Abstract
The BOLD signal measured in fMRI studies depends not only on neuronal activity, but also on other parameters like tissue vascularization, which may vary between subjects and between brain regions. A correction for variance from vascularization effects can thus lead to improved group statistics by reducing inter-subject variability. The fractional amplitude of low-frequency fluctuations (fALFF) as determined in a resting-state scan has been shown to be dependent on vascularization. Here we present a correction method termed RESCALE (REsting-state based SCALing of parameter Estimates) that uses local information to compute a voxel-wise scaling factor based on the correlation structure of fALFF and task activation parameter estimates from within a cube of 3 × 3 × 3 surrounding that voxel. The scaling method was used on a visuo-motor paradigm and resulted in a consistent increase in t-values in all task-activated cortical regions, with increases in peak t-values of 37.0% in the visual cortex and 12.7% in the left motor cortex. The RESCALE method as proposed herein can be easily applied to all task-based fMRI group studies provided that resting-state data for the same subject group is also acquired.
Keywords: Functional magnetic resonance imaging, Resting-state, fALFF, Scaling
Highlights
► RESCALE is a scaling method to reduce between-subject variance in task-fMRI. ► At single-subject level, local scaling factors are computed for each voxel. ► Scaling factors are based on linear relation between fALFF and parameter estimates. ► RESCALE was evaluated in a visuo-motor paradigm. ► RESCALE increased peak t-values by 37% in visual areas and 12.7% in motor areas.
Introduction
Functional magnetic resonance imaging (fMRI) is an established method used to investigate brain activity by acquiring blood oxygenation level dependent (BOLD) MR signal. The BOLD signal reflects changes in the concentration of oxygenated and deoxygenated hemoglobin, which in turn are robustly associated with neural activity (Logothetis and Wandell, 2004). Still, a significant amount of BOLD signal variability can be attributed to physiological factors such as local differences in vascularization of tissue, arterial CO2 concentration, or cardio-respiratory activity (Logothetis, 2008). As most fMRI studies focus on neural activity, a correction for physiological influence factors is desirable to extract from the measured data a signal more specific to the signal of interest (Harvey et al., 2008; Hutton et al., 2011; Lu et al., 2010).
In fMRI studies, a general distinction can be made between task-based and resting-state fMRI experiments. While the former concentrate on neural activation induced by external stimuli or performance of specific tasks, the latter aim at investigating intrinsic neural activity in the absence of external intervention. On this basis, resting-state fMRI (rs-fMRI) has been shown to reflect deep patterns of brain organization, and substantial effort has been made to investigate and interpret correlations between BOLD signal time courses of spatially remote brain regions in rs-fMRI, leading to the identification of so-called resting-state networks (Biswal et al., 2010; Kalcher et al., 2012). These resting-state networks represent partitions of the brain characterized by the similarity of their signal time courses in the low-frequency range (typically between 0.01 < f < 0.1 Hz). The most commonly used approaches to assess similarity, however, only take into account the correlation structure of signal time courses in various volume elements and leave aside other aspects like signal amplitude. A distinct approach to the analysis of rs-fMRI data lies in examining measures relating to the single voxel instead of the relation between voxels — one instance being the Amplitude Of Low Frequency Fluctuations (ALFF) of the measured BOLD signal (Zang et al., 2007), which have been demonstrated to be related to physiological properties of brain tissues (Kannurpatti et al., 2011). Based on the assumption that physiological effects on the BOLD signal during resting-state can be directly related to physiological effects during task-based experiments, a logical next step would be to use information gained from rs-fMRI scans for reducing the amount of unexplained variance in task-based fMRI measurements. Indeed, strong evidence for a correspondence between inter-subject variability of resting-state fluctuations and task activation magnitudes has been put forward by Mennes et al. (2010), (2011), who explored this relationship for both ALFF and functional connectivity.
The attempt to compensate for individual non-neural variability in order to improve on comparability of task activation magnitude can be made by scaling the BOLD response, reducing variability both between different regions in a single brain and between different subjects. Increasing comparability is of particular interest in the light of the current trend toward data sharing (Biswal et al., 2010) and meta-analyses comparing heterogeneous datasets from different centers (Huf et al., 2011). Early work on this topic concentrated on specific paradigms aimed at directly indentifying physiological variations for the correction of task-induced activity, for example by periodic inhalation of CO2-enriched air using breathing masks (Kannurpatti and Biswal, 2008) — a method perceived as excessively invasive by some subjects. In contrast, Thomason et al. (2007) used a design based on alternating periods of breath holding and normal respiration as a less invasive method to assess effects of changes in relative O2 and CO2 blood concentrations on the BOLD response. Using this design, they established a relationship, both on group-level between subjects and on single-subject level between voxels and ROIs, of specific brain regions' BOLD reactivity to global alterations of blood oxygenation on the one hand and magnitude of task-induced activation in these regions on the other. Of note, the correlation on single-subject level was only present in voxels activated by the task, and could not be observed in voxels inactive during the task.
Both the correction methods based on CO2-inhalation and on breath holding, however, are typically inapplicable in clinical populations due to limited subject compliance for the former or due to the extreme sensitivity to motion artifacts for the latter. In contrast, scaling of fMRI data based on resting-state scans bears the advantage that it can be easily implemented since rs-fMRI can be employed in most non-clinical and clinical subject populations and, indeed, already is a widely used method.
