Abstract
Background:
Hypercapnic respiratory failure in patients with COPD frequently requires mechanical ventilatory support. Extracorporeal CO2 removal (ECCO2R) techniques have not been systematically evaluated in these patients.
Methods:
This is a pilot study of a novel ECCO2R device that utilizes a single venous catheter with high CO2 removal rates at low blood flows. Twenty hypercapnic patients with COPD received ECCO2R. Group 1 (n = 7) consisted of patients receiving noninvasive ventilation with a high likelihood of requiring invasive ventilation, group 2 (n = 2) consisted of patients who could not be weaned from noninvasive ventilation, and group 3 (n = 11) consisted of patients on invasive ventilation who had failed attempts to wean.
Results:
The device was well tolerated, with complications and rates similar to those seen with central venous catheterization. Blood flow through the system was 430.5 ± 73.7 mL/min, and ECCO2R was 82.5 ± 15.6 mL/min and did not change significantly with time. Invasive ventilation was avoided in all patients in group 1 and both patients in group 2 were weaned; PaCO2 decreased significantly (P < .003) with application of the device from 78.9 ± 16.8 mm Hg to 65.9 ± 11.5 mm Hg. In group 3, three patients were weaned, while the level of invasive ventilatory support was reduced in three patients. One patient in group 3 died due to a retroperitoneal bleed following catheterization.
Conclusions:
This single-catheter, low-flow ECCO2R system provided clinically useful levels of CO2 removal in these patients with COPD. The system appears to be a potentially valuable additional modality for the treatment of hypercapnic respiratory failure.
Trial registry:
ClinicalTrials.gov; No.: NCT00987740 and 01021605; URL: www.clinicaltrials.gov
COPD causes significant morbidity and mortality, and is the fourth leading cause of death in the world.1 Hypercapnic respiratory failure in COPD worsens the prognosis and increases the mortality.2‐5 Noninvasive positive pressure ventilation (NIPPV) is now established as the standard treatment of respiratory failure in acute exacerbation of COPD6; however, 26% to 54% of these patients still require invasive positive pressure ventilation (IPPV).7,8 The prognosis for patients with COPD who require IPPV is poor with hospital survival ranging from 31% to 76%.6,9,10
As an adjunct to IPPV, extracorporeal CO2 removal (ECCO2R) techniques were first applied to patients with hypoxic respiratory failure over three decades ago11,12 but have not achieved widespread use. Curiously, the application of ECCO2R specifically for hypercapnic respiratory failure has not been well studied. We describe here a pilot study of the application of a novel, single venous catheter ECCO2R system in patients with COPD with hypercapnic respiratory failure conducted in India (single center) and in Germany (five centers).
Materials and Methods
ECCO2R Device
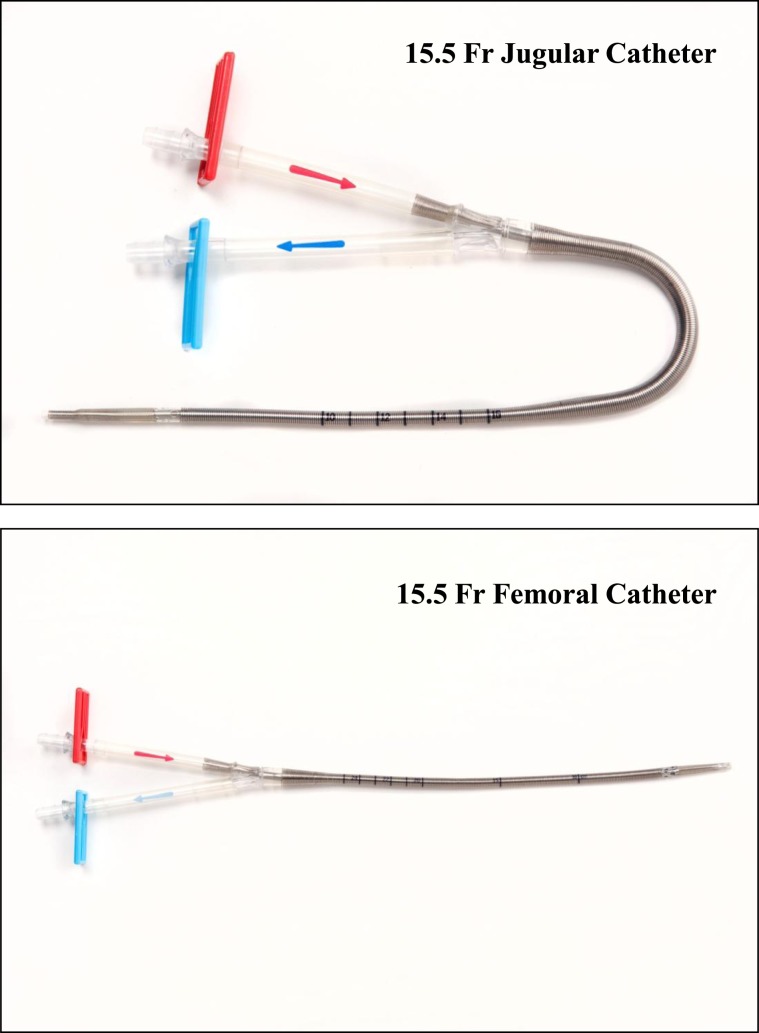
The ECCO2R device (Hemolung Respiratory Assist System; ALung Technologies Inc) was applied in three groups of patients with COPD with hypercapnic respiratory failure. This ECCO2R device has been described in detail previously and consists of a catheter, an integrated pump/gas-exchange cartridge, and a controller assembly.13 The size 15.5F dual-lumen catheter is available in either a femoral or internal jugular configuration (Fig 1), and is inserted percutaneously and advanced to the inferior or superior vena cava. Venous blood is withdrawn through the double-lumen catheter by a centrifugal pump magnetically driven by the Hemolung controller unit, where it flows past a cylindrical bundle of hollow fibers assembled around a rotating core. Sweep gas (air or oxygen) is drawn through the hollow fibers by a vacuum pump (to prevent air embolism in the event of fiber breakage), creating a diffusion gradient for gas exchange across the membrane. The venous blood is returned to the patient through the double-lumen catheter. Unlike conventional passive oxygenators, the pump-driven enhanced flow past the membranes markedly increases gas-exchange efficiency, allowing for significant CO2 removal at relatively low blood flow in the range of 300 to 500 mL/min. The rate of CO2 removal is calculated and displayed on the controller monitor based on internal measurements of CO2 percentage and flow in the hollow-fiber membrane sweep gas. This device has been studied in animals for up to 8 days, and was, therefore, limited in the clinical feasibility study to a maximum of 7 days (or 168 h) of therapy.
