Background: Previous in vitro studies indicated that dentin matrix protein 1 (DMP1) might regulate dentin sialophosphoprotein (DSPP) expression in odontoblasts.
Results: Transgenic expression of DSPP rescued the tooth defects of Dmp1 knockout mice.
Conclusion: DSPP is a downstream effector molecule of DMP1 in dentinogenesis.
Significance: This study has identified the in vivo functional relationship of DMP1 and DSPP in dentinogenesis.
Keywords: Bone, Dentin, Gene Regulation, Tooth, Transgenic Mice, Dentin Matrix Protein 1, Dentin Sialophosphoprotein, Dentinogenesis
Abstract
Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) are essential for the formation of dentin. Previous in vitro studies have indicated that DMP1 might regulate the expression of DSPP during dentinogenesis. To examine whether DMP1 controls dentinogenesis through the regulation of DSPP in vivo, we cross-bred transgenic mice expressing normal DSPP driven by a 3.6-kb rat Col1a1 promoter with Dmp1 KO mice to generate mice expressing the DSPP transgene in the Dmp1 KO genetic background (referred to as “Dmp1 KO/DSPP Tg mice”). We used morphological, histological, and biochemical techniques to characterize the dentin and alveolar bone of Dmp1 KO/DSPP Tg mice compared with Dmp1 KO and wild-type mice. Our analyses showed that the expression of endogenous DSPP was remarkably reduced in the Dmp1 KO mice. Furthermore, the transgenic expression of DSPP rescued the tooth and alveolar bone defects of the Dmp1 KO mice. In addition, our in vitro analyses showed that DMP1 and its 57-kDa C-terminal fragment significantly up-regulated the Dspp promoter activities in a mesenchymal cell line. In contrast, the expression of DMP1 was not altered in the Dspp KO mice. These results provide strong evidence that DSPP is a downstream effector molecule that mediates the roles of DMP1 in dentinogenesis.
Introduction
Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP)3 belong to the small integrin-binding ligand N-linked glycoprotein family. These two non-collagenous proteins share several unique features in addition to the common biochemical and structural characteristics of the small integrin-binding ligand N-linked glycoprotein family members (1, 2). DMP1 cDNA was originally cloned from the total RNA isolated from the odontoblast-pulp fibroblast complex of a rat incisor (3). Later it was found to be highly expressed in odontoblasts in tooth and in the osteoblasts/osteocytes in bone (4–6). DMP1 plays crucial roles in the formation of the mineralized tissues (7–9). DSPP was first discovered by cDNA cloning using a mouse odontoblast cDNA library (10) and was expressed at a high level in the dentin and at a very low level in the long bone (11). Mouse and human genetic studies have indicated the association of DSPP mutations or its ablation with dentinogenesis imperfecta and severe tooth decay (12–18).
Several lines of evidence suggest a possible functional relationship between DMP1 and DSPP. 1) They are both highly expressed in the odontoblasts in tooth, and the expression of mouse DMP1 takes place ∼1.5 days earlier than DSPP (2, 4–6). 2) The dentin defects in the Dspp KO mice are similar to those in Dmp1 KO mice. Both Dspp and Dmp1 KO mice display dentin hypomineralization with reduced dentin thickness, increased width of the predentin zone, and enlarged pulp chambers (7, 18). 3) DSPP expression is reduced in the dentin of Dmp1 KO mice compared with that of the WT mice (7). 4) In addition to being secreted into the dentin matrix, DMP1 also localizes in the nucleus during the differentiation of odontoblasts and is bound to and activate the DSPP promoter in an odontoblast cell line (19). These findings suggest that DMP1 might regulate DSPP expression during dentinogenesis. However, there is no convincing in vivo evidence to date that supports the hypothesis that DSPP is a downstream target gene of DMP1.
We recently generated DSPP transgenic mice expressing normal DSPP under the control of a 3.6-kb rat Collagen (Col) 1a1 promoter (20, 21). In this study, we bred DSPP transgenic mice with Dmp1 KO mice to generate mice expressing the DSPP transgene but lacking DMP1. These animals were named “Dmp1 KO/DSPP Tg mice.” We employed a multipronged approach to determine whether the DSPP transgene would rescue the tooth defects of Dmp1 KO mice in these Dmp1 KO/DSPP Tg mice compared with the WT and Dmp1 KO mice. Our analyses showed that the transgenic expression of DSPP rescued the tooth and alveolar bone defects of the Dmp1 KO mice. Additionally, our in vitro analyses showed that full-length DMP1 and its 57-kDa C-terminal fragment significantly stimulated Dspp promoter activities in a mesenchymal cell line. These findings lend support to the belief that DSPP is a downstream effector molecule regulated by DMP1 during dentinogenesis.
EXPERIMENTAL PROCEDURES
Transgenic mice
The transgenic mice expressing the DSPP transgene were described previously (20, 21). The DSPP transgene in this mouse line is downstream to the 3.6-kb rat Col1a1 promoter that directs the expression of DSPP in the tissues expressing type I collagen, including bone and dentin. The mouse line showing the highest expression level of normal DSPP transgene (20, 21) was crossbred with the Dmp1 KO mice (7, 8, 22) to obtain mice expressing the DSPP transgene in the Dmp1 KO background (i.e. without the endogenous Dmp1 gene). These mice were referred to as “Dmp1 KO/DSPP Tg” mice. The polymerase chain reaction (PCR) primers for detecting the DSPP transgene were: forward, 5′-CCAGTTAGTACCACTGGAAAGAGAC-3′; reverse, 5′-TCATGGTTGGTGCTATTCTTGATGC-3′ (the expected PCR products when using mouse genomic DNA as the template were 521 bp for the transgene and 676 bp for the WT). The genotyping of the Dmp1 KO alleles was described previously (22). The generation of Dspp KO mice has been described elsewhere (18, 21). The animal protocols for this study were approved by the Animal Welfare Committee of Texas A&M Health Science Center, Baylor College of Dentistry (Dallas, TX).
Expression Levels of the DSPP Transgene in Teeth
Quantitative real-time PCR was performed to evaluate the relative levels of DSPP mRNA in the incisors of 1-month-old WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice. Detailed descriptions of the primers, protocols, and PCR reaction conditions were provided in our previous reports (20, 21, 23).
Plain X-ray Radiography and Microcomputed Tomography (μ-CT)
The mandibles and long bones from 1- and 3-month-old mice were dissected and analyzed with a Faxitron MX-20 specimen radiography system (Faxitron X-ray Corp., Buffalo Grove, IL). For the μ-CT analyses, the mandibles were scanned using a μ-CT35 imaging system (Scanco Medical, Basserdorf, Switzerland) as described previously (24). The whole mandible was scanned in 7.0-μm slice increments at medium resolution to assess the overall shape and structure. Following this evaluation, a high-resolution scan of the alveolar bone in 3.5-μm slice increments was made for quantification purposes. For this analysis, a cylindrical region with a fixed radius beginning at the furcation area between the mesial and distal root of the mandibular molar was chosen. The data acquired from the high-resolution scans of five samples/group were used for the quantitative analyses.