Following this line of reasoning, Kannurpatti et al. investigated the relationship between task activation magnitude and resting-state fluctuation amplitude (RSFA) from resting epochs during the paradigm of interest (Kannurpatti and Biswal, 2008) as well as from separate resting-state scans (Kannurpatti et al., 2011, 2012). In a complementary approach, scaling based on the power spectrum of low frequency fluctuations as a correlate of physiological factors influencing the BOLD signal has been developed by Biswal et al. (2007). In their study, the power spectrum of the fMRI signal of the paradigm was obtained by applying discrete Fourier transformation on the signal time course and calculating the square of the modulus of the Fourier spectrum for each frequency component; the resulting low frequency spectral amplitude (LSFA) was then used for signal scaling.
Other measures based on the power spectrum of separate resting-state scans have been proposed for use in task-fMRI scaling. Probably the most widely used are a measure termed Amplitude of Low Frequency Fluctuations (ALFF), the mean square root of low frequency components (0.01 < f < 0.08 Hz) of a signal's power spectrum, and the related fractional ALFF (fALFF) (Zou et al., 2008). The latter, based on the proportion of the power of the frequency components between 0.01 and 0.08 Hz to the total power of the signal, can be seen as a form of normalization leading to improved between-subject comparability: ALFF values have been shown to increase in the proximity of brain vessels or CSF, probably due to pulsations in these areas, while fALFF values are more robust with respect to this source of variability (Mennes et al., 2011; Zou et al., 2008, 2012). In particular, Mennes et al. (2011) explored the inter-subject correlation linking activation magnitude in task-based fMRI experiments using fALFF, revealing the potential of inter-subject scaling based on spectral measures gained from rs-fMRI datasets. Furthermore, in comparing the results from the prediction of task activation by fALFF with the prediction based on ALFF, they also found that fALFF was more robustly associated with task activations than ALFF. Thus, the evidence converges towards the conclusion that fALFF is best suited for the purpose of scaling fMRI based on spectral resting state amplitude measures.
Recently, Di et al. (2012) proposed a more specific approach to using ALFF for the correction of fMRI task activations by not only scaling on a global level with a constant scaling factor for the whole brain, or with a scaling factor taking into account one voxel only, but to use information from all voxels in one subject to assess the strength of relationship between ALFF and task activation magnitude individually for each subject, thus gaining a more specific scaling factor. They computed a scaling factor based on an estimate for a linear relationship between task activation magnitude and ALFF across all voxels of each subject's brain, leading to some improvement in t-values. Their results suggest that the correlation structure over multiple voxels can be taken into account to obtain more precise information for scaling, and the subject-wise correlation coefficient between ALFF and task activation proved useful in this respect. Still, the correlation between ALFF and task activation magnitude was not uniform in all regions of the brain, and the improvement achieved by their scaling method was limited by the simplification of modeling the complex relationship between task activation and ALFF as a single linear model for each subject, disregarding variation across the brain.
In the present study, we investigated a novel scaling approach termed RESCALE (REsting-state based SCALing of parameter Estimates) that is based on the local linear relationships between fALFF and task activation magnitude. More specifically, a scaling factor was calculated for each voxel based on the linear model fitted on the data from the 27 voxels in its immediate vicinity, predicting task activation magnitude from fALFF values: the slope of the regression model thus obtained can be seen as a quantification of the hemodynamic reactivity of this specific subject in this specific brain area. Individual-subject data can then be divided by a scaling factor based on this slope to reduce voxelwise between-subject heterogeneity.
Methods
Subjects
Forty-seven healthy right-handed subjects (21 males, age 24.1 ± 4.7) were recruited at Medical University of Vienna (Vienna, Austria). Exclusion criteria included previous psychiatric or neurological illness as well as the usual exclusion criteria for MRI studies. The subjects gave written informed consent prior to measurements and the study was approved by the institutional review board.
Tasks
Each subject underwent two fMRI paradigms: first, a right-hand finger tapping task for which subjects were instructed to alternately touch their right index and middle finger with their right thumb when shown a flickering checkerboard (8 Hz) on the projection screen. This visuo-motor paradigm (VMP) consisted of 6 blocks of finger tapping, with a duration of 10 s each, alternating with 7 blocks of rest of the same duration where subjects had to focus on a fixation cross. Second, subjects underwent a 6 min resting-state scan with eyes open, again looking at a fixation cross. All subjects also performed other fMRI paradigms during the scan sessions. The VMP was always the first task, the other fMRI measurements (including the resting-state measurement) were performed in random order. In total, each subject performed between 2 and 6 other fMRI paradigms between the VMP and the resting-state measurement.
Data acquisition
MRI measurements were performed on a Siemens Magnetom TIM TRIO 3T scanner using a 32-channel head coil. High-resolution anatomical images were acquired using an MPRAGE sequence with 1 × 1 × 1.1 mm resolution with 160 sagittal slices (TE / TR = 4.21 / 2300 ms, flip angle 9, inversion time 900 ms). Functional images were acquired with a gradient-echo EPI sequence (TE / TR = 42 / 1800 ms, flip angle 90), with 75 volumes for the VMP and 200 volumes for the resting-state measurement.