Figure 1.
The Hemolung15.5F double-lumen extracorporeal CO2 removal (ECCO2R) catheter with an optional femoral or jugular configuration.
Subjects
Three groups of patients, aged 21 to 80 years, with COPD and hypercapnic respiratory failure (PaCO2 > 50 mm Hg, pH < 7.3) were considered eligible for study inclusion. Group 1 (n = 7) consisted of patients with acute exacerbation of COPD on NIPPV therapy with a very high likelihood (> 50%) of requiring intubation and IPPV.7,14 Patients in this group had been on NIPPV for at least 1 h with either PaCO2 > 55 mmHg and pH < 7.25 or pH < 7.30 and PaCO2 > 55 mm Hg, with < 5 mmHg PaCO2 decrease from baseline following NIPPV application. Group 2 (n = 2) consisted of patients with hypercapnic respiratory failure requiring NIPPV who had failed two weaning attempts and did not wish to be invasively mechanically ventilated. Group 3 (n = 11) consisted of patients with hypercapnic respiratory failure already on invasive mechanical ventilation who had either failed two or more weaning attempts or failed one weaning attempt and did not wish to continue invasive mechanical ventilation.
For each subject, written informed consent (as approved by each site’s institutional review board) was obtained (institutional review board committee names and project approval numbers for all centers are included as a data supplement in e-Table 1 (475.6KB, pdf) ). Consecutive patients with COPD presenting with hypercapnic respiratory failure were evaluated, and subjects meeting the inclusion criteria were enrolled. The diagnosis of COPD was based on medical history, physical examination, and pulmonary function tests indicating airways obstruction.1 Patients with hemodynamic instability, sensitivity to heparin, recent major surgery, uncontrolled arrhythmia, thrombocytopenia (platelets < 100,000/mm3), a bleeding diathesis, or coma from any cause were excluded from the study.
The double-lumen catheter was placed in either the femoral (n = 13) or jugular vein (n = 7) and advanced to the vena cava. A loading dose of heparin (80 units/kg) was administered, and the heparin dose was adjusted to maintain the activated partial thromboplastin time at 1.5 to 2.3 times baseline as long as the catheter was in situ. Blood flow through the catheter was initiated at 400 mL to 500 mL/min to achieve a CO2 removal rate > 50 mL/min. The CO2 removal rate and blood flow, displayed on the Hemolung controller, were continuously monitored and recorded. Baseline blood samples for measurements of hematologic parameters, electrolytes, and liver function tests, as well as arterial blood gas measurements, were made. These measurements were repeated serially at intervals throughout the period as long as the ECCO2R catheter was in situ. In addition, daily measurements of dyspnea using a visual analog scale (VAS) were made when possible.15 Group 1 patients on ECCO2R and NIPPV were considered for intubation if there was a worsening or lack of improvement of the arterial blood gases, defined as an arterial pH < 7.25 or an increase in PaCO2 > 10 mmHg after 2 h of treatment, or worsening of respiratory distress, deterioration of neurologic status, intolerance of face/nasal mask, inability to clear secretions, or life-threatening cardiovascular instability. In each case, the final decision was made by the attending physician/principal investigator. All patients were followed to hospital discharge or 30 days after discontinuance of ECCO2R.
