0 immediately before the second pulse of each pair.
Data analysis
Combining data, even for single developmental days, from the period during which there are rapid changes in [Ca2+]res dynamics is expected to introduce variability into measured parameters. Indeed, our data from early developmental points varied substantially and this variability decreased with increasing age. We have interpreted this to be an indication of the narrow temporal window over which some of these maturation processes occur.
The ΔF/F0 signal was digitally filtered with a five-point center-weighted filter and fit using a least-squares regression routine to a single exponential decay beginning 50 ms after the stimulus initiation. In addition, the ΔF/F0 signal was numerically integrated before (∫ΔF/F0) or after being normalized to the ΔF/F0 peak value (∫νΔF/F0). The rise time was measured by subtracting the time of the ΔF/F0 peak value and the time of stimulation. Fitting and statistical analysis were carried out with MATLAB 7.0 (Mathworks, Natick, MA, USA), PROSTAT 4.0 (Polysoft International, Pearl River, NY, USA) or PRISM (GraphPad Software, La Jolla, CA, USA). Coefficient of determination (COD) was used as a measure of goodness of fit for linear regressions. Average data are presented as means ± SEM, and statistical significance was determined at P < 0.05.
Drugs
Drugs were stored frozen in aliquots and diluted to the appropriate concentration in ACSF on the day of the experiment. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), d-(1)-2-amino-5-phosphonopentanoic acid (d-AP5), bicuculline, thapsigargin and tetrodotoxin (TTX) were obtained from Tocris (Ellisville, MO, USA) and all other drugs and reagents were obtained from Sigma-Aldrich (St Louis, MO, USA). Drugs were applied through a bath perfusion system for a minimum of 10 min (> 2.5 bath exchanges) before recording commenced. The timecourse experiments were continuously recorded during bath exchange and the time for complete response was expected to result from a combination of the bath exchange time constant (70 s) and the time necessary for drug permeation into the slice.
Results
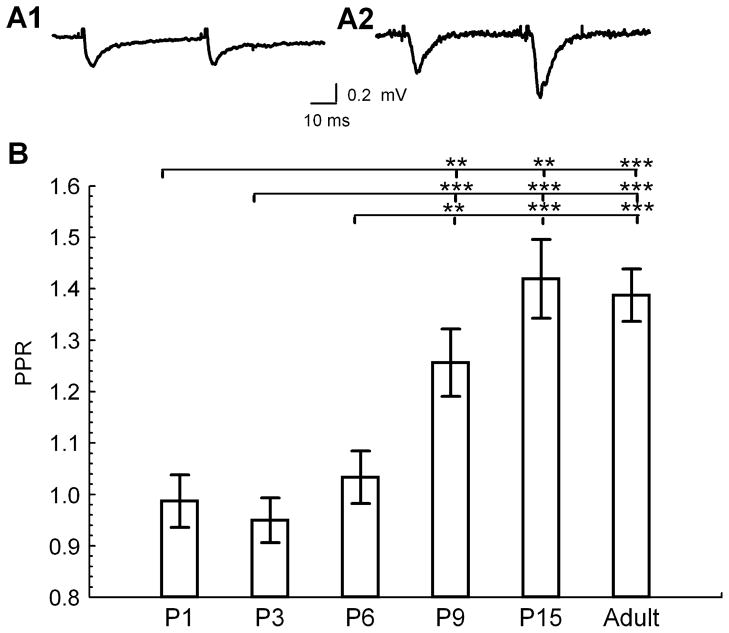
Previous studies have shown that there is a developmental increase in hippocampal short-term facilitation during the first few postnatal weeks in rats (Wasling et al., 2004). To determine whether a similar developmental change occurs in mice we measured the PPR of fEPSPs in response to paired stimuli at 50-ms interpulse intervals in hippocampal slice preparations from P1 to adult (> P60; Fig. 1A). Similar to the findings in rats, we found a developmental increase in PPR at the SC-CA1 synapse in the mouse between P6 and P9 (Fig. 1B); however, no significant additional change was observed between P15 and adult animals.
Fig 1.
Developmental increase in short-term synaptic plasticity. (A) Representative traces of fEPSP slopes at half maximum at two developmental stages during paired stimulation at 50-ms interpulse interval, average of five traces [A1, immature (P6); A2, adult (P114)]. (B) PPR at different developmental ages (ANOVA with Fisher’s post hoc comparison of significance of PPR vs. age between groups, F4,25 = 8.71, P = 0.0002; P1, n = 4; P3, n = 6; P6, n = 11; P9, n = 6; P15, n = 4; adult, n = 3 animals). **P < 0.01 and ***P < 0.001.
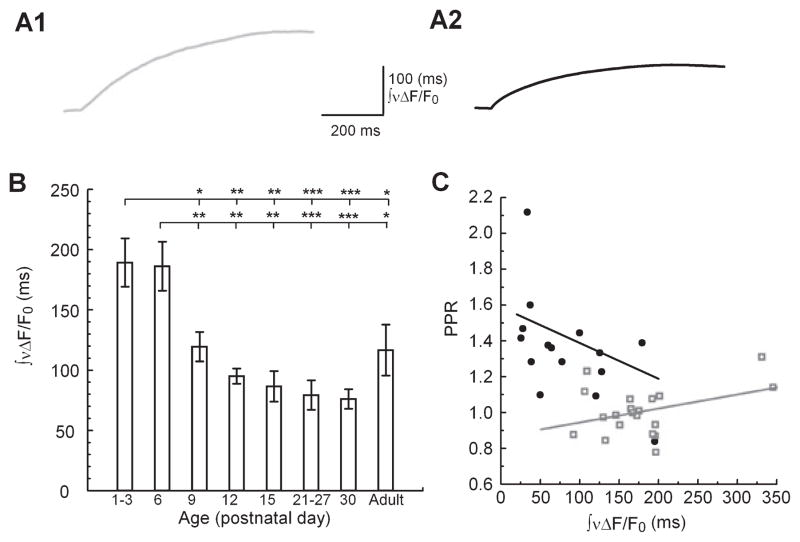
As PPF is believed to be dependent on [Ca2+]res, we next chose to measure the kinetics of [Ca2+]res during this developmental period. To characterize the developmental changes of presynaptic Ca2+ kinetics in the SC-CA1 synapse, we used Ca2+-sensitive fluorescent dyes to measure presynaptic [Ca2+]res following stimuli in the stratum radiatum in brain slices from animals aged between P1 and P120. For this purpose, presynaptic terminals were loaded with AM-derivatives of either Mg Green for millisecond kinetic analysis or Fura-2 for more sensitive analysis of [Ca2+]pre on a timescale of seconds (see Materials and Methods). Previous investigations in adult hippocampus in our lab (Schiess et al., 2006) and other labs (Regehr & Tank, 1991; Wu & Saggau, 1994) have demonstrated that this method reports almost exclusively Ca2+ dynamics of presynaptic terminals (see Materials and Methods, and Discussion). We evaluated quantitatively the changes in [Ca2+]res kinetics by first measuring the change in fluorophore fluorescence of the Mg Green signal (ΔF/F0) in response to a single stimulus. To obtain a measure of [Ca2+]res timecourse that made no assumptions about the specific underlying kinetic processes we calculated the integral of ΔF/F0 after it had been normalized to its peak; this provides a measure of the overall kinetic behavior of the [Ca2+]res transient (Fig. 2, A1 and A2; ∫νΔF/F0). We found a significant age-dependent decrease in the ∫νΔF/F0 (Fig. 2B), which is consistent with a more rapid [Ca2+]res clearance kinetics in older animals.