Preprocessing
All data were preprocessed using a combination of AFNI (Cox, 1996) and FSL (Smith et al., 2004). Anatomical images were skullstripped and normalized to the MNI152 standard space. Functional images from both scans underwent slice-timing correction (Sladky et al., 2011), intensity bias correction (using the bias field map estimated by FSL FAST) and volume registration, were coregistered to the anatomical images in the native space, resampled to 1.484 mm (the in-plane resolution of the EPI images) isotropic voxels, despiked (using AFNI 3dDespike), and masked with the skullstripped anatomical image. Following these steps common for the preprocessing of both scans, the pipeline for task and resting-state data diverged. Task time courses were transformed to percent signal change and activation maps were computed using a general linear model (GLM) in AFNI. Stimulus regressors were modeled as a superposition of boxcar functions each convolved with a canonical hemodynamic response function, and motion parameters were included as nuisance regressors of no interest. Resting-state data were corrected via a GLM approach, using white matter, CSF and global signal as well as motion parameters as nuisance regressors (Weissenbacher et al., 2009). Global signal regression (GSR), though debated as a preprocessing step in resting-state studies (Murphy et al., 2009; Saad et al., 2012), has been used to reduce the effect of global signal fluctuations on local fALFF estimates. The reason to include GSR nonetheless was that its main criticism relates to its introduction of negative correlations between time courses, which are not of interest in this study, and there is as yet no evidence of bias introduced by GSR with respect to spectral measures like fALFF. However, GSR reduces the influence of global signal confounders and thus improves the specificity of the signal on a local level. Note that smoothing of the data was not generally part of the preprocessing for all analyses, as will be detailed below.
Fractional ALFF
From the resting-state data, fractional ALFF (Zou et al., 2008) maps for each subject were computed using R 2.14.1 (R Development Core Team, 2011) on Ubuntu Linux 10.04.4 Lucid Lynx, employing the computational framework described in Boubela et al. (2012). For each voxel, resting-state time courses underwent discrete Fourier transformation, and the proportion of the power spectrum between 0.01 and 0.08 Hz relative to the sum of the power of all frequencies (from 0.0028 to 0.28 Hz, the Nyquist frequency for the TR of 1.8 s) was computed as the fALFF value.
Standard group analyses
To assess task activation and fALFF distribution on group level, standard group analyses were performed on both datasets. For the VMP data, smoothing with a 6 mm FWHM Gaussian kernel was performed on single-subject results and voxelwise t-tests were computed over all subjects. For the resting-state data, single-subject fALFF maps were analogously smoothed with a 6 mm FWHM Gaussian kernel and voxelwise group mean values were computed.
Voxelwise inter-subject correlation
To assess the relationship between task activation magnitude and fALFF at inter-subject level, voxelwise correlations between all subjects task activation beta coefficients and fALFF, both smoothed with a 6 mm FWHM Gaussian kernel, were computed. Correlation coefficients were transformed to z-scores using the Fisher transformation and p-values computed for these z-scores were used to assess the significance of correlation strength.
Intra-subject correlation
The relationship between task activation magnitude and fALFF at single-subject level was evaluated at a local level using the original, non-smoothed data. For each voxel, a linear model was fit for the 27 fALFF and task activation values of the voxel itself and its direct neighbors (forming a cube of 3 × 3 × 3 voxels) with the task activation beta coefficients as the dependent and the fALFF values as the explanatory variables. The correlation between these two variables as well as the slope of the linear model were interpreted as local measures of the relationship between fALFF and task activation magnitude. Note that non-smoothed data were used for the assessment of local correlation since smoothing would bias the correlation structure of neighboring voxels.
RESCALE task activation magnitude correction
Cubewise local slopes (i.e. the local slopes within the cubes of 3 × 3 × 3 voxels described above) were used for voxelwise signal scaling at single-subject level. For each subject, right-hand finger tapping task activation parameter estimate maps were corrected with a normalization factor, termed slope correction coefficient (SCC), based on the (cubewise) local slope between fALFF and task activation. Each voxel's slope correction coefficient was calculated as the absolute value of the local slope relative to the 99%-quantile of all local slopes of the subject:
The normalization of the SCC by the division of the slope by the 99% quantile was used to account for global between-subject differences in local slopes. The SCC itself, however, can be problematic as a scaling factor since most values (i.e. all voxels from regions not activated during the task) are near 0, inflating corrected betas to arbitrarily large values. To avoid these problems, the corrected beta values are computed as:
This means that the denominator of this last formula lies between 1 and 2 for 99% of the voxels, and larger values for the remaining 1%. Thus, the βcorrected can only take values between 0 and the original β, avoiding the risk of introducing outliers in the dataset in the process of rescaling.
Up to this point, single-subject data were always used without smoothing. For subsequent second-level analysis, the corrected task activation maps were smoothed with a 6 mm FWHM Gaussian kernel and a group t-test was performed for each voxel. Results of unscaled and scaled second-level analyses were compared on a voxelwise basis and in two regions of interest, the primary motor and the visual cortex.