Results
Twenty patients with COPD with hypercapnic respiratory failure received ECCO2R therapy (Table 1) for a mean of 104.2 ± 59.7 h (range, 0.2-192 h). The individual ventilatory parameters are shown in Table 2. The device was well tolerated by the patients and invasive ventilation was avoided in association with ECCO2R therapy in all patients in group 1; in one patient, ECCO2R was stopped after 20 min due to inadequate anticoagulation. Three patients in this group died within 30 days after completion of ECCO2R therapy due to their underlying disease states. Both patients in group 2 were weaned off continuous NIPPV with the application of ECCO2R, but remained on intermittent NIPPV support, and were alive 30 days after completion of therapy.
Table 1.
—Study Groups
| Subject | Age, y (Sex) | FEV1 % Predicted | FEV1/FVC % | Clinical Dataa | Days on NIPPV/IPPV Prior to ECCO2R | Hours on ECCO2R | Clinical Effects During ECCO2R | Status 30 d Post-ECCO2R |
| Group 1: Failing NIPPV | ||||||||
| 1 | 76 (F) | … | … | Stage IV COPD, obesity, OSA, renal insufficiency | 2 | 78 | ↓PaCO2 ↓ dyspnea, remained on NIPPV | Died day 26: septic shock |
| 2 | 69 (F) | 38 | 50 | Stage III COPD, no known comorbidities | 4 h | 66 | ↓PaCO2 ↓dyspnea cessation of NIPPV support | On nocturnal NIPPV |
| 3 | 68 (F) | 18 | 27 | Stage IV COPD with bilateral lower-lobe emphysema | 7 | 142 | ↓PaCO2 ↓dyspnea ↓NIPPV support | Died, day 1: cardiopulmonary arrest |
| 4 | 72(M) | 21 | 34 | Stage IV COPD, LTOT > 2 y, bilateral LVRS 1 y | 16 | 160 | ↓PaCO2 ↓dyspnea | Died, day 10: respiratory failure, refused intubation |
| 5 | 49 (M) | 14 | 20 | Stage IV COPD, resection bulla right upper lobe 1991, on lung transplant list 2 y | 4 | 140 | ↓PaCO2 ↓dyspnea ↓NIPPV support | Bilateral lung transplant day 31 |
| 6 | 50 (F) | … | … | Stage IV COPD, atypical mycobacterial infection (on antimicrobial therapy since 2009) | 3 | 0.2 | Catheter clotted, insufficient anticoagulation | On NIPPV |
| 7 | 78 (M) | 38 | 39 | Stage III COPD, LTOT 1 y | 1 | 41 | ↓PaCO2 ECCO2R stopped because of ↓blood flow | Died, day 7: pneumonia, sepsis |
| Group 2: Unable to wean from NIPPV | ||||||||
| 1 | 78 (M) | 22 | 30 | Stage IV COPD, right lung lobectomy 1998, polycystic kidney disease | 1 | 48 | ↓PaCO2 ↓dyspnea, stopped NIPPV | On intermittent NIPPV |
| 2 | 59 (F) | 18 | 53 | Stage IV COPD, pulmonary hypertension LTOT 5 y, bilateral upper lobe endoscopic LVRS 2 y, compete endoscopic closure of right upper lobe 1 y | 7 | 192 | ↓PaCO2 ↓dyspnea,↓NIPPV support | On intermittent NIPPV |
| Group 3: Unable to wean from IPPV | ||||||||
| 1 | 66 (M) | 19 | 32 | Stage IV COPD, LTOT 2 y, nocturnal NIPPV 1 y, endoscopic LVRS 4 y | 9 | 168 | ↓PaCO2 extubated day 2, reintubated after 37 h, tracheostomized and placed on NIPPV | On nocturnal NIPPV |
| 2 | 64 (M) | 13 | 28 | Stage IV COPD, bullous emphysema, bilateral LVRS 2 y, on NIPPV 2 y, LTOT 2 y | 4 | 177 | Extubated in 3 h; reintubated after 94 h, then extubated | On intermittent NIPPV |
| 3 | 61 (F) | 28 | 31 | Stage IV COPD, on lung transplant list since 2007 | 15 | 95 | ↓PaCO2; remained on IPPV; ECCO2R discontinued because of coagulopathy (von Willebrand disease) | Died, day 17: pneumonia, septic shock |
| 4 | 64 (F) | … | … | … | 30 | 3 | Vascular perforation during catheter placement | Died while on Hemolung at approximately 3 h |
| 5 | 61 (M) | 45 | 60 | Stage III COPD, OSA on home NIPPV, myelodysplastic syndrome, cardiac arrest 1 mo prior, tracheostomy 18 d prior | 30 | 79 | ↓PaCO2 ↓dyspnea; spontaneous breathing | Extubated; on intermittent NIPPV |
| 6 | 55 (F) | … | … | Stage III COPD, anxiety-panic disorder, hypertension, tracheostomy 10 d prior | 17 | 168 | ↓PaCO2 ↓dyspnea, ↓ventilator support | Continued IPPV with ↓ventilator support |
| 7 | 73 (M) | … | … | Stage IV COPD, LTOT, hypertension, tracheostomy 2 mo prior | 27 | 42 | ↓PaCO2 ↓ventilator support | Continued IPPV with ↓ventilator support |
| 8 | 46 (F) | 12 | 52 | Stage IV COPD, obesity, LTOT 10 y, on NIPPV 3 y | 22 | 168 | ↓PaCO2; remained on IPPV | Died, day 26: abdominal sepsis, respiratory failure |
| 9 | 70 (M) | … | … | Stage IV COPD, Cor Pulmonale | 62 | 118 | ↓PaCO2; remained on IPPV; panic/anxiety attacks requiring sedation | Continued IPPV |
| 10 | 67 (M) | 28 | 45 | Stage IV COPD | 15 | 74 | ↓PaCO2; ↓ ventilator support; ↓blood flow due to inadequate anticoagulation | Died, day 21: pneumonia, respiratory failure |
| 11 | 71 (M) | 16 | 34 | Stage IV COPD, hypertension | 72 | 129 | ↓PaCO2 ↓dyspnea, extubated; reintubated day 4 | Continued IPPV with ↓ventilator support |
ECCO2R = extracorporeal CO2 removal; F = female; IPPV = invasive positive pressure ventilation; LTOT = long-term oxygen therapy; LVRS = lung volume reduction; M = male; NIPPV = noninvasive positive pressure ventilation; OSA = obstructive sleep apnea.