Histology and Immunohistochemistry
Under anesthesia, the WT, Dmp1 KO, Dmp1 KO/DSPP Tg, and Dspp KO mice at 1 and/or 3 months of age were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 m phosphate buffer. The mandibles were then dissected and fixed again in the same fixative for 24 h. Following this period, the samples were decalcified in 8% EDTA containing 0.18 m sucrose (pH 7.4) at 4 °C for approximately 2 weeks. The tissues were then processed for paraffin embedding, and 5-μm serial sections were cut and prepared for analysis. The sections were either stained with H&E or used for immunohistochemistry analyses. For the immunohistochemistry analyses, four antibodies were employed. Anti-DSP-2C12.3 monoclonal antibody was used at a dilution of 1:800 to detect DSPP and dentin sialoprotein (DSP, the N-terminal fragment of DSPP) (25). Anti-biglycan antibody “LF-159” (a gift from Dr. Larry Fisher, National Institutes of Health, Bethesda, MD) was diluted at a ratio of 1:1000. Anti-BSP antibody 10D9.2 was diluted at 1:200 (26). Anti-DMP1 polyclonal antibody (857-3), which recognizes the C-terminal region of DMP1 (27, 28), was used at a dilution of 1:250. All the immunohistochemistry experiments were carried out using the mouse on mouse immunoglobulin kit and 3, 3′-diaminobenzidine kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer.
Double Fluorochrome Labeling of the Dentin
Double fluorescence labeling was performed to assess the mineral deposition rate of the dentin in the mice. A detailed protocol was reported in our previous studies (9, 24). Briefly, calcein (green) label (Sigma Aldrich, St. Louis, MO) at a 5 mg/kg concentration and Alizarin red label (Sigma Aldrich) at a 20 mg/kg concentration were administered intraperitoneally 1 week apart. The mice were sacrificed 48 h after the Alizarin red was injected. The mandibles were then dissected and fixed in 70% ethanol for 48 h. Following this step, the samples were dehydrated through ascending concentrations of ethanol and embedded in methyl-methacrylate. Sections (10 μm thick) were cut, and the incisor dentin region under the mesial root of the first molar was viewed under epifluorescent illumination with a Nikon E800 microscope interfaced with Osteomeasure histomorphometry software (version 4.1, Atlanta, GA).
Backscattered and Resin-casted Scanning Electron Microscopy
The mandibles from all groups of 3-month-old mice were dissected and fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 m cacodylate buffer solution (pH 7.4) at room temperature. Four hours later, the samples were immersed in 0.1 m cacodylate solution. They were then dehydrated in ascending concentrations of ethanol and embedded in methyl-methacrylate resin and oriented to expose the mandibular molar region (Buehler, Lake Bluff, IL). Microcloths with Metadi Supreme polycrystalline diamond suspensions of decreasing sizes (Buehler) were used to polish the surfaces of the samples. For backscattered scanning electron microscopy analysis, the samples were coated with carbon. For resin-casted scanning electron microscopy, the dentin surface was first acid etched with 37% phosphoric acid for 2–10 s, washed with 5.25% sodium hypochlorite for 5 min, and coated with gold and palladium. A FEI/Philips XL30 field emission environmental scanning electron microscope (Philips, Hillsboro, OR) was used to perform the analyses as reported previously (9, 29).
In Situ Hybridization Analysis
In situ hybridization was performed to assess the expression and distribution of Col1a1 and Dlx3 homeodomain protein in 1-month-old samples from all three groups. The RNA probes were labeled with digoxigenin using a RNA labeling kit (Roche) as described in our previous report (30). Following the instructions of the manufacturer, digoxigenin-labeled RNA probes were detected by an enzyme-linked immunoassay with a specific anti-digoxigenin-alkaline phosphatase antibody conjugate (Roche) and a VECTOR red alkaline phosphatase substrate (Vector Laboratories), which produced a red color indicating positive signaling. Methyl green was used for counterstaining.
Serum Biochemistry Analyses
The serum FGF23 and phosphorus levels in the 3-month-old WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice were determined as described previously (9). Briefly, the serum FGF23 level was determined using a full-length FGF23 ELISA kit (Kainos Laboratories). Serum phosphorus was measured according to the phosphomolybdate-ascorbic acid method (9).
Transient Transfection and Promoter Luciferase Assay
The generation of the 5.7-kb DSPP promoter luciferase construct has been described previously (31). For the promoter luciferase assay, C3H10T1/2 mesenchymal cells in 24-well plates were transiently transfected with 0.1 μg of 5.7-kb DSPP promoter luciferase construct together with 0.6 μg of a construct expressing the full-length normal DMP1 (FL-DMP1) or the 57-kDa C-terminal fragment preceded by an endoplasmic reticulum entry signal peptide (SP-57kD) (32), or a pCDNA3 empty vector (Ctrl) with FuGENE 6 transfection reagent (Roche). The pRL-TK construct, which expressed Renilla luciferase, was cotransfected as an internal control to monitor transfection efficiency. The transfected cells were harvested using passive lysis buffer 48 h after transfection. The firefly and Renilla luciferase activities were assayed using a dual luciferase reporter assay system (Promega, Madison, WI). The promoter activities were expressed as luciferase activities relative to the control activities. A luciferase assay was performed in triplicate, and representative data are presented.
Statistical Analysis
The data analyses were performed with one-way analysis of variance for multiple group comparison. If significant differences were found with the one-way analysis of variance, Student's t test was used to determine which groups were significantly different from the others. The quantified results were expressed as the mean ± S.D. p < 0.05 was considered to be statistically significant.
RESULTS
Transgenic Expression of Dspp in Dmp1 KO Mice
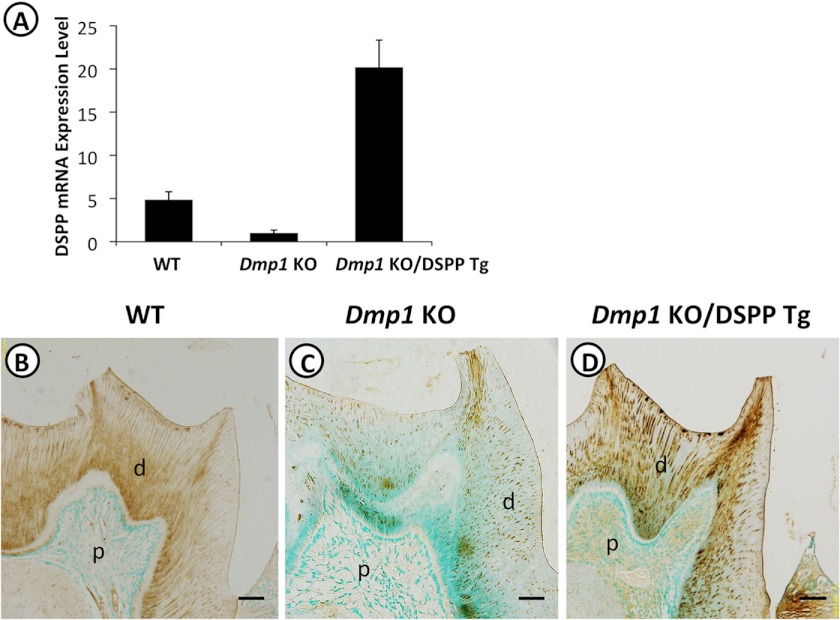
The Dmp1 KO/DSPP Tg mice were generated by breeding Dmp1 KO mice with DSPP transgenic mice. Quantitative real-time PCR analyses and immunohistochemical staining were performed to compare the DSPP expression in the teeth of the WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice. Real-time PCR analyses showed that the DSPP expression was 4-fold lower in the teeth of the Dmp1 KO mice compared with that in the WT mice (Fig. 1A). The expression level of DSPP in the teeth of the Dmp1 KO/DSPP Tg mice was ∼20-fold greater than that in the Dmp1 KO mice or ∼5-fold more than that in the WT mice (Fig. 1A). Immunohistochemical staining with an anti-DSP antibody demonstrated that the DSP staining signals were observed predominantly in the dentin matrix of the WT (Fig. 1B), Dmp1KO (C), and Dmp1 KO/DSPP Tg mice (D). Consistent with the real-time PCR results, the Dmp1 KO/DSPP Tg mice had the strongest DSP staining signals (Fig. 1D), and the Dmp1 KO mice showed the weakest signals (C). Together, these results suggest that the DSPP transgene conferred high levels of DSPP expression in the Dmp1 KO mice and that its distribution was comparable with that of the endogenous Dspp gene in dentin.