Results
Standard group analyses
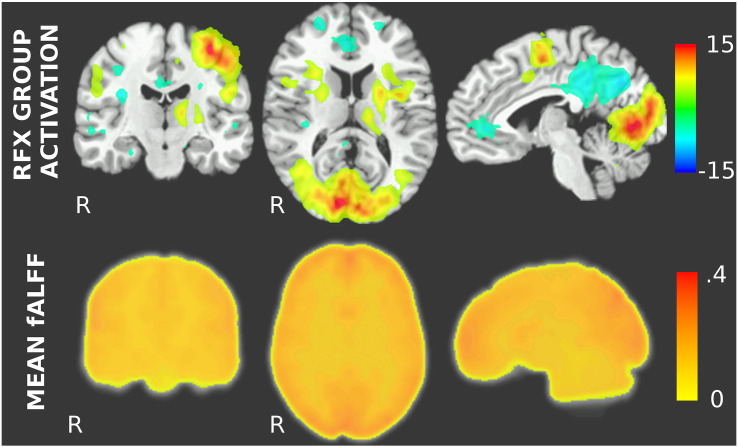
Standard analysis shows activation during the visuo-motor task located in clusters in the left primary motor cortex (M1), the supplementary motor areas (SMA), the occipital visual cortex as well as in the basal ganglia. Task-induced deactivations were found in anterior and posterior cingulate cortex and in the right postcentralgyrus (see top part of Fig. 1). Mean fALFF values (shown in the bottom part of Fig. 1) were found to be highest in the gray matter, with slightly higher values in prefrontal and occipital cortex than in more central brain regions. This might be related to the use of a 32-channel head coil, which introduces some intensity inhomogeneity (more signal in the most anterior and the most posterior regions). The intensity inhomogeneity correction greatly reduces this influence, but some residual intensity differences between the most anterior and posterior brain regions as compared to more central ones can be seen in the distribution of the fALFF values.
Fig. 1.
Top: Random effect group activation maps of visuo-motor task using standard analysis. The maps show t-values from voxelwise group t-tests thresholded at |t| = 4.25. Activation can be seen in occipital cortex, primary motor cortex as well as in the basal ganglia, while deactivations can be seen in anterior and posterior cingulate cortex as well as in parts of the prefrontal cortex, all of these regions being part of the default-mode network. Bottom: Mean fALFF map calculated from all subjects' smoothed fALFF maps.
Voxelwise inter-subject correlation
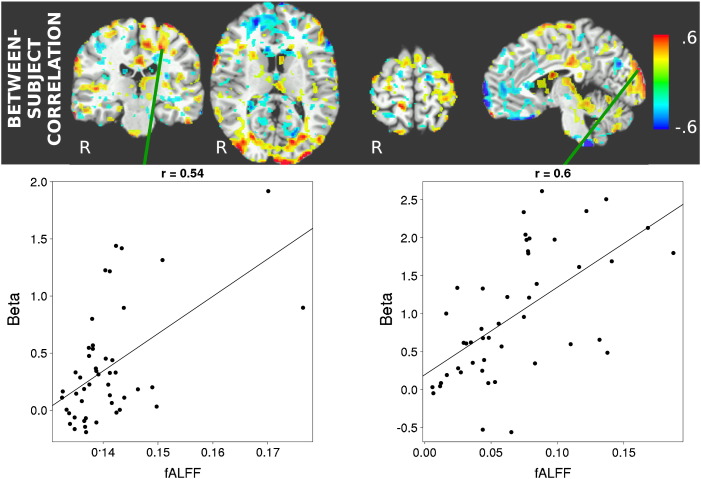
Voxelwise inter-subject correlation between fALFF and magnitude of task activation was found to be statistically significant mainly in task active areas of the brain, with positive correlations in task positive areas, and negative correlations in task negative areas. Fig. 2 shows the localization of voxels with high inter-subject correlation, as well as typical correlation patterns between task activation amplitude and fALFF between subjects in left motor as well as occipital cortex. It can be seen that, while a general tendency towards improvement in task-activated areas can be seen, the general distribution pattern of the correlation coefficients is not limited to these areas and can be described as patchy. Notably, there is no correlation in the subcortical areas identified as activated in Fig. 1, i.e. in the basal ganglia and the thalamus.
Fig. 2.
Voxelwise inter-subject correlation between fALFF and task activation. Top: Correlation coefficient map thresholded at |r| = 0.2. Bottom: Scatter plots of this relationship for two selected voxels in the left motor (r = 0.54, p = 3 ⋅ 10− 5 uncorrected) and occipital cortex (r = 0.6, p = 2.1 ⋅ 10− 6 uncorrected), each point representing one of the 47 subjects. Strongest positive correlations are observed in occipital and left motor cortex, while negative correlations occur mostly in the prefrontal and cingulate cortex.
Intra-subject local correlation
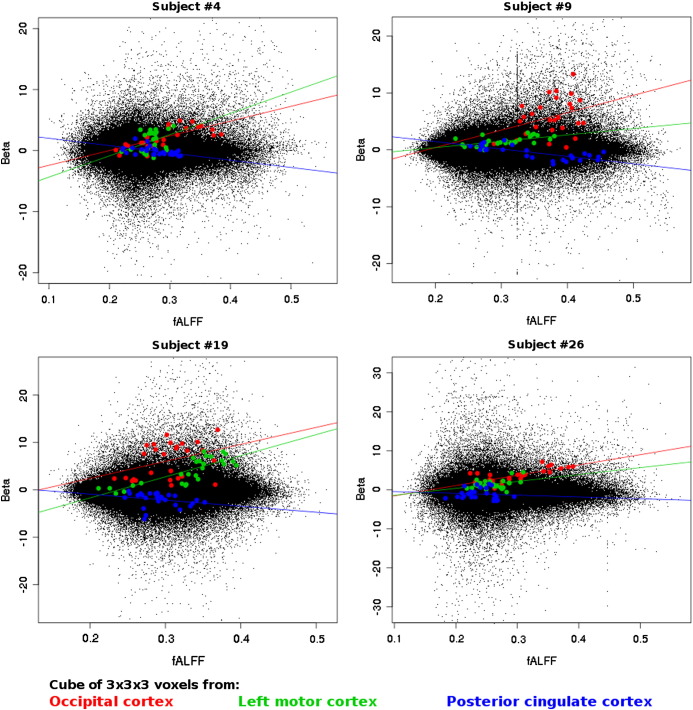
Single-subject level correlation patterns between magnitude of activation in the visuo-motor task and resting-state fALFF are shown for four representative subjects in Fig. 3. Within task-positive regions, a positive correlation between these two variables can be observed in almost all subjects (all subjects had significantly increased slopes in the motor cortex, 46 out of 47 subjects had significantly increased slopes in the visual cortex), as exemplified by the points highlighted in green and red, representing voxels in the left motor cortex and occipital cortex respectively. Conversely, in task-deactivated regions (exemplified by the points highlighted in blue in Fig. 3), local correlations were found to be negative (the slopes in the posterior cortex of 45 out of 47 subjects were significantly negative).
