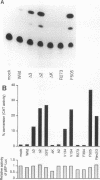
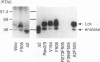
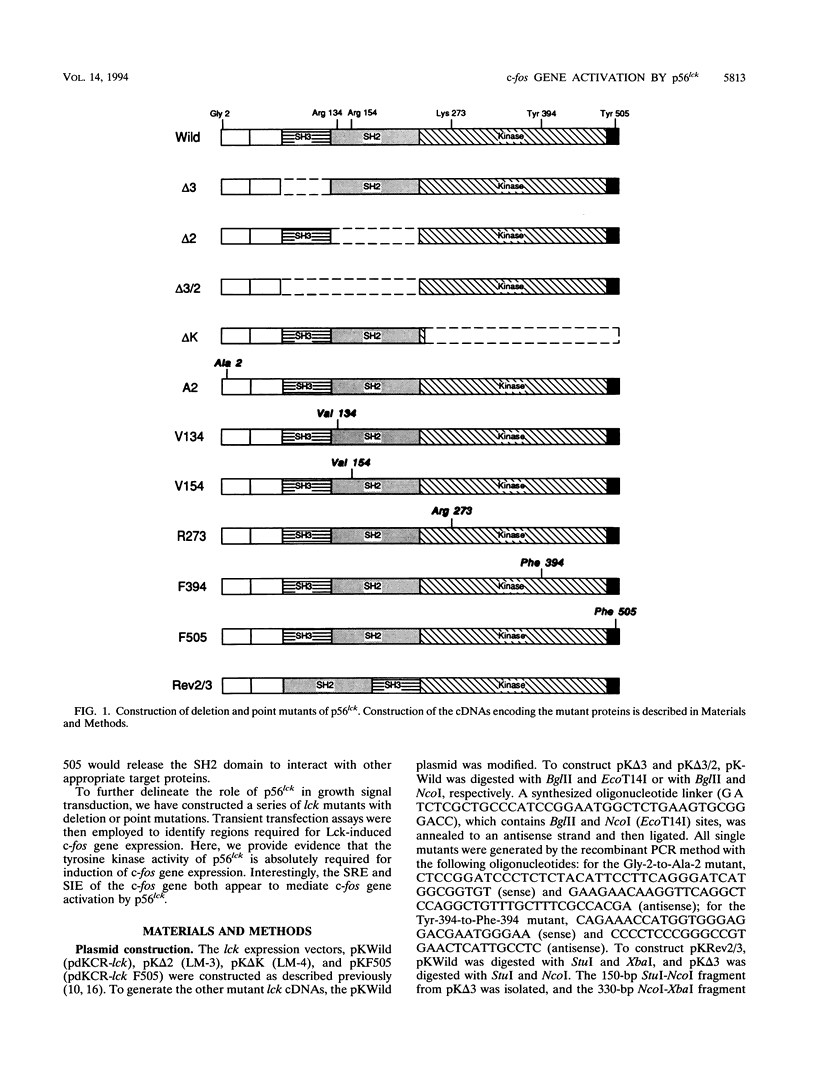
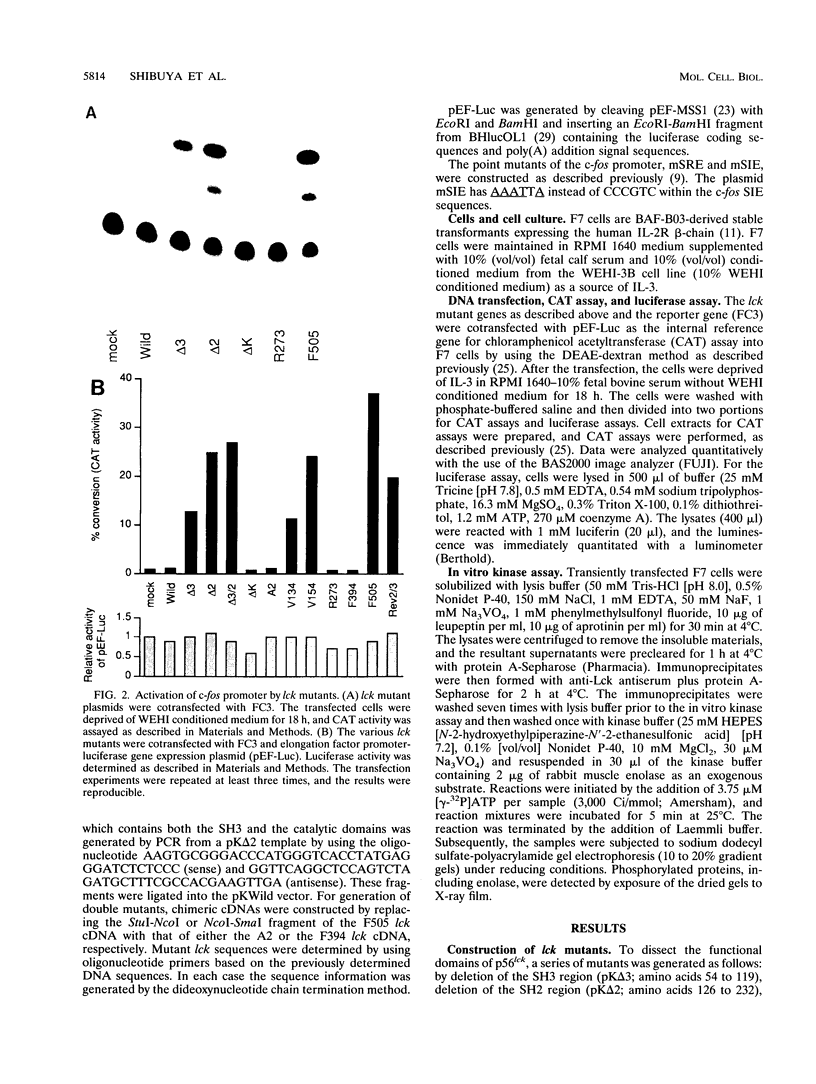
Abstract
Members of the newly identified receptor family for cytokines characteristically lack the intrinsic protein tyrosine kinase domain that is a hallmark of other growth factor receptors. Instead, accumulating evidence suggests that these receptors utilize nonreceptor-type protein tyrosine kinases for downstream signal transduction by cytokines. We have shown previously that the interleukin-2 receptor beta-chain interacts both physically and functionally with a Src family member, p56lck, and that p56lck activation leads to induction of the c-fos gene. However, the mechanism linking p56lck activation with c-fos induction remains unelucidated. In the present study, we systematically examined the extent of c-fos promoter activation by expression of a series of p56lck mutants, using a transient cotransfection assay. The results define a set of the essential amino acid residues that regulate p56lck induction of the c-fos promoter. We also provide evidence that the serum-responsive element and sis-inducible element are both targets through which p56lck controls c-fos gene activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990 Oct;10(10):5197–5206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., Meijlink F., Verma I. M. Identification of a transcriptional enhancer element upstream from the proto-oncogene fos. Science. 1985 Dec 6;230(4730):1174–1177. doi: 10.1126/science.3865371. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Atwell S. K., Shoelson S. E., Harrison S. C. Structure of the regulatory domains of the Src-family tyrosine kinase Lck. Nature. 1994 Apr 21;368(6473):764–769. doi: 10.1038/368764a0. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kawahara A., Mori H., Shibuya H., Taniguchi T. c-fos gene induction by interleukin 2: identification of the critical cytoplasmic regions within the interleukin 2 receptor beta chain. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2022–2026. doi: 10.1073/pnas.89.6.