FIGURE 1.
Transgenic expression of DSPP in Dmp1 KO mice. A, real-time PCR was performed with total RNA isolated from the incisors of 1-month-old WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice. The DSPP mRNA level in the Dmp1 KO mouse incisor was taken as 1, and the mRNA levels of the WT and Dmp1 KO/DSPP Tg mice were expressed as folds of that in the Dmp1 KO mice. The data represented five analyses (n = 5) for each group. B–D, immunohistochemistry was performed on 3-month-old mandibular molars with anti-DSP antibody (signal in brown). The Dmp1 KO mice (C) showed remarkably reduced DSP signals compared with the WT mice (B), and intense DSP signals were observed in the Dmp1 KO/DSPP Tg mice (D). d, dentin; p, pulp. Scale bars = 100 μm.
Transgenic Expression of Dspp Rescued the Dentin and Alveolar Bone Defects of Dmp1 KO Mice
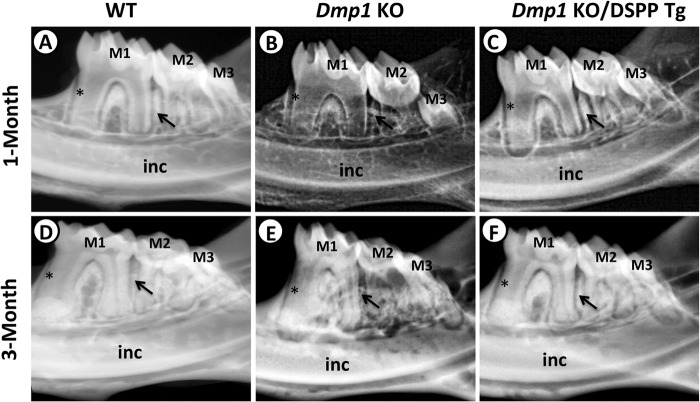
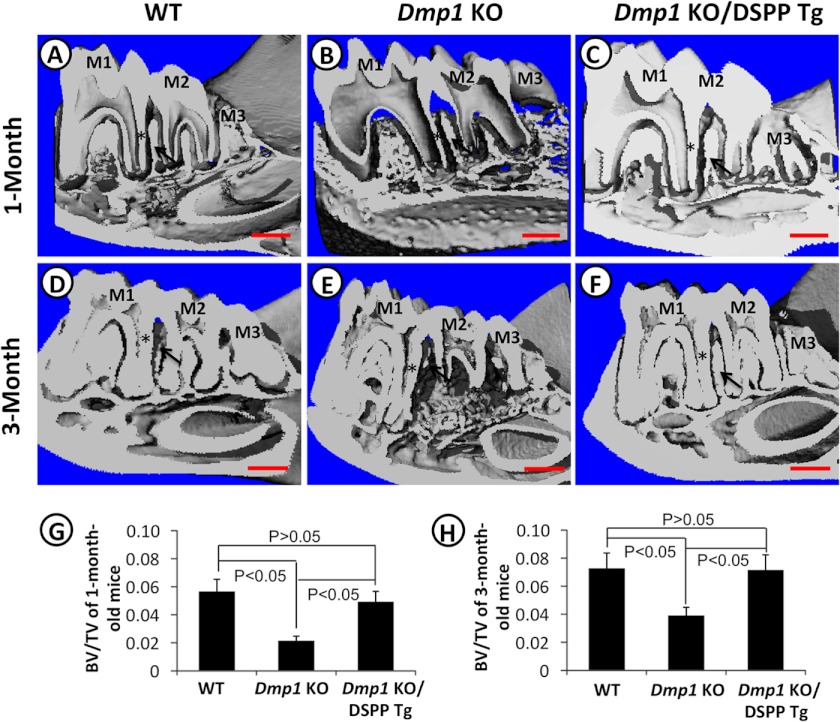
To determine whether the transgenic expression of DSPP would rescue the tooth and bone defects of the Dmp1 KO mice, we first performed plain x-ray radiography (Fig. 2) and μ-CT (Fig. 3) analyses of the mandibular jaws of 1- and 3-month-old mice to assess the overall dentin and alveolar bone structures. At both ages, the Dmp1 KO mice exhibited several dentin and alveolar bone anomalies, including reduced dentin thickness, enlarged pulp chambers and increased porosity in the alveolar bone (Figs. 2, B and E, and 3, B and E). At one month of age, the transgenic expression of DSPP was able to significantly rescue these Dmp1-deficient defects (Figs. 2C and 3C). However, the rescue was not complete, and there was still a noticeable difference between the radio opacity of the dentin and alveolar bones of the Dmp1 KO/DSPP Tg (Figs. 2C and 3C) and the WT mice (Figs. 2A and 3A) at 1 month of age. At three months, the dentin and alveolar bone in the Dmp1 KO/DSPP Tg mice were very similar to those of the WT mice, indicating that the DSPP transgene fully rescued the Dmp1-deficient dentin and alveolar bone defects (Figs. 2, D and F, and 3, D and F). Quantitative analyses showed that the expression of the DSPP transgene reversed the bone volume fraction in the alveolar bone region of the Dmp1 KO mice to the same level as in the WT mice at both 1 month (Fig. 3G) and 3 months (H). However, x-ray radiographic analyses showed that the transgenic expression of DSPP failed to fully rescue the long bone defects of the Dmp1 KO mice (supplemental Fig. 1), despite the fact that the DSPP transgene was highly active in the long bone (data not shown).
FIGURE 2.
Plain x-ray analyses of the mandibular molars from 1- and 3-month-old mice. Shown are representative radiographs of the mandibular molars from 1- and 3-month-old WT mice (A and D), Dmp1 KO mice (B and E), and Dmp1 KO/DSPP Tg mice (C and F). The Dmp1 KO mice had thinner dentin and reduced radio-opacity (B and E). The Dmp1 KO/DSPP Tg mice showed significantly increased dentin thickness and radio-opacity at the age of 1 month (C) compared with the Dmp1 KO mice. At the age of 3 months, the dentin of the Dmp1 KO/DSPP Tg mice (F) was comparable with that of the WT mice (D). The asterisks mark the radicular dentin of the mesial side of the mesial root of the first molar. The arrows point to the interdental alveolar bone between the first and second molars. M1, first molar; M2, second molar; M3, third molar; inc, incisor.
FIGURE 3.
μ-CT analyses of mandibles from 1- and 3-month-old mice. Shown are representative μ-CT images of the mandibles from 1- and 3-month-old WT mice (A and D), Dmp1 KO mice (B and E), and Dmp1 KO/DSPP Tg (C and F). Quantitative analyses of the bone volume fraction (bone volume (BV)/total volume (TV)) showed that the DSPP transgene was able to rescue the alveolar bone defects of Dmp1 KO mice at the ages of both 1 month (G) and 3 months (H). n = 5 in each group. p < 0.05 was considered statistically significant. The asterisks mark the radicular dentin of the distal side of the distal root of the first molar. The arrows point to the interdental alveolar bone between the first and second molars. M1, first molar; M2, second molar; M3, third molar. Scale bars = 200 μm.