COPD stages based on the Global Initiative for Chronic Obstructive Lung Disease.16
Table 2.
—Ventilatory Parameters
| Subject | Group 1 | Group 2 | Group 3 | |||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Minute ventilation, L/min | ||||||||||||||||||||
| Baseline, pre-ECCO2R | 2.5 | 2.5 | 6.5 | … | 7.1 | … | … | … | … | 7.4 | 7.0 | 6.9 | 4.7 | 8.6 | 4.5 | 11.0 | 8.4 | 8.3 | 7.8 | 5.9 |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | 2.0 | 3.4 | 4.0 | 15.0 | 6.0 | … | … | 6.8 | 9.7 | 8.0 | 6.9 | 3.7 | 3.5 | … | 6.8 | 10.1 | 7.7 | 7.1 | 6.8 | 9.7 |
| 2 h | 4.0 | 3.5 | 8.0 | 16.0 | 8.0 | … | … | 7.3 | 10 | 6.6 | 7.3 | 3.5 | 3.41 | … | 3.8 | 6.8 | 5.5 | … | 7.3 | 10.2 |
| Final | 6.8 | … | 4.9 | 8.3 | 8.7 | … | … | 10.4 | … | 11.7 | 7.4 | 8.2 | … | 8.5 | 3.4 | 8.7 | 8.3 | 7.6 | 10.4 | … |
| Frequency, min | ||||||||||||||||||||
| Baseline, pre-ECCO2R | 18 | 20 | 18 | 22 | 16 | 18 | … | 25 | 15 | 15 | 16 | 19 | 15 | 16 | 16 | 20 | 23 | 22 | 25 | 15 |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | 21 | 24 | 13 | 22 | 16 | … | 26 | 15 | 15 | 16 | 12 | 12 | 29 | 20 | 33 | 38 | 26 | 26 | 15 | |
| 2 h | 18 | 19 | 13 | 22 | … | … | … | 26 | 15 | 15 | 16 | … | 11 | 30 | 14 | 20 | 23 | 30 | 26 | 15 |
| Final | 23 | 24 | 20 | 22 | … | … | … | 26 | 16 | 24 | 11 | 16 | … | 11 | 20 | 20 | 24 | 25 | 26 | 16 |
| FIO2, % | ||||||||||||||||||||
| Baseline, pre-ECCO2R | … | … | 30 | 44 | 28 | 30 | 44 | 40 | 35 | 35 | 35 | 45 | 35 | 30 | 30 | 40 | 50 | 32 | 36 | 30 |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | … | … | 30 | 44 | … | … | … | 50 | 35 | 36 | 35 | 50 | 35 | 30 | 30 | 50 | 50 | 40 | … | 30 |
| 2 h | … | … | 30 | 44 | 32 | … | 28 | … | 35 | 36 | 35 | 50 | 35 | 30 | 30 | 50 | 50 | … | … | 30 |
| Final | … | … | 30 | 80 | 28 | … | 40 | 40 | 35 | 45 | 40 | 75 | 35 | 35 | 30 | 30 | 40 | 40 | 44 | 30 |
| IPAP, cm H2O | ||||||||||||||||||||
| Baseline pre-ECCO2R | … | … | 28 | 30 | 20 | 20 | … | 15 | 32 | … | … | … | … | … | … | … | … | … | … | … |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | … | … | … | … | 20 | … | … | … | 32 | … | … | … | … | … | … | … | … | … | … | … |
| 2 h | … | … | 12 | … | 20 | … | … | 24 | 32 | … | … | … | … | … | … | … | … | … | … | … |
| Final | … | … | 30 | 32 | 20 | … | … | 24 | 30 | … | … | … | … | … | … | … | … | … | … | … |
| EPAP, cm H2O | ||||||||||||||||||||
| Baseline, pre-ECCO2R | … | … | 12 | 4 | 6 | 8 | … | 5 | 10 | … | … | … | … | … | … | … | … | … | … | … |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | … | … | … | … | 6 | … | … | … | 10 | … | … | … | … | … | … | … | … | … | … | … |
| 2 h | … | … | 12 | … | 6 | … | … | 5 | 10 | … | … | … | … | … | … | … | … | … | … | … |
| Final | … | … | 10 | 6 | 5 | … | … | 5 | 8 | … | … | … | … | … | … | … | … | … | … | … |
| PEEP, cm H2O | ||||||||||||||||||||
| Baseline, pre-ECCO2R | … | … | … | … | … | … | … | … | … | 4 | 5 | 9 | 10 | 15 | 8 | 7 | 8 | 5 | 6 | 5 |
| Time after ECCO2R application | ||||||||||||||||||||
| 1 h | … | … | … | … | … | … | … | … | … | 4 | 5 | … | 10 | 15 | 8 | 10 | 8 | 5 | 6 | 5 |
| 2 h | … | … | … | … | … | … | … | … | … | 4 | 6 | 8 | 9 | 15 | 10 | 10 | 8 | 5 | 6 | 5 |
| Final | … | … | … | … | … | … | … | … | … | 4 | 8 | 10 | … | 7 | 8 | 10 | 6 | 5 | 6 | 5 |
EPAP = expiratory positive airway pressure; IPAP = inspiratory positive airway pressure; PEEP = positive end-expiratory pressure. See Table 1 for expansion of other abbreviations.