Fig. 3.
Exemplary single-subject scatter plots of the relationship between fALFF and activation magnitude in the visuo-motor task. Each plot represents values for all in-brain voxels of a different subject (out of 47), with each point corresponding to one voxel. Voxels highlighted in red, green and blue correspond to the voxels in a 3 × 3 × 3 cube around an example voxel in the occipital (red), left motor cortex (green) and posterior cingulate cortex (blue). Voxels in the left motor cortex as well as those in the occipital cortex consistently show a positive linear relationship, while voxels in the (task-deactivated) posterior cingulate cortex show a negative relationship.
Voxelwise SCC
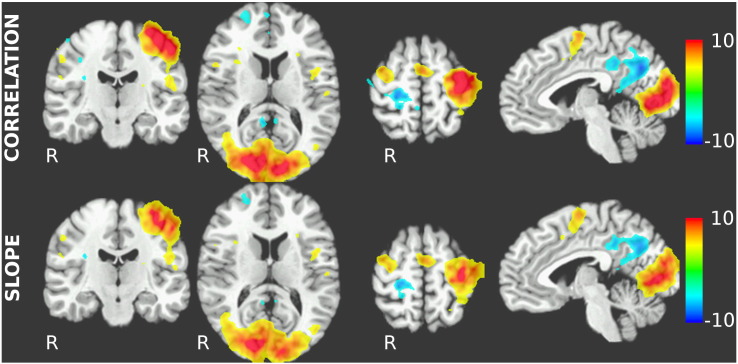
Local correlation strengths within 3 × 3 × 3 voxel cubes are highest in cubes centered on task-activated voxels, with positive correlations in task-activated areas and negative correlations in deactivated areas. The t-maps of both local slopes and correlation coefficients at group level are shown in Fig. 4. In both maps, the broad pattern of the distribution of the task activation itself can be recognized: positive correlations and slopes are located in the left primary motor cortex as well as occipital and supplementary motor cortices, and negative correlations occur in the default-mode regions deactivated during the VMP task. In comparison with the task activation maps in Fig. 1, clusters in task-deactivated regions are smaller in the SCC and local correlation maps, and there are no subcortical activation clusters in the basal ganglia in the SCC and local correlation maps.
Fig. 4.
Strength of local relationship between fALFF and activation magnitude, measured by correlation (top) and slope (bottom). Maps presented here are t-values from group t-test of smoothed single-subject z-transformed local correlation coefficients and regression slopes, thresholded at |t| = 4.25. Regions with positive correlations and slopes broadly correspond to regions with positive task activation, with the notable exception of the basal ganglia, and regions with negative correlations and slopes correspond to task-deactivated regions (cf. Fig. 1).
RESCALE scaled group statistics
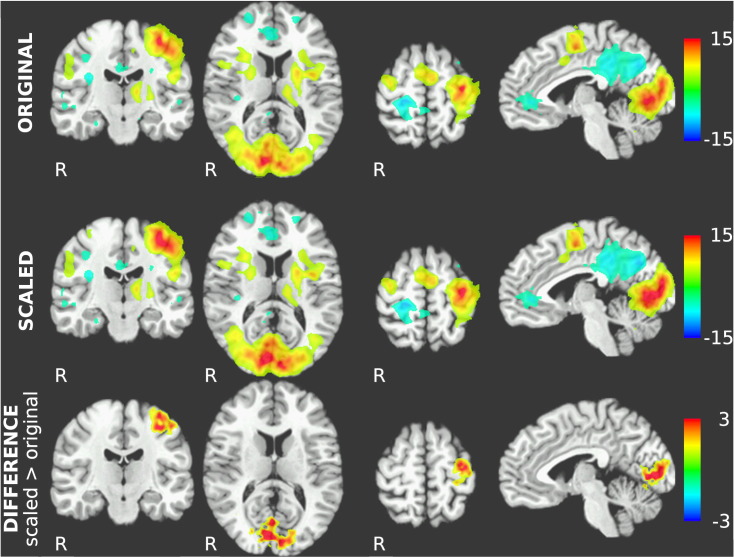
Group t-tests of scaled single-subject results yield the activation map in Fig. 5, next to the original, unscaled group results. Direct comparison of unscaled and scaled t-maps shows that while the location and extent of activated regions remain the same, t-values in activated regions tend to increase in the scaled maps: within regions of significant positive local correlation, scaled t-values are visibly higher than unscaled t-values. The map of differences between unscaled and scaled t-values (see Fig. 5, bottom) shows greatest improvement of t-values in left primary and secondary motor cortices as well as in the visual cortex.
Fig. 5.