Transgenic Expression of DSPP Rescued the Dentin Mineralization Defects and the Abnormalities in Dentinal Tubules
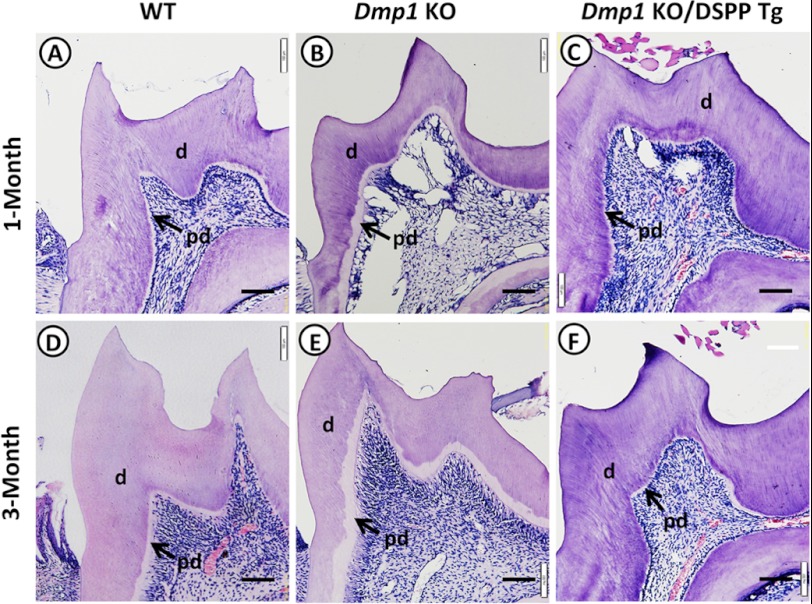
We conducted a series of detailed analyses of the rescue of dentin defects in Dmp1 KO mice caused by the DSPP transgene. First, histological analyses confirmed that the mandibular molars of the Dmp1 KO mice displayed an enlarged predentin zone, reduced dentin thickness, and enlarged pulp chambers (Figs. 4, B and E) compared with those of the WT mice (A and D) at the ages of 1 and 3 months. The molars of the Dmp1 KO/DSPP Tg mice (Fig. 4, C and F) appeared to be similar to those of the WT mice (A and D) at both ages, suggesting that the transgenic expression of DSPP might rescue the predentin/dentin mineralization defects of the Dmp1 KO mice.
FIGURE 4.
Histological analyses of molars from 1- and 3-month-old mice. Shown is H&E staining of the mandibular first molars from 1- and 3-month-old WT mice (A and D), Dmp1 KO mice (B and E), and Dmp1 KO/DSPP Tg (C and F). Note that the widened predentin zones, reduced dentin thickness, and enlarged pulp chambers in the Dmp1 KO mice (B and E) were rescued in the Dmp1 KO/DSPP Tg mice (C and F). The arrows point to the predentin zone. d, dentin; pd, predentin. Scale bars = 100 μm.
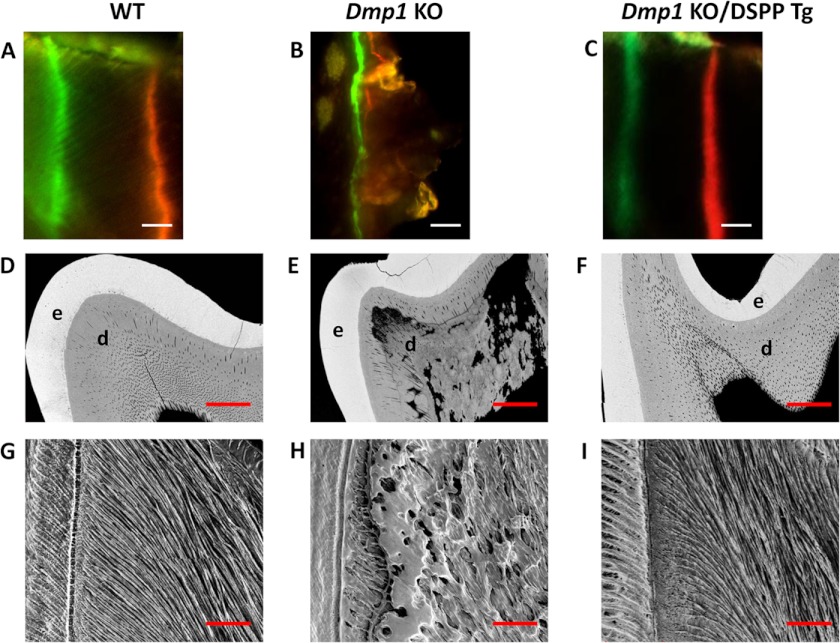
Therefore, we analyzed the predentin/dentin mineralization and dentinal tubular structures of the three groups of 3-month-old mice. Double fluorochrome labeling was employed to assess the dentin deposition rate (Figs. 5, A–C). Two distinct and well separated fluorescent labels were observed in both the WT (Fig. 5A) and Dmp1 KO/DSPP Tg mice (C), whereas both labels appeared to be blurry and fused in the Dmp1 KO mice (B). The quantitative analyses showed that the dentin deposition rate was ∼7 μm/day for both Dmp1 KO/DSPP Tg and WT mice (n = 3 per group), and no significant differences were observed. The quantitative analysis for the Dmp1 KO samples was not performed because of the lack of a clear distinction between the two labels. In addition, the backscattered scanning electron microscopy analysis was performed to analyze the dentin mineralization (Figs. 5, D–F). This testing revealed that the Dmp1 KO mice displayed large areas of unmineralized or hypomineralized dentin (Fig. 5E) compared with the WT mice (D) and that the transgenic expression of DSPP remarkably reduced the areas of the hypomineralized dentin in the Dmp1 KO mice (F). Furthermore, the resin-casted scanning electron microscopy analysis was used to examine the dentinal tubular structures (Fig. 5, G–I). It showed that the dentinal tubules were well organized and evenly distributed in the Dmp1/DSPP Tg mice (Fig. 5I) and WT mice (G) in contrast to the completely disorganized tubules in the Dmp1 KO mice (H). Altogether, these results demonstrate that the transgenic expression of DSPP rescued the predentin/dentin mineralization defects as well as the abnormal dentinal tubules observed in the Dmp1 KO mice.
FIGURE 5.
Analyses of the dentin mineralization and dentinal tubular structures of 3-month-old mice. Double fluorochrome labeling was performed to determine the dentin apposition rate of 6-week-old WT (A), Dmp1 KO (B), and Dmp1 KO/DSPP Tg mice (C). Mice were first injected with calcein (green). Seven days later, they were injected with Alizarin red (red). The representative images were taken from the incisor dentin region under the mesial root of the first molar. The distance between these two labels represented the dentin matrix deposited over a period of 7 days. The labels for the Dmp1 KO mice were very blurry and were not distinct from each other (B). A Dmp1 KO/DSPP Tg sample (C) showed clear-cut green and red labels, similar to those of WT mice (A). Scale bars = 10 μm. Backscattered scanning electron microscopy images (D–F) show the enamel in white, dentin in gray, and low mineral-density areas in black. The Dmp1 KO molars had irregular dentin with several black/dark areas, indicating hypomineralization (E). The enamel and dentin of the Dmp1 KO/DSPP Tg molars were evenly mineralized and distributed (F), comparable with those of the WT mice (D). e, enamel; d, dentin. Scale bars = 100 μm. Also shown are resin-casted and acid-etched scanning electron microscopy analyses of the dentinal tubules of WT mice (G), Dmp1 KO mice (H), and Dmp1 KO/DSPP Tg mice (I). Note that the dentinal tubules of the Dmp1 KO mice were unevenly distributed (H). The dentinal tubules of the Dmp1 KO/DSPP Tg mice were evenly distributed parallel to each other (I), similar to those of WT mice (G). Scale bars = 50 μm.