In group 3, nine of 11 patients had been on invasive mechanical ventilation for > 15 days prior to ECCO2R. Three patients were weaned off invasive mechanical ventilation, and in three further patients, the level of invasive ventilatory support was reduced. Only one patient remained on the same level of ventilatory support. One patient in group 3 died as a result of a retroperitoneal bleed following catheterization and received ECCO2R support for < 3 h. At follow-up, after the cessation of ECCO2R therapy, seven patients died (Table 1). One patient in group 1 (patient 4, Table 1) suffered a cardiopulmonary arrest within 24 h of ECCO2R cessation, and the other patients died of varying causes between 7 and 26 days after completion of ECCO2R. None of these deaths could be attributed to the ECCO2R, and this mortality rate of 35% is not unexpected, given the severity of COPD and respiratory failure in these patients.6,9,10 This mortality rate compares with in-hospital mortality rates of 29.3%,6,10 and 1-year mortality of 49.1% to 62%9,10 in similar patients.
The application of ECCO2R resulted in a decrease in the sensation of breathlessness. Systematic assessment of breathlessness by VAS was available in five subjects in whom there was a significant decrease in breathlessness: baseline VAS score, 7.2 ± 2.2 vs 3.56 ± 1.59 at 48 h (n = 5, P < .027).
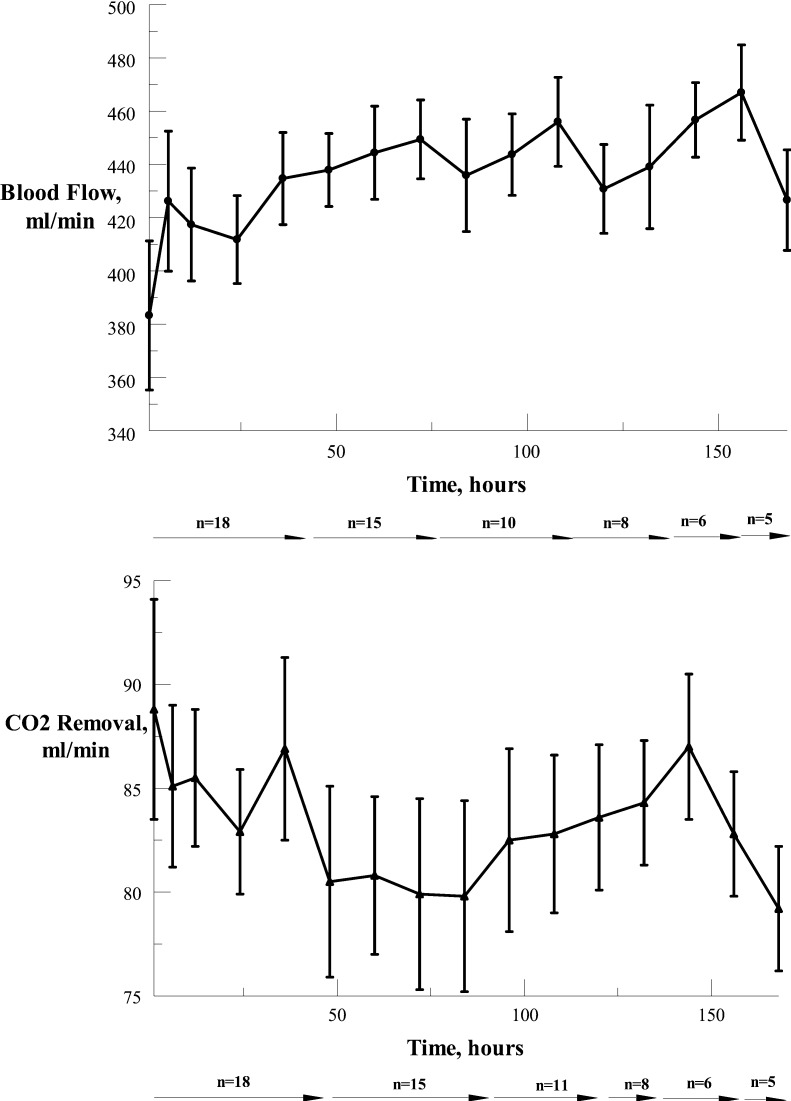
Blood flow through the system (Fig 2) ranged between 117 and 587 mL/min (mean ± SD of 430.5 ± 73.7 mL/min). ECCO2R ranged from 14 to 121 mL/min (mean ± SD of 82.0 ± 16.3 mL/min) with the lower figures representing weaning from the device. Over the course of ECCO2R therapy, blood flow and CO2 removal did not change significantly in these patients.