Fig 2.
Developmental changes in presynaptic ∫νΔF/F0. (A) Representative traces of ∫νΔF/F0 at two developmental ages (A1, P6; A2, Adult (P85); Mg Green) (B) Summary data of ∫νΔF/F0 vs. age. ANOVA with Fisher’s post hoc comparison of significance of ∫νΔF/F0 vs. age between groups: F40,7 = 6.65; P = 0.00001; P1–P3, n = 8; P6, n = 12; P9, n = 5; P12, n = 4; P15, n = 3; P21–P27, n = 9; P30, n = 3; adult, n = 4 animals. (C) Concurrent recordings of fEPSP PPF and ∫νΔF/F0 (P1–P6, grey open squares, n = 20 animals; P9–adult, black circles, n = 15 animals) fit by least-squares linear regression. *P < 0.05, **P < 0.01 and ***P < 0.001.
To assess the relationship between presynaptic Ca2+ kinetics and facilitation we plotted the ∫νΔF/F0, following a single pulse against the PPR observed in concurrent postsynaptic recordings (Fig. 2C). Because there appeared to be a distinct transition in PPR between P6 and P9, we grouped the data into two groups: P1–P6 and P9–adult. We found a negative slope in the P9–adult group, consistent with the dependence of facilitation on ∫νΔF/F0 with decreased probability of release and enhanced facilitation (slope = −1.36 × 10−5, COD = 0.29). In contrast, however, the P1–P6 age group did not exhibit a negative slope and the data were more poorly correlated (slope = 0.521 × 10−5, COD = 0.14). This suggests a developmental transition between P6 and P9 during which presynaptic [Ca2+]res assumes a role in short-term facilitation. Typically, increased [Ca2+]res correlates with increased facilitation and depletion of vesicles correlates with decreased facilitation (Liley & North, 1953; Takeuchi, 1958; Elmqvist & Quastel, 1965; Thies, 1965; Betz, 1970; Zucker & Regehr, 2002). However, the negative correlation between ∫νΔF/F0 and PPR suggests [Ca2+]res removal mechanisms are important in the amount of facilitation. Interestingly, a more rapid [Ca2+]res removal is reflected by decreased ∫νΔF/F0 and this is correlated with increased facilitation. To determine the processes that contribute to the regulation of [Ca2+]res during this important developmental period, we further investigated the kinetics of [Ca2+]res clearance.
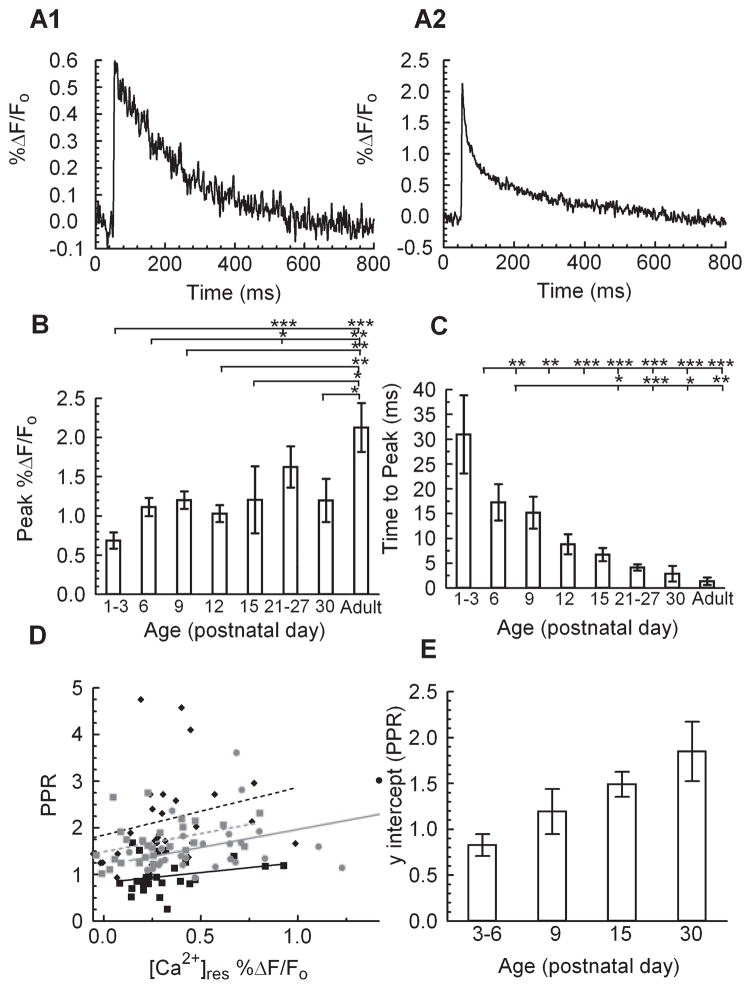
As shown in Fig. 3A, there were distinct age-dependent differences in the decay of the Mg Green fluorescence signal, indicative of a change in the rate of [Ca2+]res clearance between P6 and adult mice. We first measured the kinetic components responsible for Ca2+ accumulation, peak amplitude of ΔF/F0, and time to peak of ΔF/F0. There was an approximate doubling of the peak amplitude of ΔF/F0 from P1 to adult (Fig. 3B). Because this could have resulted in part from a difference in Ca2+ influx or in the kinetics of an internal store contribution, we also compared the time at which the peak ΔF/F0 occurred at the different ages. This analysis revealed a dramatic monotonic 20-fold decrease in the time to peak ΔF/F0 from P1 to adult (Fig. 3C). Taken together, the age-dependent decrease in the ∫νΔF/F0 signal must therefore reflect an overall increase in the rate of [Ca2+]res clearance, as there was a simultaneous increase in the peak ΔF/F0 amplitude.