Transgenic Expression of DSPP Rescued the Odontoblast Differentiation Defects
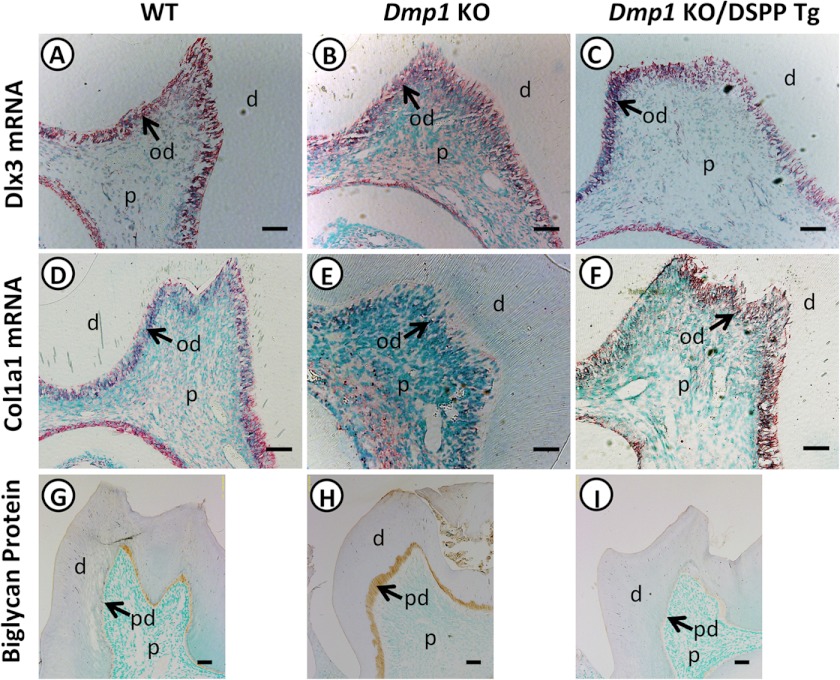
The rescue of the predentin/dentin mineralization defects by the DSPP transgene led us to examine whether the transgenic expression of DSPP also rescued the odontoblast differentiation defects. Therefore, we tested the expressions of three molecular markers: a homeodomain-containing transcription factor, Dlx3, and two odontoblast differentiation markers, Col1a1 and biglycan. Dlx3 has been shown to regulate dentinogenesis through directly regulating Dspp expression (33). We found that Dlx3 was expressed predominantly in the odontoblasts of WT (Fig. 6A), Dmp1 KO (B), and Dmp1/DSPP Tg (C) mice with no apparent differences noticed among the groups. This result suggests that the regulation of Dspp by Dlx3 may be independent of DMP1. In contrast, the terminal differentiation marker Col1a1 was markedly reduced in the Dmp1 KO mice (Fig. 6E) compared with that in WT mice (D). The transgenic expression of DSPP (Fig. 6F) increased the Col1a1 expression to a level similar to that of the WT mice. Biglycan is another odontoblast terminal differentiation marker and is primarily localized in the predentin zone of the teeth (34, 35). In contrast to the type I collagen, immunohistochemical staining showed that strong biglycan staining signals were localized in the widened predentin zone of Dmp1 KO mice (Fig. 6H) compared with WT mice (G). The transgenic expression of DSPP reduced the biglycan expression and the predentin thickness (Fig. 6I). These data suggest that the transgenic expression of DSPP rescued the altered gene expression profile of the odontoblasts in Dmp1 KO mice.
FIGURE 6.
Expression of Dlx3, Col1a1, and biglycan in the teeth of WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice. In situ hybridization was performed to assess the expression of Dlx3 and type 1 collagen in the three groups of mice. Both Dlx3 and Col1a1 mRNA were localized predominantly in the odontoblast layer. No apparent difference was noted for Dlx3 expression between each group of these mice (A–C). However, the Col1a1 mRNA was markedly lower in Dmp1 KO mice (E) than in WT (D) and Dmp1 KO/DSPP Tg mice (F). Immunohistochemical staining showed that the biglycan signals (brown) were restricted to the predentin zone in the mandibular molars. The Dmp1 KO mice displayed a widened zone of biglycan signals, reflecting a widened predentin zone (H). The Dmp1 KO/DSPP Tg mice had a low level of biglycan immunostaining signals (I), similar to that of WT mice (G). d, dentin; p, dental pulp; od, odontoblast. Scale bars = 50 μm (A–F) and 100 μm (G–I).
Transgenic Expression of Dspp Rescued the Defects in the Osteocyte Lacunocanalicular System in the Alveolar Bone
Both plain x-ray radiography (Fig. 2) and μ-CT (Fig. 3) analyses demonstrated that the transgenic expression of DSPP rescued the alveolar bone defects. To confirm the rescue effects of DSPP at the cellular level, we analyzed the osteocyte lacunocanalicular system of the alveolar bones using the resin-casted scanning electron microscopy approach. The osteocyte lacunae in the alveolar bones of the Dmp1 KO mice appeared to be disorganized with fewer canaliculi (Fig. 7B), whereas the osteocyte lacunae in the WT and Dmp1 KO/DSPP Tg mice were well organized with regularly spaced canaliculi (A and C). This observation implies that the transgenic expression of DSPP rescued the defects in the osteocyte lacunocanalicular system of Dmp1 KO mice.
FIGURE 7.

The osteocyte lacunocanalicular system in the alveolar bones of 3-month-old mice. The osteocyte lacunocanalicular system was examined using resin-casted and acid-etched scanning electron microscopy analyses. Shown are the representative images of the resin-casted osteocytes from the alveolar bones of WT mice (A), Dmp1 KO mice (B), and Dmp1 KO/DSPP Tg mice (C). The osteocyte lacunae of Dmp1 KO mice (B) appeared larger with fewer short canaliculi compared with those of the WT mice (A). The osteocyte lacunae of the Dmp1 KO/DSPP Tg (C) mice had regularly spaced canaliculi radiating outwards into the surrounding bone, similar to those of the WT mice (A). The asterisks mark the resin-casted lacunae. Scale bar = 5 μm.