Figure 2.
Blood flow (mean ± SEM) and CO2 removal (mean ± SEM) through the catheter over time, where n = number of subjects at various time points.
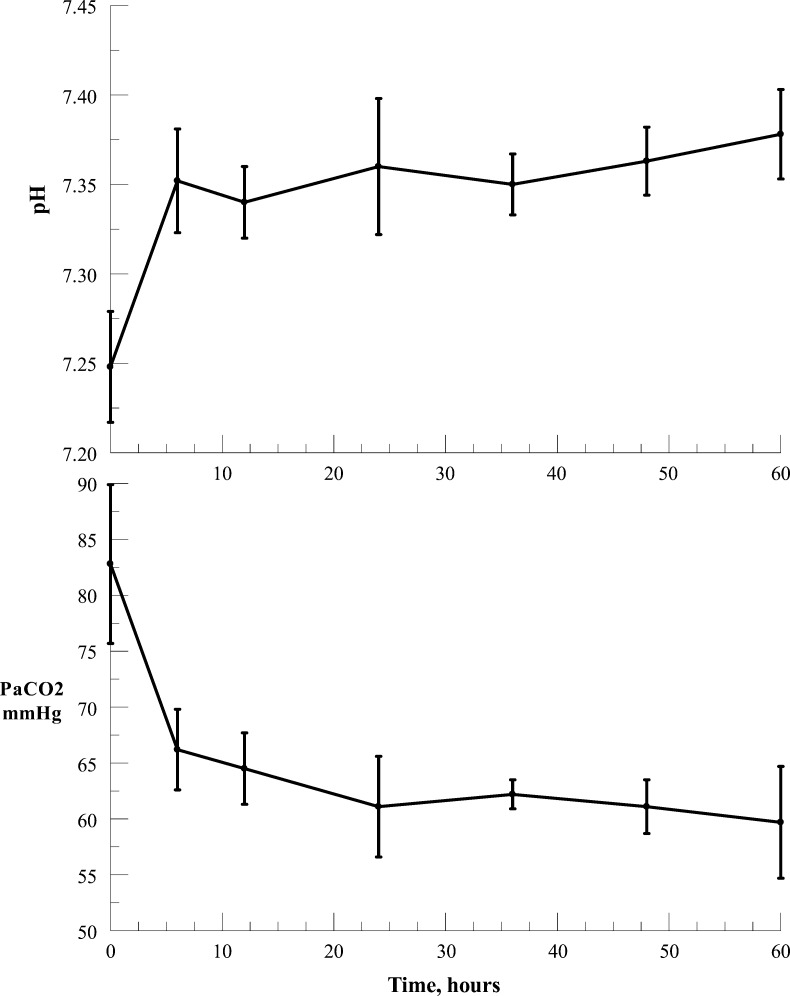
In patients from groups 1 and 2 on NIPPV, there was a significant decrease (P < .003, paired t test) in PaCO2 within 1 h of the application of the device from 78.9 ± 16.8 mm Hg to 65.9 ± 11.5 mm Hg, and a significant increase (P < .0002, paired t test) in pH from 7.28 ± 0.08 to 7.34 ± 0.07. The effects of the application of ECCO2R in group 1 patients (n = 6) are shown in Figure 3, indicating continuing decrease in PaCO2 and increase in pH with time. Arterial PO2 was maintained within acceptable values with appropriate adjustment of the FIO2. In group 3 patients, who were on invasive mechanical ventilation, precise changes in PaCO2 and pH specifically due to the application of ECCO2R cannot be accurately assessed because of ventilator setting adjustments; nevertheless, the data on CO2 removal rates (Fig 2) indicate that the PaCO2 would very likely have decreased with constant ventilator settings.
Figure 3.
Changes in PaCO2 (mean ± SEM) and pH (mean ± SEM) with ECCO2R in patients in group 1 (acute exacerbation of COPD on noninvasive positive pressure ventilation [NIPPV]). n = number of subjects; 0 h on abscissa represents baseline values. See Figure 1 legend for expansion of other abbreviations.