Comparison between unscaled (top) and RESCALE-processed (center) group-level activation maps. Both maps show t-values thresholded at |t| = 4.25. Activation clusters remain the same, but (absolute) t-values within the clusters are generally increased after the RESCALE procedure. Bottom: Difference map showing voxels with absolute t-value changes greater than 1. Improvements can be seen in left primary and supplementary motor cortex as well as in the occipital cortex, with no significant differences in t-values in other regions.
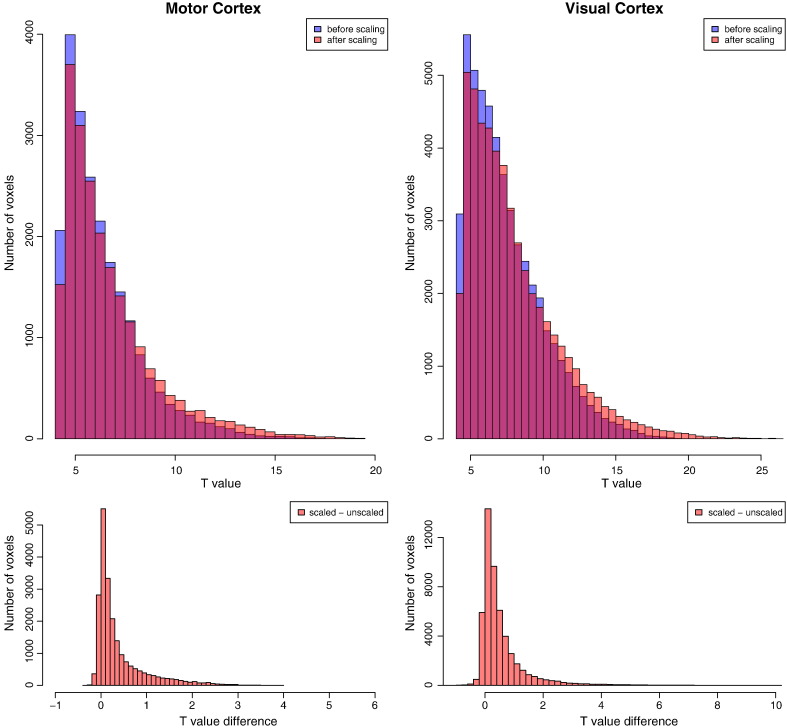
For a more detailed quantification, the two main activation areas, the left motor cortex as well as the occipital cortex (as defined by masks from the unscaled activation maps, thresholded at T = 4.25/p = 0.0001 uncorrected/q = 0.0005 FDR-corrected), were examined. The distribution of unscaled and scaled t-values in these regions is given in Fig. 6, showing a clear right-shift of the distribution. This is corroborated by the histogram of the voxelwise t-value differences within the two regions with positive task activation in the bottom part of this figure: changes in t-values are mainly positive, the distribution being skewed to the right. Changes in mean activation magnitude in both regions between unscaled and scaled t-values are of the order of 10% of unscaled activation magnitude and are highly significant (a t-test over all voxels yields a p-value of ). Peak t-values increased by 12.7% in the motor cluster and 37.0% in the occipital visual cluster, as summarized in Table 1.
Fig. 6.
Top: Distribution of T values for the voxels in the motor (left) and visual (right) ROIs. Bottom: Distribution of differences between scaled and unscaled t-values within the same two ROIs. The scaling increases T values in the activated regions, as can be seen in the right-shift in the histograms in the top row as well as in the mainly positive values in the histograms in the bottom row. Note that the ROI masks are defined by the original (unscaled) functional activation clusters seen in Fig. 1, and that the motor cluster includes the basal ganglia.
Table 1.
Peak T values and their MNI coordinates before and after scaling in three regions of interest.
| Unscaled | Scaled | Change | |||
|---|---|---|---|---|---|
| Motor | 17.3 | (39 17 63) | 19.5 | (39 15 64) | 12.7% |
| Visual | 19.2 | (0 82 –3) | 26.3 | (− 1 82 2) | 37.0% |
| Basal ganglia | 9.6 | (25 –3 12) | 9.6 | (25 –3 14) | 0.0% |
Discussion
This study shows that fractional Amplitude of Low Frequency Fluctuations during resting-state fMRI can be employed to significantly reduce the inter-subject variability of task-based activation maps. More specifically, assuming that fALFF values as determined from resting-state scans are correlated with the local responsivity of brain tissue to neural activity, we can use these fALFF maps to perform voxel-wise scaling of parameter estimates to reduce effects from spatial variations in the amplitude of the hemodynamic response. As such, local correlation between fALFF and activation magnitude in each voxel's immediate neighborhood of 26 adjacent voxels can be used to quantify the strength of the relationship between neurovascular responsivity and magnitude of BOLD signal change due to task-induced activation. Thus, this measure can be seen as a factor for scaling BOLD activation by which single-subject data can be corrected to more clearly distinguish the signal of interest. Smoothing can then be applied to scaled single-subject data for second-level analysis in the presence of anatomical differences between subjects.
RESCALE group statistics yield higher sensitivity for the detection of task-based activations, as can be seen in the increase in t-values shown in Fig. 6. Regions in the occipital and motor cortex showed increases of t-values and thus higher significance levels, whereas the basal ganglia, which were also activated in the original analysis, had no significant correlation of activation magnitude and fALFF and unchanged significance in the scaled dataset. Thus, applying the RESCALE approach seems to be most effective in cortical regions, and perhaps less so in subcortical regions.