Fig 3.
Developmental changes in presynaptic [Ca2+]res kinetics. (A) Representative traces of ΔF/F0 during single stimulus pulses at two developmental ages (top, P6; bottom, P114; Mg Green). (B) Summary data of maximum ΔF/F0 vs. age. ANOVA with Fisher’s post hoc comparison of significance of peak ΔF/F0 between age groups: F40,7 = 4.12, P = 0.0017. (C) Time to peak ΔF/F0 vs. age. ANOVA with Fisher’s post hoc comparison of significance of time to peak between age groups: F40,7 = 4.21, P = 0.0016. P1–P3, n = 8; P6, n = 12; P9, n = 5; P12, n = 4; P15, n = 3; P21–P27, n = 9; P30, n = 3; adult, n = 4 animals. Further analysis of this data is shown in Figs 2 and 4. (D) Relationship of PPR vs. [Ca2+]res measured as % ΔF/F0 before stimulation compared to the PPR at IPIs of 50, 100, 150, 200, 300 and 500 ms; least-squares linear regression yields a nonsignificant difference in slope between groups (slope= 0.7756; F99,3 = 0.18, P = 0.91; P3–P6, black squares, black line, n = 4; P9, gray squares, gray line, n = 4; P15, gray circles, dashed gray line, n = 4; P30, black diamonds, dashed black lines, n = 4 animals) (E) Y-intercept vs. age from the linear regression of PPR vs. [Ca2+]res; significant difference of age vs. intercept (F102,3 = 17.97, P < 0.0001). *P < 0.05, **P < 0.01 and ***P < 0.001.
As the [Ca2+]res has been associated with the degree of PPF, we next compared the relationship between [Ca2+]res and the simultaneously measured PPF in the different aged groups by changing the interpulse interval while keeping the stimulation strength constant (Fig. 3D). Analysis of these data demonstrated there was a significant positive slope of this relationship in slices from older animals: P9 (slope=0.96 PPR/%ΔF/F0, COD = 0.23, F1,25 = 7.47, P = 0.011), P15 (slope = 0.97 PPR/%ΔF/F0, COD = 0.27, F1,24 = 8.68, P = 0.0071) and adult mice (slope = 1.17 PPR/%ΔF/F0, COD = 0.1752, F1,25 = 5.31, P = 0.023). While the slopes of this relationship were not significantly different (Fig. 3D, P = 0.93) among all age groups, the intercept was found to increase significantly with age (Fig. 3E). By contrast, the slope of this relationship for slices in the younger P3 and P6 mice, which do not exhibit significant PPF, did not differ significantly from zero (P3–6 slope = −0.23, COD = 0.06, F1,26 = 1.78, P = 0.19). This suggests that the mechanisms that underlie the [Ca2+]res-dependent facilitation are not estabilised or are disengaged until the second postnatal week of hippocampal development (see Disscusion).
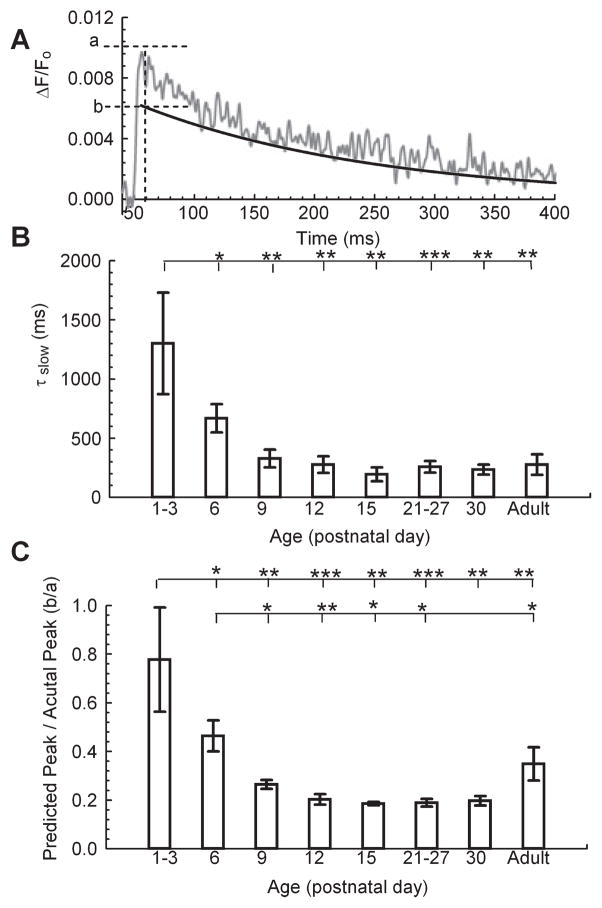
As we reported previously for presynaptic SC-CA1 terminals in adult rats (Schiess et al., 2006), the ΔF/F0 decay in these terminals in adult mice followed a double exponential timecourse whose time constants differed by ~ 10-fold (not shown). In contrast, the presynaptic ΔF/F0 decay in younger mice could not be accurately fit with the sum of two decaying exponential processes and we attributed this to the significantly delayed ΔF/F0 peak time in the younger animals. In adult mice, the fast ΔF/F0 clearance process had a time constant of ~20 ms. Consequently, the significantly slower rising kinetic component in the younger animals (time to peak = 30.95 ± 8.31 ms) markedly overlapped this portion of [Ca2+]res clearance (Fig. 3). Therefore, in order to assess developmental changes in [Ca2+]res clearance kinetics independently of the initial Ca2+ accumulation, we measured the decay of ΔF/F0 beginning 50 ms after its peak when the fast rise and decay contributions were minimal. We reasoned that changes in either the rate or the proportion of this slower component of Ca2+ decay would have considerable impact on the overall response. To obtain an estimate of this kinetic component, we fit the slow component of the remaining ΔF/F0 decay as a single exponential process (Fig. 4A). The rate of this slow decay component was then extrapolated to the time of the peak of ΔF/F0 in order to obtain the value indicated as ‘b’ in the rise time in Fig. 4A. The ratio of this value to the actual peak (‘a’ in Fig. 4A) provided an estimate of the contribution of the slow component to the peak ΔF/F0 signal. We found an age-dependent decrease in this ratio (b/a, Fig. 4C) indicating an age-dependent increase in the relative contribution of a process with a fast time constant. Interestingly, the increased peak ΔF/F0 (Fig. 3B) and decreased time to peak (Fig. 3C) offset the decreased slow time constant (Fig. 4B) to minimize any difference in ∫νΔF/F0 between the P1–P3 and P6 age groups (Fig. 2B). Thus, the most dramatic reduction in ∫νΔF/F0 occurred between P6 and P9 (Fig. 2B). These data further indicate that the most dramatic change in [Ca2+]res timecourse occurred over the developmental period of P6 to P9, strongly suggesting a significant transition in the underlying mechanisms of [Ca2+]res clearance during this period. Importantly, the developmental changes that led to a decrease in total [Ca2+]res, as represented by the ∫νΔF/F0, coincided with the simultaneously recorded increase in the amount of PPF (see Discussion).