Transgenic Expression of DSPP Rescued the Osteoblast Differentiation Defects in the Alveolar Bone
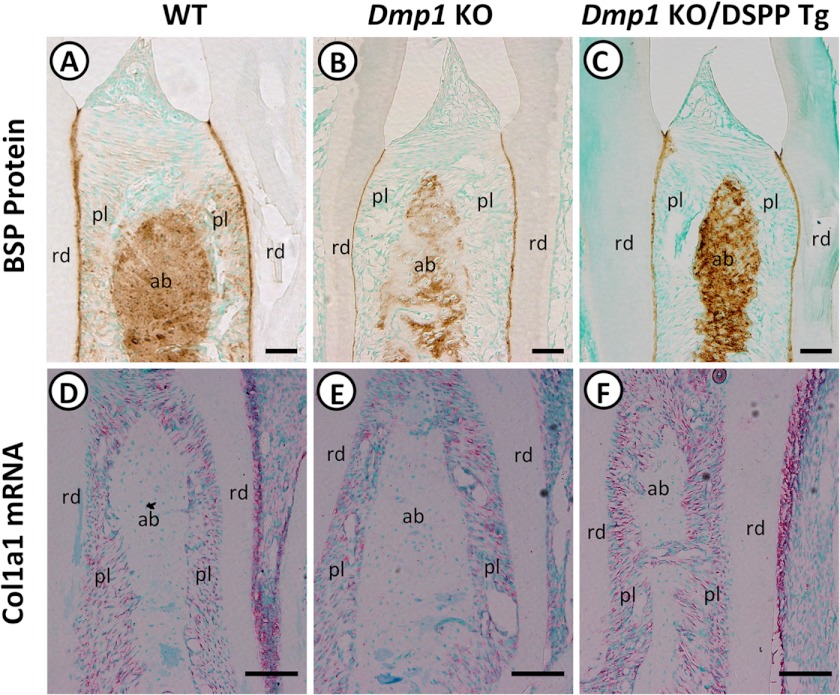
To further determine whether the transgenic expression of DSPP also rescued the osteoblast differentiation defects in the alveolar bones of the Dmp1 KO mice, we examined the expressions of two osteoblast differentiation markers, BSP and Col1a1. Immunohistochemical staining demonstrated that both WT (Fig. 8A) and Dmp1 KO/DSPP Tg mice (C) displayed strong BSP staining signals in the interdental alveolar bones between the mandibular first and second molars compared with the weak staining signals in the Dmp1 KO mice (B). In situ hybridization demonstrated that the Col1a1 mRNAs were mainly restricted to the periodontal ligament in the three groups of mice. Their expression was reduced in the Dmp1 KO mice (Fig. 8E) compared with that in the WT mice (D), and the DSPP transgene prominently increased Col1a1 expression in the Dmp1/DSPP Tg mice (F). It should be noted that the periodontal ligament serves as a periosteum to the alveolar bone proper (36). These findings suggest that transgenic expression of DSPP rescued the osteoblast differentiation defects in the alveolar bones of Dmp1 KO mice.
FIGURE 8.
Expression of BSP and Col1a1 in the interdental alveolar bones. Immunohistochemical staining was performed on tooth sections of 3-month-old WT mice (A), Dmp1 KO mice (B), and Dmp1 KO/DSPP Tg mice (C) with an anti-BSP antibody. Strong BSP signals (brown) were observed in the interdental alveolar bone between the first and second mandibular molars of WT (A) and Dmp1 KO/DSPP Tg mice (C), whereas Dmp1 KO mice (B) displayed markedly reduced signals. In situ hybridization was performed to assess the relative expression of Col1a1 from 1-month-old WT mice (D), Dmp1 KO mice (E), and Dmp1 KO/DSPP Tg (F) mice. The Col1a1 mRNA (signal in red) was restricted predominantly to the periodontal ligament. Its expression was reduced in Dmp1 KO mice (E) compared with WT mice (D). The transgenic expression DSPP (F) increased Col1a1 expression to a level similar to that in the WT mice. rd, radicular dentin; pl, periodontal ligament; ab, interdental alveolar bone. Scale bars = 100 μm (A, B, and C) and 50 μm (D, E, and F).
Transgenic Expression of DSPP Had No Apparent Effects on Serum FGF23 and Phosphorus Levels
Dmp1 KO mice postnatally developed hypophosphatemic rickets accompanied by elevated FGF23 levels (9). Re-expression of either full-length DMP1 or a 57-kDa C-terminal fragment in Dmp1 KO mice not only rescued skeletal abnormalities but also restored the serum FGF23 and phosphorus levels (37). To determine whether the DSPP transgene had any systemic effects, we analyzed the serum FGF23 and phosphorus levels of the three groups of mice. Interestingly, serum biochemical analyses revealed that Dmp1 KO mice displayed significantly elevated serum FGF23 levels and reduced serum phosphorus levels compared with the WT mice. The analyses also revealed that the transgenic expression of DSPP in Dmp1 KO mice failed to restore the altered serum FGF23 and phosphorus levels to the normal ranges (Fig. 9). These findings imply that the rescue of the tooth and alveolar bone defects was solely due to the local expression of DSPP but was not attributed to the systemic effects of DSPP on the serum FGF23 and phosphorus levels.
FIGURE 9.
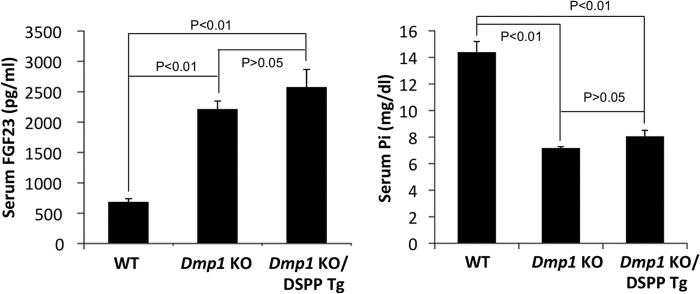
Serum FGF23 and phosphorus levels. Serum FGF23 and phosphorus levels of 3-month-old WT, Dmp1 KO, and Dmp1 KO/DSPP Tg mice are shown. The Dmp1 KO mice had significantly elevated FGF23 and reduced phosphorus levels compared with WT mice. The transgenic expression of DSPP failed to restore the serum FGF23 and phosphorus levels to the ranges comparable with those of WT mice. All values are mean ± S.D. n = 3 in each group.
DMP1 Stimulated 5.7 kb Dspp Promoter Activity
Having established that the transgenic expression of DSPP rescued the tooth and alveolar bone abnormalities of the Dmp1 KO mice, we next examined whether DMP1 regulates the 5.7-kb DSPP promoter luciferase activities in the C3H10T1/2 mesenchymal cells (Fig. 10). This 5.7-kb Dspp promoter fragment contains all the necessary regulatory elements that confer the tissue-specific expression of a reporter gene similar to that of the endogenous Dspp (49). The C3H10T1/2 cells are pluripotent stem cells that can differentiate into odontoblast-like cells (50). We found that the full-length DMP1 significantly stimulated Dspp promoter activity (Fig. 10). Moreover, the 57-kDa C-terminal fragment induced a 3-fold increase in Dspp promoter activity. Importantly, we showed previously that the 57-kDa C-terminal fragment could rescue the Dmp1-deficient defects in both bone and tooth (37).
FIGURE 10.
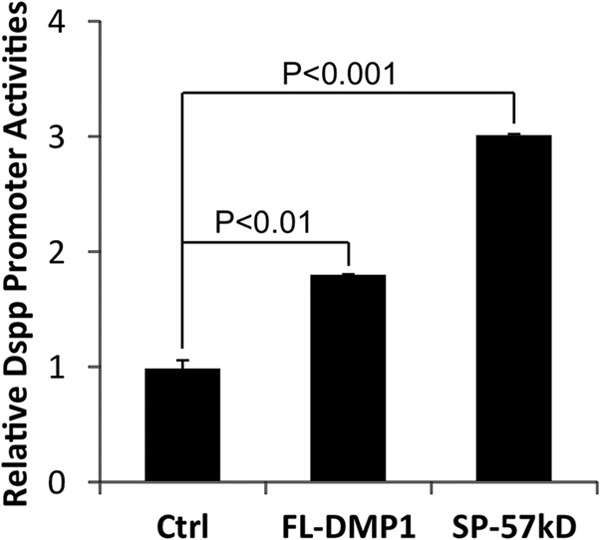
Effects of DMP1 on 5. 7 kb Dspp promoter activities in C3H10T1/2 cells. C3H10T1/2 cells were transiently cotransfected with a 5.7-kb Dspp-luciferase construct and the indicated expression constructs along with the pRL-TK construct as an internal control. The luciferase activities were determined by the Promega dual luciferase assay system, and the promoter activities were expressed as luciferase activities relative to that of the control. The values are the mean ± S.D. n = 3 for each group. Ctrl, the pCDNA3 empty expression vector; FL-DMP1, full-length normal DMP; SP-57kD, 57-kDa C-terminal fragment preceded by an endoplasmic reticulum entry signal peptide.
