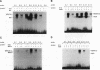
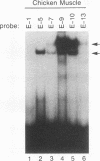
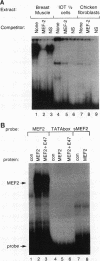
Abstract
Members of the MyoD family of gene-regulatory proteins (MyoD, myogenin, myf5, and MRF4) have all been shown not only to regulate the transcription of numerous muscle-specific genes but also to positively autoregulate and cross activate each other's transcription. In the case of muscle-specific genes, this transcriptional regulation can often be correlated with the presence of a DNA consensus in the regulatory region CANNTG, known as an E box. Little is known about the regulatory interactions of the myogenic factors themselves; however, these interactions are thought to be important for the activation and maintenance of the muscle phenotype. We have identified the minimal region in the chicken MyoD (CMD1) promoter necessary for muscle-specific transcription in primary cultures of embryonic chicken skeletal muscle. The CMD1 promoter is silent in primary chick fibroblast cultures and in muscle cell cultures treated with the thymidine analog bromodeoxyuridine. However, CMD1 and chicken myogenin, as well as, to a lesser degree, chicken Myf5 and MRF4, expressed in trans can activate transcription from the minimal CMD1 promoter in these primary fibroblast cultures. Here we show that the CMD1 promoter contains numerous E-box binding sites for CMD1 and the other myogenic factors, as well as a MEF-2 binding site. Surprisingly, neither muscle-specific and the other myogenic factors, as well as a MEF-2 binding site. Surprisingly, neither muscle-specific expression, autoregulation, or cross activation depends upon the presence of of these E-box or MEF-2 binding sites in the CMD1 promoter. These results demonstrate that the autoregulation and cross activation of the chicken MyoD promoter through the putative direct binding of the myogenic basic helix-loop-helix regulatory factors is mediated through an indirect pathway that involves unidentified regulatory elements and/or ancillary factors.
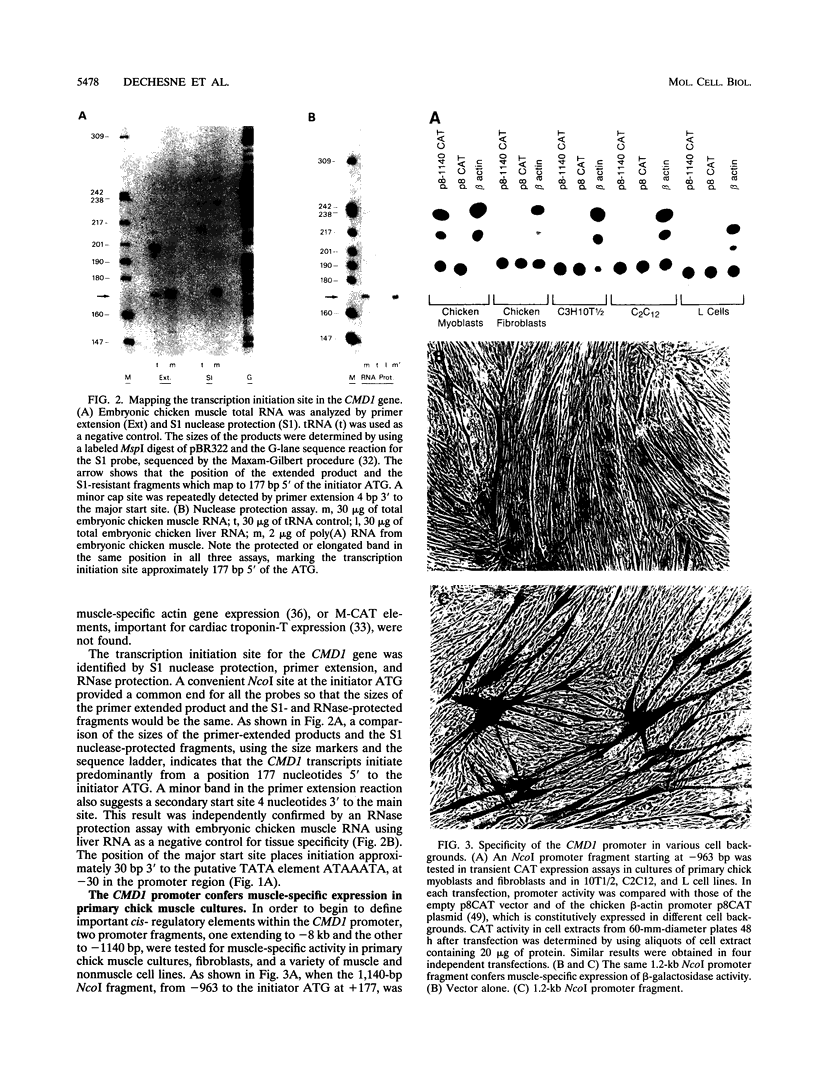
Full text
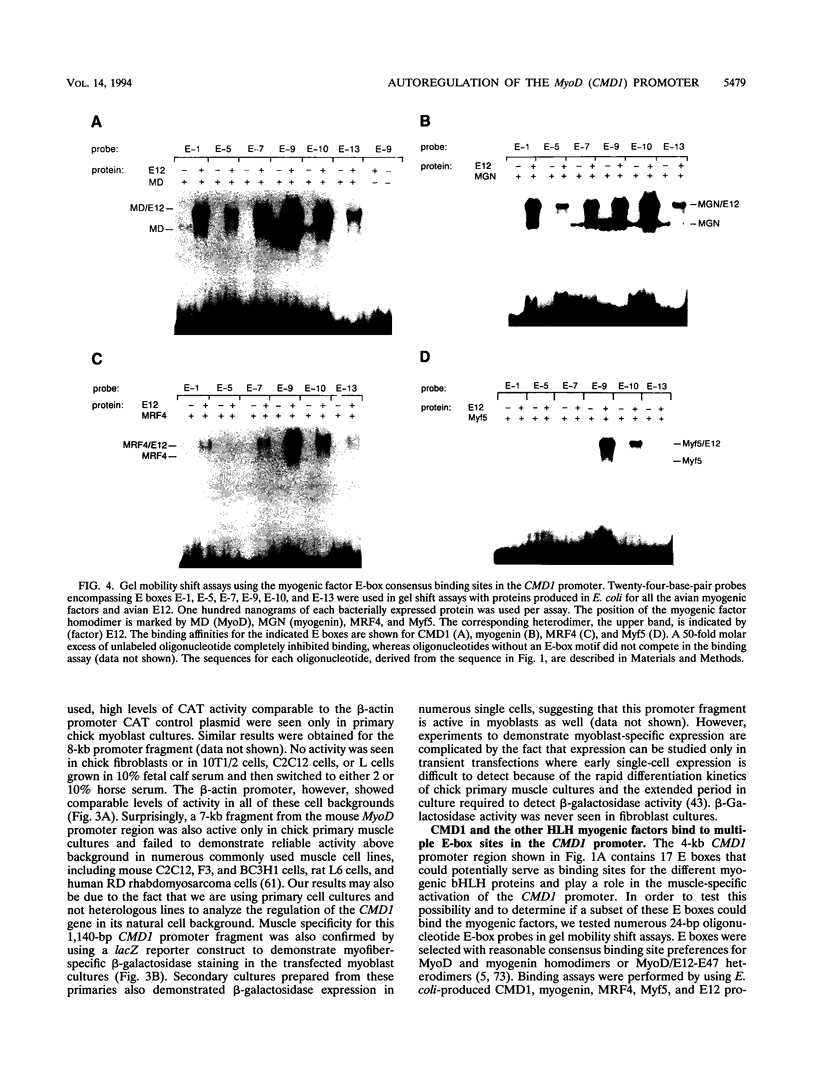
PDF




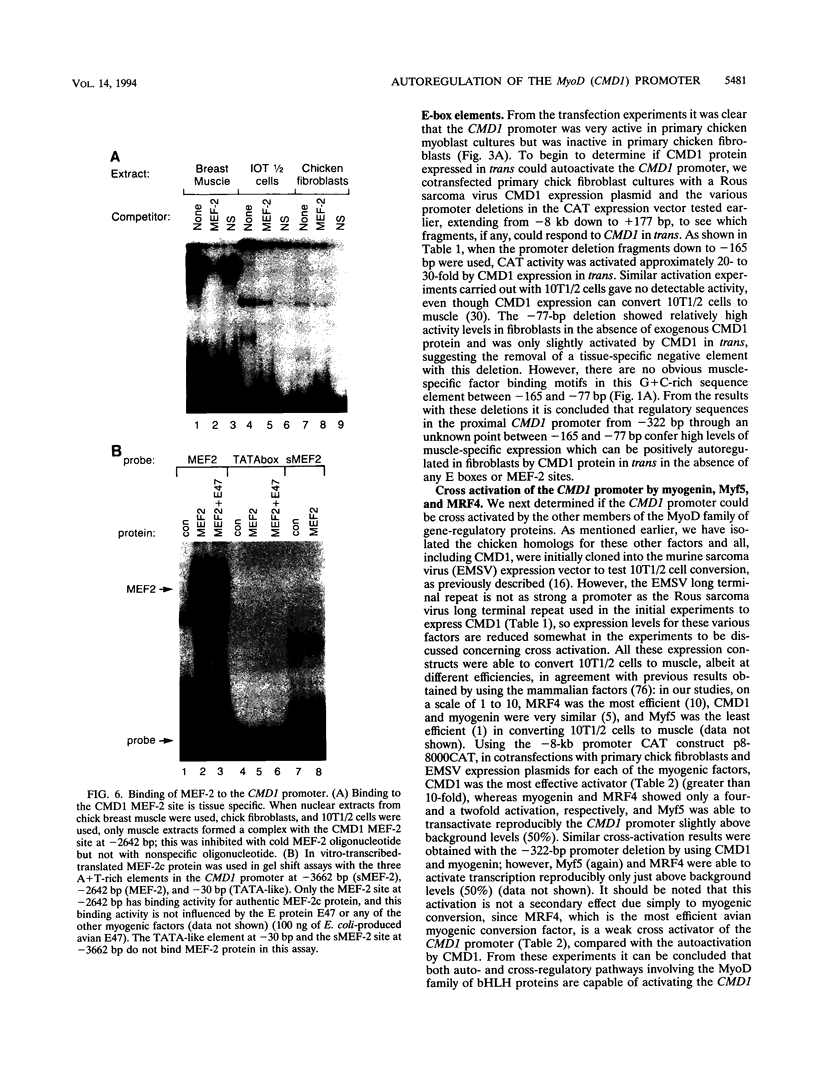




Images in this article
Selected References
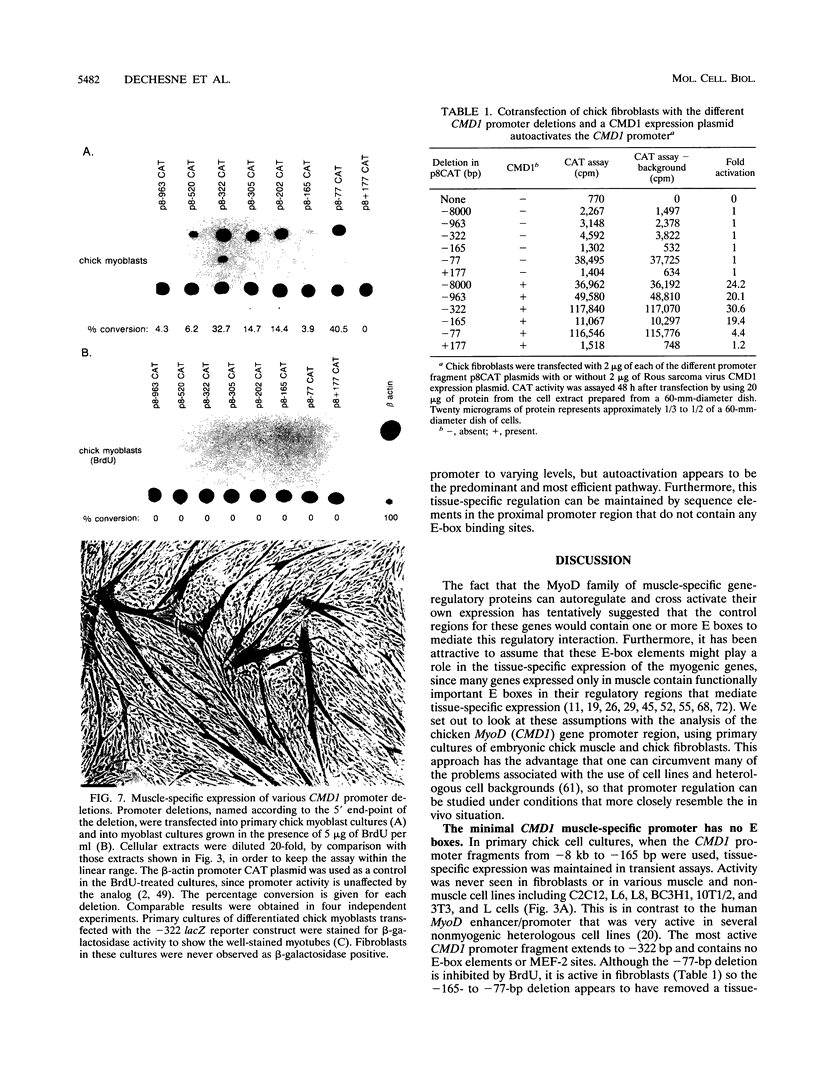
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura A., Fujisawa-Sehara A., Komiya T., Nabeshima Y., Nabeshima Y. MyoD and myogenin act on the chicken myosin light-chain 1 gene as distinct transcriptional factors. Mol Cell Biol. 1993 Nov;13(11):7153–7162. doi: 10.1128/mcb.13.11.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter R., Quitschke W., Paterson B. M. Approximately 1 kilobase of sequence 5' to the two myosin light-chain 1f/3f gene cap sites is sufficient for differentiation-dependent expression. Mol Cell Biol. 1988 Mar;8(3):1361–1365. doi: 10.1128/mcb.8.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R. K., Sanicola M., Raftery L. A., Gillevet T., Gelbart W. M. An extensive 3' cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991 Mar;111(3):657–666. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bober E., Lyons G. E., Braun T., Cossu G., Buckingham M., Arnold H. H. The muscle regulatory gene, Myf-6, has a biphasic pattern of expression during early mouse development. J Cell Biol. 1991 Jun;113(6):1255–1265. doi: 10.1083/jcb.113.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Chen J., Gonzales F., Jones P. A., Fusenig N. E. Progressive stages of "transdifferentiation" from epidermal to mesenchymal phenotype induced by MyoD1 transfection, 5-aza-2'-deoxycytidine treatment, and selection for reduced cell attachment in the human keratinocyte line HaCaT. J Cell Biol. 1992 Mar;116(5):1257–1271. doi: 10.1083/jcb.116.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Rudnicki M. A., Arnold H. H., Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992 Oct 30;71(3):369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Making muscle in mammals. Trends Genet. 1992 Apr;8(4):144–148. doi: 10.1016/0168-9525(92)90373-C. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. C., Wallace M. C., Merlie J. P., Olson E. N. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993 Jul 9;261(5118):215–218. doi: 10.1126/science.8392225. [DOI] [PubMed] [Google Scholar]