The scaling method presented here represents an extension of previous work on correction methods for physiological effects. Earlier studies have established an influence of variation of arterial CO2 concentration, as induced by inhaling CO2-enriched air or by breath holding (Kannurpatti and Biswal, 2008), as well as global influence of ALFF values on task activation (Di et al., 2012). This improved method introduces the assessment of local correlation strength of fALFF and task activations in the neighborhood of each voxel to gain a more specific correction factor.
Local scaling on single-subject level can be seen to address two challenges of earlier methods. First, anatomical differences between subjects have limited the potential of simple correction at group-level (see Fig. 2); this was resolved by computing scaling factors on single-subject level. Second, variability in the relationship between fALFF values and task activation magnitude between different brain regions precludes a meaningful global normalization of single-subject activation maps; this was avoided by the local nature of the method proposed herein. For these reasons, activation scaling probably works best on a voxel-wise single-subject level.
Between-subject correlation of (f)ALFF and task activations has proven insufficient for group-level correction of activation maps in our study because of the spatial heterogeneity in the association between these two variables. Fig. 2 clearly shows the extent of this heterogeneity: while basic patterns can be discerned, for example the generally higher correlation in visual and motor areas, the boundaries between active and inactive regions cannot be clearly identified. With the local correction based on correlations within subjects, however, the differences in correlation strength between the two variables in different subjects as well as in different brain regions can be more readily distinguished. This leads to maps of correlation strength that more specifically delineate activated regions, which in this visuo-motor experiment are mostly occipital and motor areas (see Fig. 4).
The local slope scaling method outperforms the between-subject scaling (compare Figs. 2 and 4) due to its better modeling of two sources of variability for BOLD scaling. One is variability between subjects, indicating that scaling has to be performed individually for each subject. The other is variability between different regions in the brain of each single subject, stressing the need for computing local scaling factors, which may vary from each voxel or small cluster of voxels to the next.
It remains to be investigated how the local relationship between fALFF and task activation magnitude extends to other brain areas not evaluated in this work, e.g. in working-memory or limbic areas. On the one hand, our result concerning activations in the basal ganglia, where scaling had no effect using the method presented here, hints at potentially limited effects of the method in regions with low resting-state fALFF values and may apply to other subcortical areas as well. This might be due to other sources of heterogeneity between subjects – that cannot be corrected for by using RESCALE – in these regions, perhaps related to iron accumulation in the basal ganglia (Haacke et al., 2005).
Moreover, variations in the power of different frequency bands of resting state fluctuations between regions (Baria et al., 2011) might pose limitations to the somewhat rigid use of the same frequency band of 0.01 to 0.08 Hz for the computation of the fALFF values for all brain regions employed here. For example, physiological differences between regions with higher power of resting-state fluctuations in the frequency range of 0.01 to 0.05 Hz (e.g. parietal and frontopolar regions, cf. Baria et al. (2011)) as opposed to regions with higher power in the range of 0.05 to 0.1 Hz (e.g. regions in the temporal lobes) might have implications on the best frequency band to use for scaling in these regions. Furthermore, Zou et al. (2012), in investigating the relationship between ALFF values and task activation, showed that the strength of the relationship varied depending on task load in a working memory task: this might also be the case for the relationship between fALFF and task parameter estimates presented here, but a different paradigm than the VMP employed in our study is needed to investigate this in more detail.
A further caveat to note is the potential order effect of paradigms. Duff et al. (2008) showed that resting-state connectivity and spectral measures are dependent on other paradigms used in the scan session. In our study, paradigm order was randomized for all but the first paradigm in each scan session (the first measurement was always the VMP), and the number and type of paradigm(s) preceding the resting-state scan were thus different across subjects; since the effects seen here are consistent across subjects despite different paradigm orders, it is unlikely that they are rooted in order effects. Still, all other paradigms presented to the subjects during the session in one way or another employed visual stimulations and subject responses by pressing buttons with the fingers of the right hand, so some effect from the previous paradigms on resting-state connectivity of the regions investigated here cannot entirely be ruled out, and a replication of the result using a scan session where the resting-state scan is performed before any other paradigms would be desirable. In any case, the order of paradigms should be kept in mind when considering the use of resting-state-based correction of task fMRI data.
Potential advantages of the RESCALE method are increased sensitivity in the detection of BOLD responses to stimuli in task-based fMRI experiments, which might be most effective in cortical regions, and perhaps less so in subcortical regions. While task activation of motor and visual areas in the right-hand finger tapping experiment presented here is easy to detect with standard methods as well, the scaling can be employed in tasks where one expects more subtle effects which might be more difficult to detect. Furthermore, a high across-scan reliability of low frequency fluctuations (Zuo et al., 2010) might lend itself to the use of fALFF-based scaling of fMRI data across multiple scan sessions of the same subjects, enhancing in particular the power of longitudinal fMRI studies. Still, it is not entirely clear how reliably the subject-specific structure of the relationship between activation magnitude and fALFF can be reproduced between multiple scan sessions for each subject. Investigations into this potential between-session variability might provide insights on the influence of measureable physiological confounders of the relationship between fALFF and task activation, and thus the exact nature and origin of this relationship itself.
Acknowledgments
This study was financially supported by the MAN-BIOPSY research cluster of the Medical University of Vienna and the University of Vienna, Austria (SO76300004), the Austrian Science Fund (P22813, P23021) and the Oesterreichische Nationalbank (Anniversary fund, project numbers 12982, 13890 and 14350). The authors are grateful to Jacqueline Atanelov, Anna Höflich, Georg Kranz, Andreas Hahn, Jürgen Pripfl, Ronald Sladky, Thomas Vanicek, and MikhailVotinov for their medical and technical support. The work was supported in part by the NIH grant R01 AG032088 to BB.