Expression of Dmp1 in Dspp KO Mice
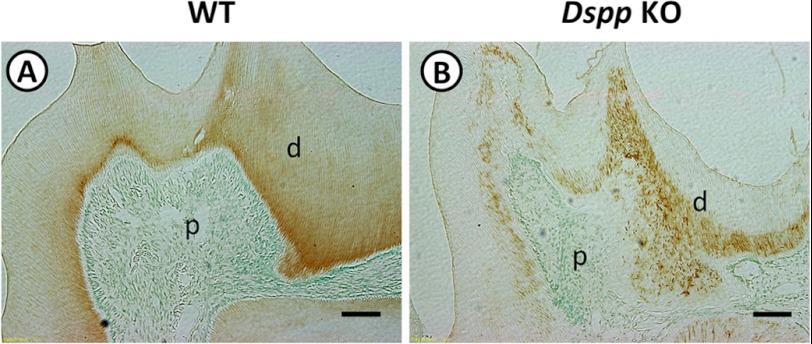
Our current findings strongly suggest that DMP1 might directly regulate Dspp expression in both tooth and alveolar bone development. If this is true, the Dmp1 expression should not be affected in the Dspp KO mice because DSPP is downstream of DMP1. To confirm this, immunohistochemical staining was performed on the tooth sections of 3-month-old WT and Dspp KO mice with a polyclonal anti-DMP1 antibody. Strong DMP1 staining signals were observed in the dentin matrixes of both WT and Dspp KO mice (Fig. 11). However, the staining signals were distributed evenly in the dentin matrix of the WT mice (Fig. 11A), whereas they appeared to form a dotted pattern in the Dspp KO mice (B). The dotted distribution of DMP1 proteins could be related to the disorganized and hypomineralized dentin in the Dspp KO mice. This finding may mean that the expression of Dmp1 gene in tooth does not depend on DSPP.
FIGURE 11.
DMP1 expression in Dspp KO mice. Immunohistochemical staining was performed on tooth sections of 3-month-old WT (A) and Dspp KO (B) mice with an anti-DMP1 antibody. Strong DMP1 staining signals (signal in brown) were observed in both WT (A) and Dspp KO (B) mice. The signals were evenly distributed in the dentin matrix of the WT mice, but they appeared to be in a speckled pattern in the Dspp KO mice. d, dentin; p, dental pulp. Scale bars = 100 μm.
DISCUSSION
Efforts have been made in recent years to define the functional relationship between DMP1 and DSPP in dentinogenesis because Dmp1 KO mice and Dspp KO mice have the same dentin mineralization defect (7, 18). In this study, we found that the Dspp expression was remarkably reduced in the Dmp1 KO mice, that the transgenic expression of DSPP rescued the tooth and alveolar bone defects of the Dmp1 KO mice, that DMP1 stimulated the Dspp promoter activity in vitro, and that Dmp1 expression was not altered in the Dspp KO mice. These findings suggest that DSPP is a downstream effector molecule of DMP1 in the development of tooth and alveolar bone.
Our previous studies have proposed that the tooth and skeletal defects of Dmp1 KO mice might result from the intrinsic differentiation defects of Dmp1-deficient cells and/or hypophosphatemia secondary to elevated circulating FGF23 levels (9, 37, 38). In this study, we found that, although the transgenic expression of DSPP rescued the mineralization defects of dentin and alveolar bone as well as the differentiation defects of odontoblasts and alveolar bone osteoblasts, the hypophosphatemia remained persistent in the Dmp1 KO/DSPP Tg mice. Therefore, the rescue of the tooth and alveolar bone defects appears to be solely associated with the local expression of DSPP in tooth and alveolar bone. However, it remains to be determined how DSPP, as a highly phosphorylated extracellular matrix protein, rescues the cell differentiation defects in the Dmp1 KO/DSPP Tg mice.
The rescue of the DMP1 KO mouse alveolar bone defects but not the long bone defects by the DSPP transgene is very intriguing. Although DMP1 is expressed in teeth, alveolar bone, and long bone, the finding indicates that the mechanism of action of DMP1 involved in the long bone development must differ from that in the formation of dentin and alveolar bone. This view is also supported by two other findings: DSPP expression is more abundant in the alveolar bone compared with that in the long bone (11, 39), and inactivation of Dspp leads to severe defects in the alveolar bone but not in the long bone (40, 41). Further work is warranted to elucidate the molecular mechanisms by which DMP1 functions differently in the dentin/alveolar bone and in the long bone.
Two different pathways may account for the regulatory function of DMP1. As an extracellular matrix protein, DMP1 contains a classical RGD motif, so the regulatory roles of DMP1 may be initiated by the binding of DMP1 to the integrin receptors, followed by activation of the MAP kinase signaling pathway (42, 43). Alternatively, extracellular non-phosphorylated full-length DMP1 may reenter the cell through its binding to the glucose-regulated protein GRP-78 receptor on the cell surface, followed by endocytosis and subsequent nuclear translocation and function as a transcription factor (44, 45). We previously reported the nuclear localization of DMP1 in the nuclei of osteocytes in bone and of cultured cells transfected with constructs expressing full-length DMP1 (46, 47). More recently, we found that DMP1 in the nucleus was restricted to the nucleoplasm but was absent in the nucleolus and that nuclear localization of DMP1 was a highly regulated event (48). These studies present evidence that DMP1 may act as either an extracellular integrin ligand or a transcription factor or both to exert its regulatory function on DSPP expression.
Functional redundancy is excluded. Although both DMP1 and DSPP share many common structural, physical, and biochemical features (1, 2), it is unlikely that the rescue of the Dmp1 KO tooth and alveolar bone defects by DSPP was due to their redundant functions. First, both Dmp1 and Dspp KO mice display similar tooth abnormalities (7, 18), hinting that they cannot compensate for the function of each other. Secondly, the DSPP transgene failed to fully rescue the long bone defects, although it was highly active in the osteoblasts/osteocytes in the long bone.
DSPP is the most abundant non-collagenous protein in dentin. Although DSPP expression is significantly reduced in Dmp1 KO mice, it does not completely disappear from these mice. These findings suggest that, in addition to DMP1, there may be other molecules/pathways involved in controlling DSPP expression, such as Dlx3 (33). These regulatory molecules together determine the final level of DSPP expression essential for normal dentin formation. The successful rescue of Dmp1 KO tooth defects by the transgenic expression of DSPP implies that the DSPP transgenic mouse model may also be used in future studies. Such work will attempt to identify other molecules regulating the expression of DSPP, which may be a common downstream factor in multiple signaling pathways essential to dentinogenesis.
Acknowledgment
We thank Jeanne Santa Cruz for assistance with editing this article.
This work was supported, in whole or in part, by National Institutes of Health Grants DE005092 (to C. Q.), DE021773 (to Y. L.), and DE015209 (to J. F.).