There was no evidence of clinically significant hemolysis in any of the patients; liver and renal function were not adversely affected by the ECCO2R (e-Table 2 (475.6KB, pdf) ). Plasma-free hemoglobin, an index of hemolysis, increased above 40 mg/100 mL in only two patients, in both of whom the increase was attributable to concomitant clinical events. Self-limited thrombocytopenia, most likely related to heparin use, was noted in eight patients. In only two patients did the platelet count drop transiently below 85,000/μL; none of the patients required platelet transfusion. A significant thrombocytopenia was seen posttherapy in one patient who received 3 units of plasma. Adverse events attributable to catheter placement consisted of one death secondary to blood loss from perforation of the left iliac vein (Table 1: group 3, subject 4), and one pneumothorax which resolved with treatment. In one subject a deep venous thrombosis was noted in the cannulated vein 3 days after the catheter had been removed, causing no further adverse effects.
Significant bleeding events requiring blood transfusion occurred in three patients: In two cases the bleeding was related to underlying disease and anticoagulation, and in one subject it was due to inadvertent excessive anticoagulation. In the two subjects with bleeding caused by their underlying disease, one (group 3, subject 3, Table 1) was attributed to a new diagnosis of von Willebrand disease, and the other (group 1, subject 3, Table 1) to an intestinal ulcer. In the patient who received excessive anticoagulation (group 1, subject 5, Table 1), bleeding occurred from the catheter insertion site and a preexisting thoracic drain.
Discussion
This pilot study supports the feasibility of using this single venous catheter ECCO2R device for removing CO2 in hypercapnic patients with COPD, with side effects primarily related to and similar to those seen with central venous catheter placement used with extracorporeal therapies.17 The device was consistently able to remove > 80 mL/min of CO2 over several days of continuous use at blood flow ranging between 300 and 550 mL/min through the extracorporeal circuit.
The overall mortality rate was very similar to that reported in comparable patients not receiving ECCO2R,6,9,10 and it could be argued that the absence of any significant improvement in mortality makes the value of this ECCO2R device questionable. However, this preliminary study was not planned to assess improvements in mortality outcomes, and the data are insufficient to provide definitive conclusions in this regard. On the other hand, mortality is significantly better in patients treated with NIPPV who avoid intubation and IPPV,6 and it is possible that ECCO2R will have a beneficial effect on mortality; however, a definitive answer must await a larger, controlled, prospective study.
The most valid data for assessing the effect of the device are those seen in patients in group 1. In these patients who were on NIPPV, the device was capable of reducing the PaCO2 and increasing pH (Fig 3), and none required invasive ventilation. We recognize that the decision to proceed to invasive ventilation is dependent on a number of factors and varies with the individual treating physician. Nevertheless, given the inclusion parameters for this group, the likelihood of these patients requiring invasive ventilation is predicted to be > 52%.14 In group 3, it could be argued that the precise effects of the device could be confounded by the degree of mechanical ventilatory support and adjustments made by the investigator. However, in each case, the patients had been on standard treatment and the application of ECCO2R resulted in clinical improvement. The data indicate that the device application was associated with a decrease in the level of perceived dyspnea.
The single death directly attributable to the catheter placement was, as noted previously, due to internal hemorrhage from vessel perforation. This is a well-known adverse event with central venous catheter placement and is not unique to this device.17 A more common side effect, thrombocytopenia, was likely related to heparin use. However, in the majority of cases, this was not associated with clinically significant bleeding. The current system is not an oxygenation device; in animal studies, the maximum oxygen uptake that can be achieved with the device at blood flow rates of 400 to 500 mL/min is 25 to 35 mL/min.
ECCO2R has been used as an adjunct to prevent lung damage in patients with ARDS.12 The original technique required a pump-driven venovenous bypass, utilizing large catheters (21–28F size) and relatively high blood flows (25%–30% of cardiac output), with a high incidence of side effects.18,19 These factors, as well as the negative results of a clinical trial,20 prevented widespread clinical application of this technique.
Further innovations have led to the development of two different CO2 removal techniques: a pumpless arteriovenous system, the interventional lung assist device (Novalung GmbH), and a pump-driven low blood flow venovenous system (Hemodec s.r.l.). The pumpless device has been used primarily in patients with ARDS as an adjunct to mechanical ventilation.21 Both an arterial and a venous catheter are required with high blood flows of 1.0 to 2.5L/min, ie, 25% to 50% of cardiac output, and concomitant use of norepinephrine to maintain the pressure gradient. Nevertheless, it is effective in CO2 removal. Unfortunately, serious complications in 12% to 25% of patients, including limb ischemia, compartment syndrome, and amputation have been reported.22 The pump-driven system uses a smaller catheter and lower blood flows (up to 400 mL/min). CO2 exchange is increased by the use of a hemodialyzer in the circuit.23 The actual CO2 removal rates have not been stated in these studies.
The present pilot study establishes the feasibility of using this single venous catheter ECCO2R device for effective removal of CO2 at relatively low blood flow rates in hypercapnic patients. It may be useful in the treatment of hypercapnic respiratory failure in patients with COPD and, possibly, may have a role in assisting protective ventilation in patients requiring extreme ventilatory support, such as in severe ARDS. A larger, prospective study may help to establish the efficacy and safety of the device and its role in the management of respiratory failure.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Burki had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Burki: contributed to the study conception and design, provided oversight of the studies and analysis of the data, and was responsible for the drafting, review, and final approval of the manuscript.