Fig 4.
Kinetic components of [Ca2+]res evaluated from fits to ΔF/F0. (A) Example ΔF/F0 trace (P9); ‘a’ is the measured peak and ‘b’ the predicted peak from extrapolated single exponential fit to ΔF/F0 data beginning 50 ms after the peak (Mg Green). (B) Slow time constant (τslow) measured by exponential fit to ΔF/F0 trace beginning 50 ms after the peak. ANOVA with Fisher’s post hoc comparison of significance of slow time constant between age groups: F40,7 = 3.03, P = 0.012. (C) Ratio of the extrapolated peak of τslow to the peak amplitude of the ΔF/F0 signal. ANOVA with Fisher’s post hoc comparison of significance of between ratio of predicted peak to actual peak between age groups: F40,7 = 3.38, P = 0.0067. P1–P3, n = 8; P6, n = 12; P9, n = 5; P12, n = 4; P15, n = 3; P21–P27, n = 9; P30, n = 3; adult, n = 4 animals; *P < 0.05, **P < 0.01 and ***P < 0.001.
We next focused on the possibility that the mechanism that drives the developmental decrease in the [Ca2+]res clearance timecourse could be linked to changes in either release from, or uptake into, internal Ca2+ stores. A contribution from Ca2+ stores would be consistent with the observation that internal stores have a contrasting developmental role in silent and active synapses and that the endoplasmic reticular SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) pump plays an important role in PPF (Cabezas & Buno, 2006).
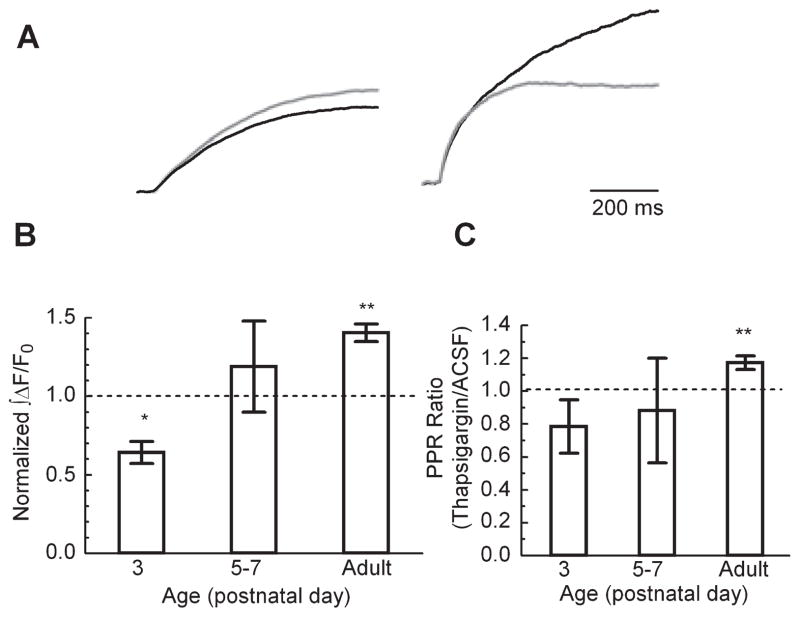
To investigate the possibility that Ca2+ stores play an important role in the regulation of presynaptic [Ca2+]res, we used thapsigargin to block the uptake of Ca2+ into the endoplasmic reticulum (ER) by the SERCA pump and measured the effect of SERCA block on the ∫ΔF/F0 signal following stimulation. To compare the timecourse of ∫ΔF/F0 at different ages, we normalized the ∫ΔF/F0 in 3 μM thapsigargin to the response in the same slice in ACSF (Fig. 5). Interestingly, at P3 thapsigargin caused a decrease in ∫ΔF/F0 while at P5–P7 it caused no change in ∫ΔF/F0, and it clearly enhanced the ∫ΔF/F0 of adults (Fig. 5A). To further test whether SERCA clearance is involved in plasticity, we recorded the PPR of fEPSPs and found that application of thapsigargin had no significant effect in the P5–P7 age group, and a trend toward a decrease in the P3 mice. However, application of thapsigargin did cause an increase in the PPR of the adult mice (Fig. 5C). This suggests that during this time period (P5–P7) there is a rapid transition in the contribution from ER Ca2+ stores at synaptic terminals. Furthermore, either release from stores or uptake by the SERCA pump may be reflected in the slow time constant of the [Ca2+]res clearance.
Fig 5.
Developmental changes in the effect of thapsigargin on [Ca2+]pre clearance. ∫ΔF/F0 following a single pulse in 3 μM thapsigargin normalized to the ∫ΔF/F0 response in ACSF. (A) Representative traces (left, P3 and right, adult; gray, ACSF and black, thapsigargin) normalized to the respective ACSF traces. (B) Summary data. P3, n = 3; P5–P7, n = 4; adult, n = 5 animals; Student’s t-test, against theoretical mean of 1. (C) Effect of application of thapsigargin on PPR vs. age (P3, n = 4, P = 0.11; P5–P7, n = 6, P = 0.34; adult, n = 5 animals); *P < 0.05 and **P < 0.01, Student’s t-test against a theoretical mean of 1.
In many cell types, thapsigargin unmasks a basal leak of Ca2+ from intracellular stores (Verkhratsky, 2005). We reasoned that thapsigargin would produce an increase in the cytoplasmic Ca2+ that would be measurable with the high affinity indicator Fura2 (ΔR/R0 signal) if the SERCA pump normally counteracted a significant leak from Ca2+ stores. This could be relevant to our findings because such a leak might contribute to the difference in [Ca2+]res timecourse which we observed between immature and adult SC-CA1 terminals. Importantly, we did not find a significant increase in ΔR/R0 following thapsigargin application in nonstimulated slices at any age (data not shown). This observation is consistent with the idea that the activity of SERCA does not continually oppose a measurable Ca2+ leak from intracellular Ca2+ stores in presynaptic terminals from either young or adult animals.
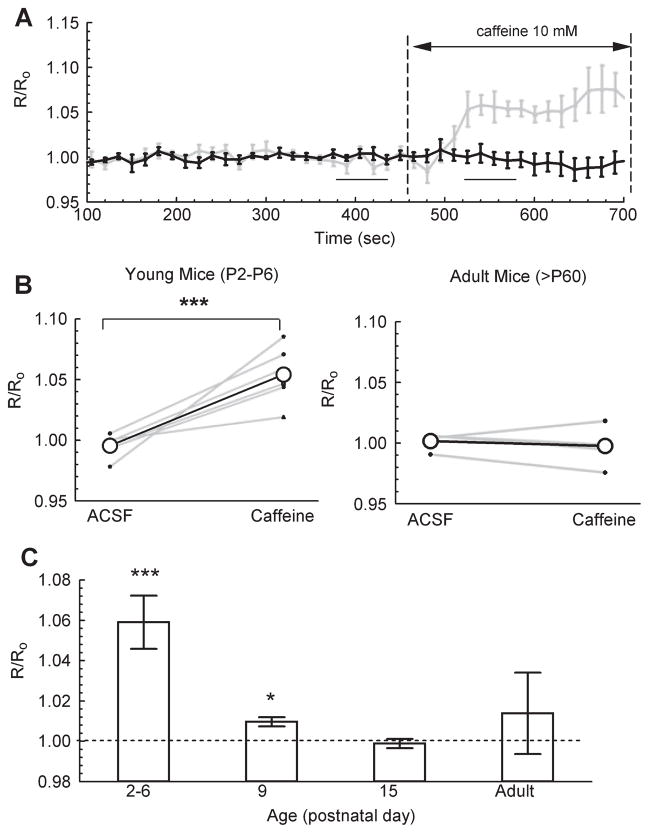
We next examined whether changes in the participation of Ca2+-dependent release from presynaptic Ca2+ stores could contribute to the observed age-dependent differences in the [Ca2+]res clearance time-course. Thus, we tested the contribution of these internal stores to [Ca2+]res in SC-CA1 terminals from both young and adult mice by measuring the relative change in ΔR/R0 in response to bath application of 10 mM caffeine. Caffeine increases the Ca2+ sensitivity of ryanodine receptors and thereby initiates release of Ca2+ from Ca2+-sensitive internal stores into the cytoplasm (Verkhratsky, 2005). As shown in Fig. 6A, there was a consistent, significant increase in ΔR/R0 following application of caffeine in SC-CA1 terminals of young mice (P2–P6) but no equivalent increase in ΔR/R0 in adults (Fig. 6B). Furthermore, the normalized change in the effectiveness of caffeine occurred during the narrow developmental time period between P6 and P9 (Fig. 6C). Both the thapsigargin and caffeine data suggest that some of the developmental decrease in the timecourse of [Ca2+]res clearance results from a decreasing effectiveness of internal stores involvement during this process.
Fig 6.
Age-dependent differences in basal loading state of Ca2+ stores. (A) Timecourse of ΔR/R0 using Fura-2 in adult (black) and young (grey) mice. (B) Average non-normalized ΔR/R0 in ACSF (four samples, between 375 and 435 s) vs. ΔR/R0 in 10 mM caffeine (four samples between 525 and 585 s). Left, young mice (P2–P6, n = 5 animals); right, adult mice (> P60, n = 5 animals; P = 0.45; paired Student’s-t test. (C) Caffeine-induced change in ΔR/R0 normalized to internal control in ACSF (at 375–435 s): P2–P6, n = 5; P9, n = 5; P15, n = 5; adult, n = 5; paired Student’s t-test. There was no significant change in baseline ΔR/R0 at any age without application of caffeine (P2–P6, P = 0.38, n = 3; P9, P = 0.22, n = 3; P15, P = 0.38, n = 3; adult, P = 0.30, n = 3); *P < 0.05 and ***P < 0.001, paired Student’s t-test.
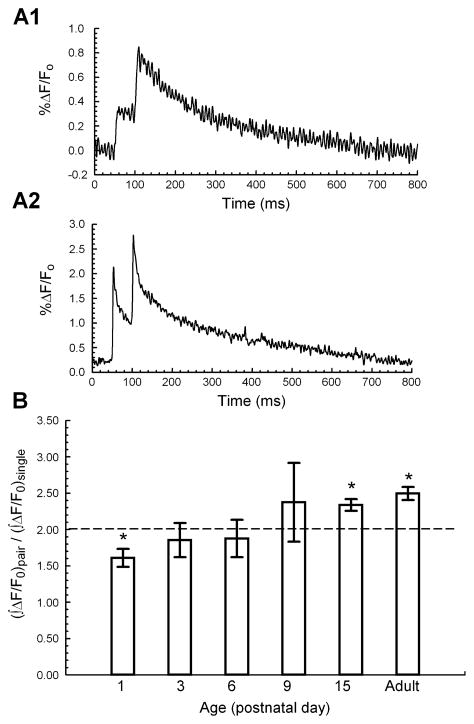
To investigate the contribution of Ca2+ clearance to short-term plasticity, we measured the ΔF/F0 response during paired pulses at a 50-ms interpulse interval (Fig. 7A). We computed the ratio of the paired-pulse ∫ΔF/F0 to the single-pulse ∫ΔF/F0 in order to compare the changes in [Ca2+]res between the first and second of paired stimuli. We have previously shown in SC-CA1 terminals from adult rats that, after subtracting the residual ΔF/F0 that remains following the first of paired pulses, there is an increase in ∫ΔF/F0 resulting from the second pulse (Schiess et al., 2006). This increase would lead us to anticipate a ratio > 2 of the paired-pulse ∫ΔF/F0 to the single-pulse ∫ΔF/F0. As expected, the ∫ΔF/F0 ratio was significantly greater than two in the adult mouse, indicating a supralinear increase in [Ca2+]res during the second pulse (Fig. 7B). Surprisingly, however, we found that at P1 the ∫ΔF/F0 ratio was significantly less than two and that between P3 and P9 the ∫ΔF/F0 ratio did not differ from two. Thus the short-term effect of [Ca2+]res on subsequent Ca2+ clearance reflects unique characteristics at P1 and the adult phenotype is not established until ~ P15.
Fig 7.
Developmental increase in [Ca2+]res during paired pulses. (A) Representative traces of ΔF/F0 during paired stimulation [A1, P6; A2, adult (P85); Mg Green]. (B) Ratio of the ∫ΔF/F0 for paired stimuli vs. ∫ΔF/F0 for single stimulus at different developmental ages (P1, n = 4; P3, n = 6; P6, n = 11; P9, n = 6; P15, n = 4; adult, n = 5 animals); *P < 0.05, Student’s t-test against a theoretical mean of 2.
Discussion
It has been proposed that developmental changes in short-term facilitation are correlated with changes in presynaptic Ca2+ (Wasling et al., 2004). We demonstrate here that there is a developmental threshold for the involvement of presynaptic [Ca2+]res in the regulation of short-term synaptic facilitation. This temporal change occurs between P6 and P9, at which time there is a marked alteration in the [Ca2+]res clearance, in the amount of [Ca2+]res following a single pulse and in the accumulation of Ca2+ in intracellular stores. This was apparent in the incremental change in the general shape of the [Ca2+]i response (Fig. 3A), in the peak amplitude of this response (Fig. 3B) and in the time that it takes to reach the peak (Fig. 3C). The developmental decrease in the ∫νΔF/F0 (Fig. 2) further indicates that there are important age-dependent changes in the time course of [Ca2+]res clearance and hence the total amount of Ca2+ available in the presynaptic terminal.
PPF at a 50-ms interpulse interval undergoes a significant developmental increase after the first postnatal week (Fig. 1). Other studies have shown an additional decrease in the PPR between juvenile (P13–P18) and young adult (P28–P42) rat SC-CA1 terminals (Speed & Dobrunz, 2008). Although we observed a small decline in the PPR between P15 and adult, this decrease was not significant. The difference in these observations could be a result of the broader range of ages of our adult animals, or of species differences between mice and rats. Additionally, while we consistently placed our stimulating electrode in the stratum radiatum, where we would expect to stimulate only Schaffer collateral fibers, it is possible that in some instances the stimulus current could have spread to temporoammonic fibers in the stratum lacunosum–moleculare. In contrast to the decrease in PPF between juvenile and adult Schaffer collateral terminals, PPF in temporoammonic fiber terminals increases during this developmental period (Speed & Dobrunz, 2009). It is conceivable, therefore, that current spread to stratum lacunosum–moleculare in some of our slices could have counteracted the expected age-dependent decrease in the SC-CA1 terminals.
Wasling et al., (2004) have shown that there is a developmental change in release probability of the first of paired responses that is indicative of this change in facilitation in rats. While our determination of spatially and temporally averaged ΔF/F0 does not directly reflect the [Ca2+] in the release domain, nor does it typically reflect influences on release proability of the initial pulse, changes in the measured rise time (Fig. 3C) do suggest a developmental change in the [Ca2+] that determines release during the first pulse. Although action potentials are a few milliseconds broader in immature than in adult neurons (Lockery & Spitzer, 1992; Gao & Ziskind-Conhaim, 1998), this increased period of depolarization is not expected to increase the duration of Ca2+ influx sufficiently to account for the observed slower rise time of [Ca2+]res. A possible explanation for the slower clearance of [Ca2+]res in slices from young animals could be maturational changes from growth cones and silent synapses to mature synapses. Growth cones differ from mature synapses in their morphology and volume, their larger number of L-type Ca2+ channels (Ohbayashi et al., 1998), and the role of Ca2+-induced Ca2+ release (Tojima et al., 2007). Functionally, influx through L-type Ca2+ channels has been shown to play a role in activation of presynaptic silent synapses (Yao et al., 2006) and in the change from silent to active synapses (Gasparini et al., 2000; Cabezas & Buno, 2006). Any of these factors might be a significant contributory factor to the observed maturational differences in [Ca2+]res clearance. However, these alterations in [Ca2+]res kinetics would not be expected to contribute to the observed changes in PPF as short-term synaptic plasticity must depend on mechanisms specific to presynaptic terminals of functional synapses.
In presynaptic terminals of the adult rodent brain, [Ca2+]res decay kinetics are accurately reflected by the sum of two exponentials (Schiess et al., 2006), which indicates either two separate populations with first-order kinetics or a multi-step process. A likely candidate for such a multi-step process is the presynaptic Ca2+ clearance by the endoplasmic reticular SERCA pump (Higgins et al., 2006). The decay kinetics for [Ca2+]res in presynaptic terminals of immature SC-CA1 synapses is slower, and is not accurately fit with a double exponential. We thus chose to analyze the timecourse of [Ca2+]res decay in immature synapses by dividing it into the temporally distinct components of peak time, slow time constant, and the ratio of the peak slow component to the peak amplitude. As shown in Figs 3C, 4A and 4B, each of these components showed an age-dependent decrease. This indicates a developmental change in the underlying kinetic processes and, consequently, marked differences in the mechanisms of [Ca2+]res clearance in immature and mature synapses that are coincident with the establishment of the adult phenotype of short-term plasticity (Wasling et al., 2004; Dumas, 2005). Importantly, expression levels and isoform expression of proteins with demonstrated roles in synaptic plasticity and Ca2+ sensitivity have been shown to change during this maturational period, including, for example, adenylyl cyclases 1 and 8 (Conti et al., 2007), CDK1/4 (Li et al., 2007), and SNAP-25 (Bark et al., 1995).
As noted above, the multi-step kinetics of [Ca2+]res clearance are consistent with the established multi-step kinetics of SERCA sequestration, making changes in this pathway likely candidates for the mechanism for our observations. There are conflicting findings regarding the role of Ca2+-induced Ca2+ release from internal stores in short-term plasticity (Emptage et al., 2001; Carter et al., 2002; Cabezas & Buno, 2006). Furthermore, age-dependent changes in ryanodine receptor expression levels have been shown to play a role in endogenous cannabinod mobilization (Isokawa & Alger, 2006) and the maintenance of presynaptic forms of LTP (Martin & Buno, 2003). We therefore reasoned that developmental changes in the ability to sequester Ca2+ in intracellular stores could affect the timecourse of [Ca2+]res clearance directly through [Ca2+]res uptake from the cytoplasm, or indirectly because of a counteracting Ca2+ release into the cytoplasm. We found that blocking SERCA-dependent Ca2+ sequestration increased the ∫ΔF/F0 in adult presynaptic terminals while causing a decrease at P3 and no difference in the P5–P6 age group. Even with the more sensitive Fura2 measurements we were unable to discern a passive Ca2+ release from stores induced by thapsigargin in slices from either young or adult animals; however, thapsigargin slowed [Ca2+]res clearance in slices from animals older than P3, but not in slices from those that were younger than P3. Additionally, we found that caffeine, which can release Ca2+ from ryanodine-sensitive stores, caused an increase in presynaptic [Ca2+]i in the younger, but not in more mature, mice. These results suggest that, while SERCA uptake and Ca2+ -induced Ca2+ release are effective at all ages, there are developmental differences in the passive leak from stores and in the processes that participate in [Ca2+]res clearance. Taken together, these data suggest that the basal filling state of intracellular Ca2+ stores could account for much of the developmental differences in [Ca2+]res clearance. Although SERCA sequestration is effective in both immature and mature terminals, a more effective passive leak in mature neurons would yield a lower basal store of Ca2+ in these terminals. As a consequence of the minimal store filling state, SERCA uptake would play a more significant role in speeding [Ca2+]res clearance in mature terminals; yet the more effectively filled stores of immature terminals would allow Ca2+-induced Ca2+ release to effectively slow [Ca2+]res clearance. This interpretation is consistent with observations of the involvement of Ca2+-induced Ca2+ release in organotypic slice cultures from P8 animals (Emptage et al., 2001) and P15 animals (Cabezas & Buno, 2006) and the contrasting minimal effectiveness of Ca2+-sensitive stores in adult synapses (Carter et al., 2002).
The [Ca2+]res decay kinetics in the adult rodent SC-CA1 synapse have been shown to be unaffected by Ca2+ buffer saturation (Blatow et al., 2003). In contrast, regulation of presynaptic [Ca2+]i in immature SC-CA1 synapses (Dekay et al., 2006) has been reported to be somewhat more sensitive to Ca2+ buffer saturation. Our results provide additional support for the notion that the development of mature [Ca2+]res clearance dynamics is not a single mechanistic change, but rather a complex series of modifications that include buffering, SERCA-dependent sequestration and Ca2+ dependent release from intracellular Ca2+ stores.
We find that although SC-CA1 presynaptic terminals of younger mice have greater total integrated [Ca2+]res than adults (Fig. 2), these synapses exhibit less short-term facilitation (Fig. 1). Interestingly, although slices from mature animals did exhibit an expected positive correlation between PPF and [Ca2+]res (Fig. 3D), we unexpectedly found that the zero [Ca2+]i intercept in this relationship increased with age (Fig. 3E), consistent with the notion that this could reflect a maturation of the expression of a high affinity facilitatory site. Additionally, slices from adult and young animals differed markedly in the relationship between PPF and ∫νΔF/F0 (Fig. 2C), suggesting a developmental change in the mechanisms that regulate the time course of available Ca2+ that influences facilitation.
Clearly, the dependence of short-term facilitation on [Ca2+]res that is characteristic of adult presynaptic terminals is undeveloped before P9. As all of our pre- and postsynaptic data result from population measurements, it is impossible to draw conclusions from them about the maturation of individual synapses. It is likely that the developmental process, which we observe, does not indicate a homogenous transformation of uniform synapses but rather reflects trends in a heterogeneous population. However, regardless of their source, our data show that the developmental change in [Ca2+]res kinetics that lead to the mature phenotype of a positive correlation between PPF and [Ca2+]res is not firmly established before P9. Importantly, the lack of a significant positive correlation between PPF and [Ca2+]res (Fig. 3D) in the P3–P6 mice could be due to a change in the relative contributions of PPD and PPF in the population of synapses.
Several underlying mechanisms can be suggested that may mediate the developmental change in the relationship between [Ca2+]res and facilitation. (i) As discussed above, a varying portion of the [Ca2+]i signal in the slices from immature animals could be from electronically silent growth cones or from silent synapses. (ii) The slowed [Ca2+]res clearance in the younger animals could result in a greater [Ca2+] available to deplete the readily releasable pool of vesicles and thus counterbalance a [Ca2+]res-dependent tendency toward increased facilitation (Liley & North, 1953; Takeuchi, 1958; Elmqvist & Quastel, 1965; Thies, 1965; Betz, 1970; Zucker & Regehr, 2002). (iii) Age-dependent changes in Ca2+-dependent inhibition of the presynaptic Ca2+ currents could contribute to developmental changes in short-term synaptic plasticity (Li et al., 2006; Sullivan, 2007). (iv) Expression of an independent modulatory component of the vesicle release machinery such as the high affinity site, which has been suggested as being necessary for facilitation in SC-CA1 synapses, could mature during this time period (P6–P9) (Atluri & Regehr, 1996; Bark et al., 2004; Schiess et al., 2006). (v) Finally, the change in this relationship could reflect an alteration of the local domain of Ca2+ that facilitates release due to a shift of the contribution from internal Ca2+ stores. A potential mechanism signaling this change is a difference in the kainate-sensitive glutamate receptor activation, possibly due to BDNF signaling (Lauri et al., 2006; Sallert et al., 2009).
We report here important developmental changes in short-term plasticity and the kinetics of presynaptic [Ca2+]res clearance. These dramatic developmental changes could have important implications for synaptic filtering characteristics and their effects on information processing in the hippocampus (Partridge & Valenzuela, 2002). Understanding the molecular mechanisms that drive these changes will be essential to evaluate the neurological impact of presynaptic Ca2+ clearance on diseases that have their onset during the transitional period of the juvenile to adult nervous system.