- Choi J., Costa M. L., Mermelstein C. S., Chagas C., Holtzer S., Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusella-De Angelis M. G., Lyons G., Sonnino C., De Angelis L., Vivarelli E., Farmer K., Wright W. E., Molinaro M., Bouchè M., Buckingham M. MyoD, myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol. 1992 Mar;116(5):1243–1255. doi: 10.1083/jcb.116.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Cheng T. C., Cserjesi P., Chakraborty T., Olson E. N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992 Sep;12(9):3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B. A., Chow K. L., Olson E. N., Schwartz R. J. Heterodimers of myogenic helix-loop-helix regulatory factors and E12 bind a complex element governing myogenic induction of the avian cardiac alpha-actin promoter. Mol Cell Biol. 1991 May;11(5):2439–2450. doi: 10.1128/mcb.11.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhamer D. J., Faerman A., Shani M., Emerson C. P., Jr Regulatory elements that control the lineage-specific expression of myoD. Science. 1992 Apr 24;256(5056):538–542. doi: 10.1126/science.1315077. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon K., Smith C. K., 2nd, Bales K. R., Santerre R. F. Temporal and quantitative analysis of myogenic regulatory and growth factor gene expression in the developing mouse embryo. Dev Biol. 1992 May;151(1):137–144. doi: 10.1016/0012-1606(92)90221-2. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Krause M., Fire A., Harrison S. W., Priess J., Weintraub H. CeMyoD accumulation defines the body wall muscle cell fate during C. elegans embryogenesis. Cell. 1990 Nov 30;63(5):907–919. doi: 10.1016/0092-8674(90)90494-y. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Leibham D., Wong M. W., Cheng T. C., Schroeder S., Weil P. A., Olson E. N., Perry M. Binding of TFIID and MEF2 to the TATA element activates transcription of the Xenopus MyoDa promoter. Mol Cell Biol. 1994 Jan;14(1):686–699. doi: 10.1128/mcb.14.1.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chambard J. C., Karin M., Olson E. N. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev. 1992 Apr;6(4):676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Lin H., Yutzey K. E., Konieczny S. F. Muscle-specific expression of the troponin I gene requires interactions between helix-loop-helix muscle regulatory factors and ubiquitous transcription factors. Mol Cell Biol. 1991 Jan;11(1):267–280. doi: 10.1128/mcb.11.