References
- Baria A.T., Baliki M.N., Parrish T., Apkarian A.V. Anatomical and functional assemblies of brain BOLD oscillations. J. Neurosci. 2011;31(21):7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Kannurpatti S.S., Rypma B. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. Magn. Reson. Imaging. 2007;25(10):1358–1369. doi: 10.1016/j.mri.2007.03.022. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kötter R., Li S.-J., Lin C.-P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A.R.B., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. (Mar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubela R.N., Huf W., Kalcher K., Sladky R., Filzmoser P., Pezawas L., Kasper S., Windischberger C., Moser E. A highly parallelized framework for computationally intensive MR data analysis. Magn. Reson. Mater. Phys. 2012;25(4):313–320. doi: 10.1007/s10334-011-0290-7. (Aug) [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. (Jun) [DOI] [PubMed] [Google Scholar]
- Di X., Kannurpatti S.S., Rypma B., Biswal B.B. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs001. (Feb, e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff E.P., Johnston L.A., Xiong J., Fox P.T., Mareels I., Egan G.F. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum. Brain Mapp. 2008;29(7):778–790. doi: 10.1002/hbm.20601. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E.M., Cheng N.Y.C., House M.J., Liu Q., Neelavalli J., Ogg R.J., Khan A., Ayaz M., Kirsch W., Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging. 2005;23(1):1–25. doi: 10.1016/j.mri.2004.10.001. (Jan) [DOI] [PubMed] [Google Scholar]
- Harvey A.K., Pattinson K.T.S., Brooks J.C.W., Mayhew S.D., Jenkinson M., Wise R.G. Brainstem functional magnetic resonance imaging: disentangling signal from physiological noise. J. Magn. Reson. Imaging. 2008;28(6):1337–1344. doi: 10.1002/jmri.21623. (Dec) [DOI] [PubMed] [Google Scholar]
- Huf W., Kalcher K., Pail G., Friedrich M.-E., Filzmoser P., Kasper S. Meta-analysis: fact or fiction? How to interpret meta-analyses. World J. Biol. Psychiatry. 2011;12(3):188–200. doi: 10.3109/15622975.2010.551544. (Apr) [DOI] [PubMed] [Google Scholar]
- Hutton C., Josephs O., Stadler J., Featherstone E., Reid A., Speck O., Bernarding J., Weiskopf N. The impact of physiological noise correction on fMRI at 7 T. NeuroImage. 2011;57(1):101–112. doi: 10.1016/j.neuroimage.2011.04.018. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcher K., Huf W., Boubela R.N., Filzmoser P., Pezawas L., Biswal B., Kasper S., Moser E., Windischberger C. Fully exploratory network independent component analysis of the 1000 functional connectomes database. Front. Hum. Neurosci. 2012;6:301. doi: 10.3389/fnhum.2012.00301. (Nov) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Biswal B.B. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. NeuroImage. 2008;40(4):1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Motes M.A., Rypma B., Biswal B.B. Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum. Brain Mapp. 2011;32(7):1125–1140. doi: 10.1002/hbm.21097. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Rypma B., Biswal B.B. Prediction of task-related BOLD fMRI with amplitude signatures of resting-state fMRI. Front. Syst. Neurosci. 2012;6:7. doi: 10.3389/fnsys.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. (Jun) [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Wandell B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lu H., Yezhuvath U.S., Xiao G. Improving fMRI sensitivity by normalization of basal physiologic state. Hum. Brain Mapp. 2010;31(1):80–87. doi: 10.1002/hbm.20846. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M., Kelly C., Zuo X.-N., Martino A.D., Biswal B.B., Castellanos F.X., Milham M.P. Inter-individual differences in resting-state functional connectivity predict task-induced bold activity. NeuroImage. 2010;50(4):1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M., Zuo X.-N., Kelly C., Martino A.D., Zang Y.-F., Biswal B., Castellanos F.X., Milham M.P. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. NeuroImage. 2011;54(4):2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team R: a language and environment for statistical computing. 2011. http://www.R-project.org ISBN 3-900051-07-0.
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Friston K.J., Trstl J., Cunnington R., Moser E., Windischberger C. Slice-timing effects and their correction in functional MRI. NeuroImage. 2011;58(2):588–594. doi: 10.1016/j.neuroimage.2011.06.078. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., Luca M.D., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., Stefano N.D., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Foland L.C., Glover G.H. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum. Brain Mapp. 2007;28(1):59–68. doi: 10.1002/hbm.20241. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. (Oct) [DOI] [PubMed] [Google Scholar]
- Zang Y.-F., He Y., Zhu C.-Z., Cao Q.-J., Sui M.-Q., Liang M., Tian L.-X., Jiang T.-Z., Wang Y.-F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. (Mar) [DOI] [PubMed] [Google Scholar]
- Zou Q.-H., Zhu C.-Z., Yang Y., Zuo X.-N., Long X.-Y., Cao Q.-J., Wang Y.-F., Zang Y.-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q., Ross T.J., Gu H., Geng X., Zuo X.-N., Hong L.E., Gao J.-H., Stein E.A., Zang Y.-F., Yang Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22136. (Jun, e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.-N., Martino A.D., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F., Castellanos F.X., Biswal B.B., Milham M.P. The oscillating brain: complex and reliable. NeuroImage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]