Acknowledgments
This work was supported by grants R01 MH48989 (M.C.W.) and R01-MH07386 (L.D.P.) from the National Institutes of Health and grant DGE-0549500 from the National Science Foundation. The authors thank Rebecca Hartley for critical reading of this manuscript.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- [Ca2+]res
residual presynaptic Ca2+ concentration
- COD
coefficient of determination
- ER
endoplasmic reticulum
- ΔF/F0
change in Mg Green fluorescence divided by the pre-stimulus fluorescence
- ∫ΔF/F0
integral of ΔF/F0
- ∫νΔF/F0
integral of ΔF/F0 normalized to peak ΔF/F0
- ΔR/R0
change in Fura2 fluorescence ratio divided by the pre-stimulus fluorescence ratio
- fEPSP
field excitatory postsynaptic potential
- PPF
paired-pulse facilitation
- PPR
paired-pulse ratio
- SC-CA1
Schaffer collateral to CA1 pyramidal neuron
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
References
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark IC, Hahn KM, Ryabinin AE, Wilson MC. Differential expression of SNAP-25 protein isoforms during divergent vesicle fusion events of neural development. Proc Natl Acad Sci USA. 1995;92:1510–1514. doi: 10.1073/pnas.92.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark C, Bellinger FP, Kaushal A, Mathews JR, Partridge LD, Wilson MC. Developmentally regulated switch in alternatively spliced SNAP-25 isoforms alters facilitation of synaptic transmission. J Neurosci. 2004;24:8796–8805. doi: 10.1523/JNEUROSCI.1940-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Cabezas C, Buno W. Distinct transmitter release properties determine differences in short-term plasticity at functional and silent synapses. J Neurophysiol. 2006;95:3024–3034. doi: 10.1152/jn.00739.2005. [DOI] [PubMed] [Google Scholar]
- Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Maas JW, Jr, Muglia LM, Dave BA, Vogt SK, Tran TT, Rayhel EJ, Muglia LJ. Distinct regional and subcellular localization of adenylyl cyclases type 1 and 8 in mouse brain. Neuroscience. 2007;146:713–729. doi: 10.1016/j.neuroscience.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- Dekay JG, Chang TC, Mills N, Speed HE, Dobrunz LE. Responses of excitatory hippocampal synapses to natural stimulus patterns reveal a decrease in short-term facilitation and increase in short-term depression during postnatal development. Hippocampus. 2006;16:66–79. doi: 10.1002/hipo.20132. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. 2005;15:562–578. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Elmqvist D, Quastel DM. Presynaptic action of hemicholinium at the neuromuscular junction. J Physiol. 1965;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci USA. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Taira T, Lauri S, Groc L. Glutamate synapse in developing brain: an integrative perspective beyond the silent state. Trends Neurosci. 2009;32:532–537. doi: 10.1016/j.tins.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Higgins ER, Cannell MB, Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys J. 2006;91:151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, Alger BE. Ryanodine receptor regulates endogenous cannabinoid mobilization in the hippocampus. J Neurophysiol. 2006;95:3001–3011. doi: 10.1152/jn.00975.2005. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J Physiol. 1999;518 (Pt 2):497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529(Pt 3):625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu Y, Zhou Y. Modulation of inactivation properties of CaV2.2 channels by 14-3-3 proteins. Neuron. 2006;51:755–771. doi: 10.1016/j.neuron.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Chen W, Yu S, Chen J, Wang H, Ruan D. The different roles of cyclinD1-CDK4 in STP and mGluR-LTD during the postnatal development in mice hippocampus area CA1. BMC Dev Biol. 2007;7:57. doi: 10.1186/1471-213X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AW, North KA. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953;16:509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Lockery SR, Spitzer NC. Reconstruction of action potential development from whole-cell currents of differentiating spinal neurons. J Neurosci. 1992;12:2268–2287. doi: 10.1523/JNEUROSCI.12-06-02268.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Buno W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. 10.1152/jn.00601.2002. J Neurophysiol. 2003;89:3029–3038. doi: 10.1152/jn.00601.2002. [DOI] [PubMed] [Google Scholar]
- Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi K, Fukura H, Inoue HK, Komiya Y, Igarashi M. Stimulation of L-type Ca2+ channel in growth cones activates two independent signaling pathways. J Neurosci Res. 1998;51:682–696. doi: 10.1002/(SICI)1097-4547(19980315)51:6<682::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Neurosteroids enhance bandpass filter characteristics of the rat Schaffer collateral-to-CA1 synapse. Neurosci Lett. 2002;326:1–4. doi: 10.1016/s0304-3940(02)00295-1. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods. 1991;37:111–119. doi: 10.1016/0165-0270(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Sallert M, Rantamaki T, Vesikansa A, Anthoni H, Harju K, Yli-Kauhaluoma J, Taira T, Castren E, Lauri SE. Brain-derived neurotrophic factor controls activity-dependent maturation of CA1 synapses by downregulating tonic activation of presynaptic kainate receptors. J Neurosci. 2009;29:11294–11303. doi: 10.1523/JNEUROSCI.0560-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess AR, Scullin CS, Partridge LD. Neurosteroid-induced enhancement of short-term facilitation involves a component downstream from presynaptic calcium in hippocampal slices. J Physiol. 2006;576:833–847. doi: 10.1113/jphysiol.2006.118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SR, Wu LG, Saggau P. Presynaptic calcium dynamics and transmitter release evoked by single action potentials at mammalian central synapses. Biophys J. 1997;72:637–651. doi: 10.1016/s0006-3495(97)78702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE. Developmental decrease in short-term facilitation at Schaffer collateral synapses in hippocampus is mGluR1-sensitive. J Neurophysiol. 2008;99:799–813. doi: 10.1152/jn.00625.2007. [DOI] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus. 2009;19:187–204. doi: 10.1002/hipo.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM. A simple depletion model of the readily releasable pool of synaptic vesicles cannot account for paired-pulse depression. J Neurophysiol. 2007;97:948–950. doi: 10.1152/jn.00554.2006. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958;8:102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- Thies RE. Neuromuscular depression and the apparent depletion of transmitter in mammalian muscle. J Neurophysiol. 1965;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Wasling P, Hanse E, Gustafsson B. Developmental changes in release properties of the CA3-CA1 glutamate synapse in rat hippocampus. J Neurophysiol. 2004;92:2714–2724. doi: 10.1152/jn.00464.2004. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–8147. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]