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hayes T. E., Kitchen A. M., Cochran B. H. Inducible binding of a factor to the c-fos regulatory region. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1272–1276. doi: 10.1073/pnas.84.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Yamada K., Kobayashi N., Kawahara A., Perlmutter R. M., Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993 Feb;12(2):759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. Trying on a new pair of SH2s. Science. 1993 Sep 24;261(5129):1694–1695. doi: 10.1126/science.8397444. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Burn P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem Sci. 1993 Jun;18(6):215–220. doi: 10.1016/0968-0004(93)90192-p. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Prasad K. V., Raab M., da Silva A., Telfer J. C., Yamamoto M. src-related protein tyrosine kinases and their surface receptors. Biochim Biophys Acta. 1993 Aug 23;1155(2):239–266. doi: 10.1016/0304-419x(93)90007-y. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Gilman M. Z. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993 Mar 4;362(6415):79–83. doi: 10.1038/362079a0. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Irie K., Ninomiya-Tsuji J., Goebl M., Taniguchi T., Matsumoto K. New human gene encoding a positive modulator of HIV Tat-mediated transactivation. Nature. 1992 Jun 25;357(6380):700–702. doi: 10.1038/357700a0. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Taniguchi T. Involvement of a common transcription factor in the regulated expression of IL-2 and IL-2 receptor genes. Int Immunol. 1989;1(1):43–49. doi: 10.1093/intimm/1.1.43. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Veillette A., Caron L., Fournel M., Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992 May;7(5):971–980. [PubMed] [Google Scholar]
- Veillette A., Davidson D. Src-related protein tyrosine kinases and T-cell receptor signalling. Trends Genet. 1992 Feb;8(2):61–66. doi: 10.1016/0168-9525(92)90351-4. [DOI] [PubMed] [Google Scholar]
- Voraberger G., Schäfer R., Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5'-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991 Oct 15;147(8):2777–2786. [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]