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Y., Dechesne C. A., Eldridge J., Paterson B. M. An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 1989 Jul;3(7):986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Mak K. L., To R. Q., Kong Y., Konieczny S. F. The MRF4 activation domain is required to induce muscle-specific gene expression. Mol Cell Biol. 1992 Oct;12(10):4334–4346. doi: 10.1128/mcb.12.10.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. M-CAT binding factor, a novel trans-acting factor governing muscle-specific transcription. Mol Cell Biol. 1990 Aug;10(8):4271–4283. doi: 10.1128/mcb.10.8.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. F., Schwarz J. J., Olson E. N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Abmayr S. M., Bate M., Arias A. M., Maniatis T. Expression of a MyoD family member prefigures muscle pattern in Drosophila embryos. Genes Dev. 1990 Dec;4(12A):2086–2097. doi: 10.1101/gad.4.12a.2086. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Shirakata M., Paterson B. M. Phosphorylation inhibits the DNA-binding activity of MyoD homodimers but not MyoD-E12 heterodimers. J Biol Chem. 1993 Nov 15;268(32):24415–24420. [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I., Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993 Aug 5;364(6437):532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Navankasattusas S., Zhu H., Garcia A. V., Evans S. M., Chien K. R. A ubiquitous factor (HF-1a) and a distinct muscle factor (HF-1b/MEF-2) form an E-box-independent pathway for cardiac muscle gene expression. Mol Cell Biol. 1992 Apr;12(4):1469–1479. doi: 10.1128/mcb.12.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M. C., Stockdale F. E. 5-Bromodeoxyuridine inhibition of differentiation. Kinetics of inhibition and reversal in myoblasts. Dev Biol. 1974 Mar;37(1):117–132. doi: 10.1016/0012-1606(74)90173-0. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Walldorf U., Eldridge J., Dübendorfer A., Frasch M., Gehring W. J. The Drosophila homologue of vertebrate myogenic-determination genes encodes a transiently expressed nuclear protein marking primary myogenic cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3782–3786. doi: 10.1073/pnas.88.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Pownall M. E., Emerson C. P., Jr Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev Biol. 1992 May;151(1):67–79. doi: 10.1016/0012-1606(92)90214-2. [DOI] [PubMed] [Google Scholar]