Dr Mani: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Herth: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Schmidt: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Teschler: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Bonin: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Becker: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Randerath: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Stieglitz: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Hagmeyer: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Priegnitz: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Pfeifer: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Blaas: contributed to the induction and treatment of patients from their institution into the study; the acquisition, analysis, and interpretation of the data from these patients; and review and approval of the manuscript.
Dr Putensen: contributed to study design and review and approval of the manuscript.
Dr Theuerkauf: contributed to study design and review and approval of the manuscript.
Dr Quintel: contributed to study design and review and approval of the manuscript.
Dr Moerer: contributed to study design and review and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Burki is a consultant and medical director for ALung Technologies Inc. Drs Mani, Herth, Schmidt, Teschler, Bonin, Becker, Randerath, Stieglitz, Hagmeyer, Priegnitz, Pfeifer, Blaas, Putensen, Theuerkauf, Quintel, and Moerer have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: ALung Technologies Inc. provided funding and equipment to all the participating institutions; the equipment was provided on loan or as disposables to the participating institutions.
Other contributions: Acknowledgment is also given to Laura Lund, PhD; Alethea Wieland, BA; Tracey Dill, RRT; and Karen Brestensky, BS, of ALung Technologies Inc who provided editorial assistance and verification of data accuracy for the manuscript.
Additional information: The e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ECCO2R
extracorporeal CO2 removal
- IPPV
invasive positive pressure ventilation
- NIPPV
noninvasive positive pressure ventilation
- VAS
visual analog score
Footnotes
Funding/Support: ALung Technologies Inc provided funding and equipment to all the participating institutions.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Information for commercial entities is available online.
References
- 1.Qaseem A, Wilt TJ, Weinberger SE, et al. American College of Physicians American College of Chest Physicians American Thoracic Society European Respiratory Society Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191 [DOI] [PubMed] [Google Scholar]
- 2.Diener CF, Burrows B. Further observations on the course and prognosis of chronic obstructive lung disease. Am Rev Respir Dis. 1975;111(6):719-724 [DOI] [PubMed] [Google Scholar]
- 3.Cooper CB, Waterhouse J, Howard P. Twelve year clinical study of patients with hypoxic cor pulmonale given long term domiciliary oxygen therapy. Thorax. 1987;42(2):105-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(4 pt 1):959-967 [DOI] [PubMed] [Google Scholar]
- 5.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508-515 [DOI] [PubMed] [Google Scholar]
- 6.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoo GW, Hakimian N, Santiago SM. Hypercapnic respiratory failure in COPD patients: response to therapy. Chest. 2000;117(1):169-177 [DOI] [PubMed] [Google Scholar]
- 8.Quinnell TG, Pilsworth S, Shneerson JM, Smith IE. Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning results, survival, and the role of noninvasive ventilation. Chest. 2006;129(1):133-139 [DOI] [PubMed] [Google Scholar]
- 9.Menzies R, Gibbons W, Goldberg P. Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest. 1989;95(2):398-405 [DOI] [PubMed] [Google Scholar]
- 10.Chu CM, Chan VL, Lin AW, Wong IW, Leung WS, Lai CK. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax. 2004;59(12):1020-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2(8189):292-294 [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881-886 [PubMed] [Google Scholar]
- 13.Batchinsky AI, Jordan BS, Regn D, et al. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39(6):1382-1387 [DOI] [PubMed] [Google Scholar]
- 14.Confalonieri M, Garuti G, Cattaruzza MS, et al. Italian noninvasive positive pressure ventilation (NPPV) study group A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25(2):348-355 [DOI] [PubMed] [Google Scholar]
- 15.Burki NK, Tobin MJ, Guz A, Sharp JT, Banzett RB, Mahler DA. Dyspnea: mechanisms, evaluation and treatment. Am Rev Respir Dis. 1988;138(4):1040-1041 [DOI] [PubMed] [Google Scholar]
- 16.Global Initiative for Chronic Obstructive Lung Disease GOLD website. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed June 12, 2012.
- 17.Blaivas M. Video analysis of accidental arterial cannulation with dynamic ultrasound guidance for central venous access. J Ultrasound Med. 2009;28(9):1239-1244 [DOI] [PubMed] [Google Scholar]
- 18.Deslauriers J, Awad JA. Is extracorporeal CO2 removal an option in the treatment of adult respiratory distress syndrome?. Ann Thorac Surg. 1997;64(6):1581-1582 [DOI] [PubMed] [Google Scholar]
- 19.Conrad SA, Rycus PT, Dalton H. Extracorporeal life support registry report 2004. ASAIO J. 2005;51(1):4-10 [DOI] [PubMed] [Google Scholar]
- 20.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 pt 1):295-305 [DOI] [PubMed] [Google Scholar]
- 21.Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med. 2006;34(5):1372-1377 [DOI] [PubMed] [Google Scholar]
- 22.Terragni PP, Birocco A, Faggiano C, Ranieri VM. Extracorporeal CO2 removal. Contrib Nephrol. 2010;165185-196 [DOI] [PubMed] [Google Scholar]
- 23.Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111(4):826-835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement