Abstract
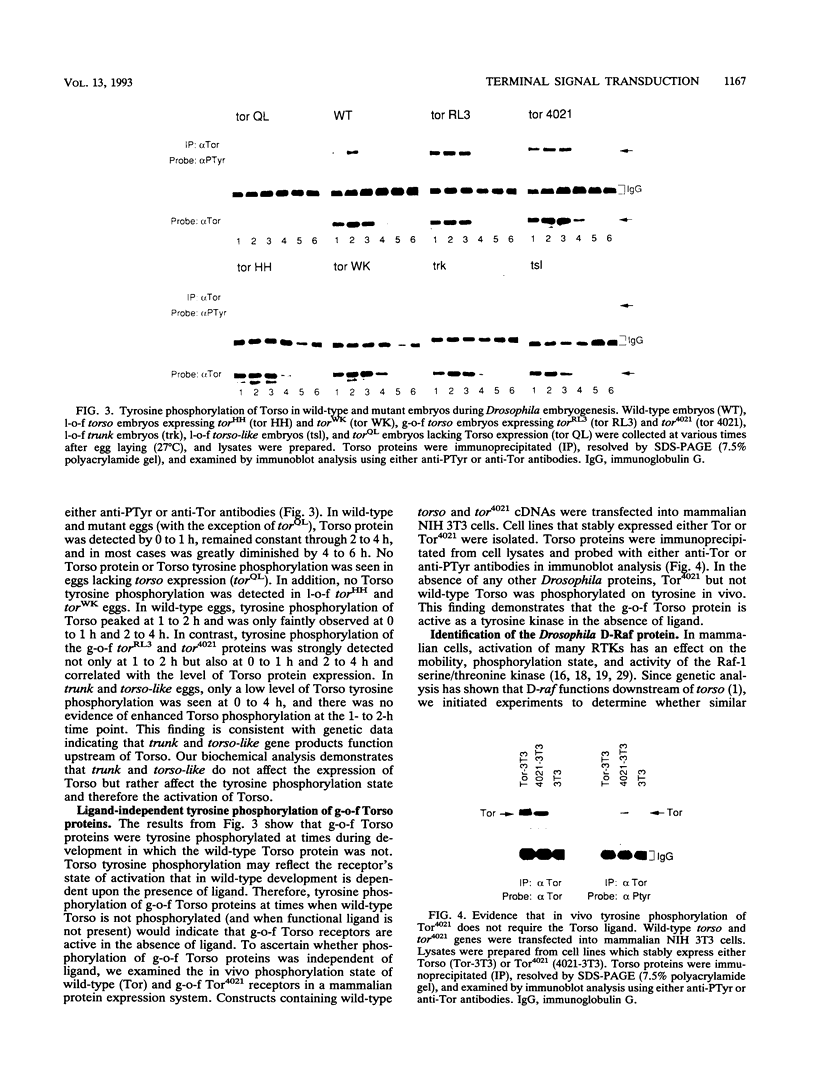
Determination of anterior and posterior terminal structures of Drosophila embryos requires activation of two genes encoding putative protein kinases, torso and D-raf. In this study, we demonstrate that Torso has intrinsic tyrosine kinase activity and show that it is transiently tyrosine phosphorylated (activated) at syncytial blastoderm stages. Torso proteins causing a gain-of-function phenotype are constitutively tyrosine phosphorylated, while Torso proteins causing a loss-of-function phenotype lack tyrosine kinase activity. The D-raf gene product, which is required for Torso function, is identified as a 90-kDa protein with intrinsic serine/threonine kinase activity. D-Raf is expressed throughout embryogenesis; however, the phosphorylation state of the protein changes during development. In wild-type embryos, D-Raf is hyperphosphorylated at 1 to 2 h after egg laying, and thereafter only the most highly phosphorylated form is detected. Embryos lacking Torso activity, however, show significant reductions in D-Raf protein expression rather than major alterations in the protein's phosphorylation state. This report provides the first biochemical analysis of the terminal signal transduction pathway in Drosophila embryos.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio L., Mahowald A. P., Perrimon N. Requirement of the Drosophila raf homologue for torso function. Nature. 1989 Nov 16;342(6247):288–291. doi: 10.1038/342288a0. [DOI] [PubMed] [Google Scholar]
- Ambrosio L., Mahowald A. P., Perrimon N. l(1)pole hole is required maternally for pattern formation in the terminal regions of the embryo. Development. 1989 May;106(1):145–158. doi: 10.1242/dev.106.1.145. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Brönner G., Jäckle H. Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mech Dev. 1991 Nov;35(3):205–211. doi: 10.1016/0925-4773(91)90019-3. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Casanova J., Struhl G. Localized surface activity of torso, a receptor tyrosine kinase, specifies terminal body pattern in Drosophila. Genes Dev. 1989 Dec;3(12B):2025–2038. doi: 10.1101/gad.3.12b.2025. [DOI] [PubMed] [Google Scholar]
- Degelmann A., Hardy P. A., Perrimon N., Mahowald A. P. Developmental analysis of the torso-like phenotype in Drosophila produced by a maternal-effect locus. Dev Biol. 1986 Jun;115(2):479–489. doi: 10.1016/0012-1606(86)90268-x. [DOI] [PubMed] [Google Scholar]
- Dickson B., Sprenger F., Morrison D., Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992 Dec 10;360(6404):600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Rabin S. J., Kaplan L., Reid S., Parada L. F., Kaplan D. R. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992 Nov;9(5):883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- Jamal S., Ziff E. Transactivation of c-fos and beta-actin genes by raf as a step in early response to transmembrane signals. Nature. 1990 Mar 29;344(6265):463–466. doi: 10.1038/344463a0. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Fukumoto Y., Oku N., Hori Y., Yamamoto T., Toyoshima K., Takai Y. Activation of the serum response element and 12-O-tetradecanoylphorbol-13-acetate response element by the activated c-raf-1 protein in a manner independent of protein kinase C. J Biol Chem. 1989 Dec 15;264(35):20855–20858. [PubMed] [Google Scholar]
- Klingler M., Erdélyi M., Szabad J., Nüsslein-Volhard C. Function of torso in determining the terminal anlagen of the Drosophila embryo. Nature. 1988 Sep 15;335(6187):275–277. doi: 10.1038/335275a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Weissinger E., Mischak H., Troppmair J., Showalter S. D., Lloyd P., Heidecker G., Rapp U. R. Probing structure and function of the raf protein kinase domain with monoclonal antibodies. Oncogene. 1990 May;5(5):713–720. [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Mark G. E., MacIntyre R. J., Digan M. E., Ambrosio L., Perrimon N. Drosophila melanogaster homologs of the raf oncogene. Mol Cell Biol. 1987 Jun;7(6):2134–2140. doi: 10.1128/mcb.7.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K. The Raf-1 kinase as a transducer of mitogenic signals. Cancer Cells. 1990 Dec;2(12):377–382. [PubMed] [Google Scholar]
- Nishida Y., Hata M., Ayaki T., Ryo H., Yamagata M., Shimizu K., Nishizuka Y. Proliferation of both somatic and germ cells is affected in the Drosophila mutants of raf proto-oncogene. EMBO J. 1988 Mar;7(3):775–781. doi: 10.1002/j.1460-2075.1988.tb02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990 Nov;6(11):350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Larsen I., Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992 Jul 24;70(2):225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. A pupal lethal mutation with a paternally influenced maternal effect on embryonic development in Drosophila melanogaster. Dev Biol. 1985 Aug;110(2):480–491. doi: 10.1016/0012-1606(85)90105-8. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. The effects of zygotic lethal mutations on female germ-line functions in Drosophila. Dev Biol. 1984 Oct;105(2):404–414. doi: 10.1016/0012-1606(84)90297-5. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Mohler D., Engstrom L., Mahowald A. P. X-linked female-sterile loci in Drosophila melanogaster. Genetics. 1986 Jul;113(3):695–712. doi: 10.1093/genetics/113.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F., Baldarelli R. M., Steingrímsson E., Diaz R. J., Patapoutian A., Merriam J. R., Lengyel J. A. The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell. 1990 Jul 13;62(1):151–163. doi: 10.1016/0092-8674(90)90249-e. [DOI] [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Schüpbach T., Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989 Jan;121(1):101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Siegfried Z., Ziff E. B. Altered transcriptional activity of c-fos promoter plasmids in v-raf-transformed NIH 3T3 cells. Mol Cell Biol. 1990 Nov;10(11):6073–6078. doi: 10.1128/mcb.10.11.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F., Nüsslein-Volhard C. Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell. 1992 Dec 11;71(6):987–1001. doi: 10.1016/0092-8674(92)90394-r. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nüsslein-Volhard C. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature. 1989 Apr 6;338(6215):478–483. doi: 10.1038/338478a0. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991 Jul 12;66(1):51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Steingrímsson E., Pignoni F., Liaw G. J., Lengyel J. A. Dual role of the Drosophila pattern gene tailless in embryonic termini. Science. 1991 Oct 18;254(5030):418–421. doi: 10.1126/science.1925599. [DOI] [PubMed] [Google Scholar]
- Stevens L. M., Frohnhöfer H. G., Klingler M., Nüsslein-Volhard C. Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature. 1990 Aug 16;346(6285):660–663. doi: 10.1038/346660a0. [DOI] [PubMed] [Google Scholar]
- Strecker T. R., Halsell S. R., Fisher W. W., Lipshitz H. D. Reciprocal effects of hyper- and hypoactivity mutations in the Drosophila pattern gene torso. Science. 1989 Feb 24;243(4894 Pt 1):1062–1066. doi: 10.1126/science.2922596. [DOI] [PubMed] [Google Scholar]
- Strecker T. R., Kongsuwan K., Lengyel J. A., Merriam J. R. The zygotic mutant tailless affects the anterior and posterior ectodermal regions of the Drosophila embryo. Dev Biol. 1986 Jan;113(1):64–76. doi: 10.1016/0012-1606(86)90108-9. [DOI] [PubMed] [Google Scholar]
- Strecker T. R., Merriam J. R., Lengyel J. A. Graded requirement for the zygotic terminal gene, tailless, in the brain and tail region of the Drosophila embryo. Development. 1988 Apr;102(4):721–734. doi: 10.1242/dev.102.4.721. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B., Heidecker G., Huleihel M., Rapp U. R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989 May;9(5):2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Klingler M., Jäckle H. Two gap genes mediate maternal terminal pattern information in Drosophila. Science. 1990 Apr 27;248(4954):495–498. doi: 10.1126/science.2158673. [DOI] [PubMed] [Google Scholar]