- Quitschke W. W., DePonti-Zilli L., Lin Z. Y., Paterson B. M. Identification of two nuclear factor-binding domains on the chicken cardiac actin promoter: implications for regulation of the gene. Mol Cell Biol. 1989 Aug;9(8):3218–3230. doi: 10.1128/mcb.9.8.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitschke W. W., Lin Z. Y., DePonti-Zilli L., Paterson B. M. The beta actin promoter. High levels of transcription depend upon a CCAAT binding factor. J Biol Chem. 1989 Jun 5;264(16):9539–9546. [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rosenthal N., Berglund E. B., Wentworth B. M., Donoghue M., Winter B., Bober E., Braun T., Arnold H. H. A highly conserved enhancer downstream of the human MLC1/3 locus is a target for multiple myogenic determination factors. Nucleic Acids Res. 1990 Nov 11;18(21):6239–6246. doi: 10.1093/nar/18.21.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Schnegelsberg P. N., Stead R. H., Braun T., Arnold H. H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993 Dec 31;75(7):1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Sassoon D. A. Myogenic regulatory factors: dissecting their role and regulation during vertebrate embryogenesis. Dev Biol. 1993 Mar;156(1):11–23. doi: 10.1006/dbio.1993.1055. [DOI] [PubMed] [Google Scholar]
- Shirakata M., Friedman F. K., Wei Q., Paterson B. M. Dimerization specificity of myogenic helix-loop-helix DNA-binding factors directed by nonconserved hydrophilic residues. Genes Dev. 1993 Dec;7(12A):2456–2470. doi: 10.1101/gad.7.12a.2456. [DOI] [PubMed] [Google Scholar]
- Smith T. H., Block N. E., Rhodes S. J., Konieczny S. F., Miller J. B. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 1993 Mar;117(3):1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Lassar A. B., Davis R. L., Weintraub H. 5-bromo-2'-deoxyuridine blocks myogenesis by extinguishing expression of MyoD1. Science. 1989 Aug 4;245(4917):532–536. doi: 10.1126/science.2547249. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Lassar A. B., Weintraub H. A novel myoblast enhancer element mediates MyoD transcription. Mol Cell Biol. 1992 Nov;12(11):4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Thompson W. R., Nadal-Ginard B., Mahdavi V. A MyoD1-independent muscle-specific enhancer controls the expression of the beta-myosin heavy chain gene in skeletal and cardiac muscle cells. J Biol Chem. 1991 Nov 25;266(33):22678–22688. [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Venuti J. M., Goldberg L., Chakraborty T., Olson E. N., Klein W. H. A myogenic factor from sea urchin embryos capable of programming muscle differentiation in mammalian cells. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6219–6223. doi: 10.1073/pnas.88.14.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Lockshon D., Lassar A. MyoD binds cooperatively to two sites in a target enhancer sequence: occupancy of two sites is required for activation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5623–5627. doi: 10.1073/pnas.87.15.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Dwarki V. J., Verma I., Davis R., Hollenberg S., Snider L., Lassar A., Tapscott S. J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991 Aug;5(8):1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wentworth B. M., Donoghue M., Engert J. C., Berglund E. B., Rosenthal N. Paired MyoD-binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1242–1246. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Binder M., Funk W. Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol. 1991 Aug;11(8):4104–4110. doi: 10.1128/mcb.11.8.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rigby P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993 Jul;7(7A):1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Rhodes S. J., Konieczny S. F. Differential trans activation associated with the muscle regulatory factors MyoD1, myogenin, and MRF4. Mol Cell Biol. 1990 Aug;10(8):3934–3944. doi: 10.1128/mcb.10.8.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg J. M., Alva G. P., Jost J. P. Characterisation of a genomic clone covering the structural mouse MyoD1 gene and its promoter region. Nucleic Acids Res. 1991 Dec 11;19(23):6433–6439. doi: 10.1093/nar/19.23.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]