- DSPP
- dentin sialophosphoprotein
- Col1a1
- collagen type 1a1
- μ-CT
- microcomputed tomography
- DSP
- dentin sialoprotein
- BSP
- bone sialoprotein.
REFERENCES
- 1. Fisher L. W., Torchia D. A., Fohr B., Young M. F., Fedarko N. S. (2001) Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 280, 460–465 [DOI] [PubMed] [Google Scholar]
- 2. Qin C., Baba O., Brunn J. C., Mckee M. D., Bonewald L., Butler W. T. (2005) in Proceedings of the 8th ICCBMT (Sodek J., Landis W., eds) pp. 174–177, University of Toronto Press, Banff, Alberta, Canada [Google Scholar]
- 3. George A., Sabsay B., Simonian P. A., Veis A. (1993) Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12624–12630 [PubMed] [Google Scholar]
- 4. D'Souza R. N., Cavender A., Sunavala G., Alvarez J., Ohshima T., Kulkarni A. B., MacDougall M. (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J. Bone Miner. Res. 12, 2040–2049 [DOI] [PubMed] [Google Scholar]
- 5. Feng J. Q., Huang H., Lu Y., Ye L., Xie Y., Tsutsui T. W., Kunieda T., Castranio T., Scott G., Bonewald L. B., Mishina Y. (2003) The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 82, 776–780 [DOI] [PubMed] [Google Scholar]
- 6. George A., Gui J., Jenkins N. A., Gilbert D. J., Copeland N. G., Veis A. (1994) In situ localization and chromosomal mapping of the AG1 (Dmp1) gene. J. Histochem. Cytochem. 42, 1527–1531 [DOI] [PubMed] [Google Scholar]
- 7. Ye L., MacDougall M., Zhang S., Xie Y., Zhang J., Li Z., Lu Y., Mishina Y., Feng J. Q. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 279, 19141–19148 [DOI] [PubMed] [Google Scholar]
- 8. Ye L., Mishina Y., Chen D., Huang H., Dallas S. L., Dallas M. R., Sivakumar P., Kunieda T., Tsutsui T. W., Boskey A., Bonewald L. F., Feng J. Q. (2005) Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J. Biol. Chem. 280, 6197–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng J. Q., Ward L. M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S. I., Zhang S., Rios H., Drezner M. K., Quarles L. D., Bonewald L. F., White K. E. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacDougall M., Simmons D., Luan X., Nydegger J., Feng J., Gu T. T. (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835–842 [DOI] [PubMed] [Google Scholar]
- 11. Qin C., Brunn J. C., Cadena E., Ridall A., Tsujigiwa H., Nagatsuka H., Nagai N., Butler W. T. (2002) The expression of dentin sialophosphoprotein gene in bone. J. Dent. Res. 81, 392–394 [DOI] [PubMed] [Google Scholar]
- 12. Xiao S., Yu C., Chou X., Yuan W., Wang Y., Bu L., Fu G., Qian M., Yang J., Shi Y., Hu L., Han B., Wang Z., Huang W., Liu J., Chen Z., Zhao G., Kong X. (2001) Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat. Genet. 27, 201–204 [DOI] [PubMed] [Google Scholar]
- 13. Zhang X., Zhao J., Li C., Gao S., Qiu C., Liu P., Wu G., Qiang B., Lo W. H., Shen Y. (2001) DSPP mutation in dentinogenesis imperfecta Shields type II. Nat. Genet. 27, 151–152 [DOI] [PubMed] [Google Scholar]
- 14. Kim J. W., Simmer J. P. (2007) Hereditary dentin defects. J. Dent. Res. 86, 392–399 [DOI] [PubMed] [Google Scholar]
- 15. Holappa H., Nieminen P., Tolva L., Lukinmaa P. L., Alaluusua S. (2006) Splicing site mutations in dentin sialophosphoprotein causing dentinogenesis imperfecta type II. Eur. J. Oral. Sci. 114, 381–384 [DOI] [PubMed] [Google Scholar]
- 16. Rajpar M. H., Koch M. J., Davies R. M., Mellody K. T., Kielty C. M., Dixon M. J. (2002) Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum. Mol. Genet. 11, 2559–2565 [DOI] [PubMed] [Google Scholar]
- 17. Kim J. W., Nam S. H., Jang K. T., Lee S. H., Kim C. C., Hahn S. H., Hu J. C., Simmer J. P. (2004) A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum. Genet. 115, 248–254 [DOI] [PubMed] [Google Scholar]
- 18. Sreenath T., Thyagarajan T., Hall B., Longenecker G., D'Souza R., Hong S., Wright J. T., MacDougall M., Sauk J., Kulkarni A. B. (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 278, 24874–24880 [DOI] [PubMed] [Google Scholar]
- 19. Narayanan K., Gajjeraman S., Ramachandran A., Hao J., George A. (2006) Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J. Biol. Chem. 281, 19064–19071 [DOI] [PubMed] [Google Scholar]
- 20. Zhu Q., Prasad M., Kong H., Lu Y., Sun Y., Wang X., Yamoah A., Feng J. Q., Qin C. (2012) Partial blocking of mouse DSPP processing by substitution of Gly(451)-Asp(452) bond suggests the presence of secondary cleavage site(s). Connect. Tissue Res. 53, 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu Q., Gibson M. P., Liu Q., Liu Y., Lu Y., Wang X., Feng J. Q., Qin C. (2012) Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J. Biol. Chem. 287, 30426–30435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Y., Ye L., Yu S., Zhang S., Xie Y., McKee M. D., Li Y. C., Kong J., Eick J. D., Dallas S. L., Feng J. Q. (2007) Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev. Biol. 303, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasad M., Zhu Q., Sun Y., Wang X., Kulkarni A., Boskey A., Feng J. Q., Qin C. (2011) Expression of dentin sialophosphoprotein in non-mineralized tissues. J. Histochem. Cytochem. 59, 1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun Y., Lu Y., Chen L., Gao T., D'Souza R., Feng J. Q., Qin C. (2011) DMP1 processing is essential to dentin and jaw formation. J. Dent. Res. 90, 619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baba O., Qin C., Brunn J. C., Jones J. E., Wygant J. N., McIntyre B. W., Butler W. T. (2004) Detection of dentin sialoprotein in rat periodontium. Eur. J. Oral. Sci. 112, 163–170 [DOI] [PubMed] [Google Scholar]
- 26. Huang B., Sun Y., Maciejewska I., Qin D., Peng T., McIntyre B., Wygant J., Butler W. T., Qin C. (2008) Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur. J. Oral. Sci. 116, 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Y., Gandhi V., Prasad M., Yu W., Wang X., Zhu Q., Feng J. Q., Hinton R. J., Qin C. (2010) Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the condylar cartilage of rat mandible. Int. J. Oral. Maxillofac. Surg. 39, 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang B., Sun Y., Chen L., Guan C., Guo L., Qin C. (2010) Expression and distribution of SIBLING proteins in the predentin/dentin and mandible of hyp mice. Oral. Dis. 16, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin D. M., Hallsworth A. S., Buckley T. (1978) A method for the study of internal spaces in hard tissue matrices by SEM, with special reference to dentine. J. Microsc. 112, 345–352 [DOI] [PubMed] [Google Scholar]
- 30. Wang X., Hao J., Xie Y., Sun Y., Hernandez B., Yamoah A. K., Prasad M., Zhu Q., Feng J. Q., Qin C. (2010) Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J. Histochem. Cytochem. 58, 957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y., Lu Y., Maciejewska I., Galler K. M., Cavender A., D'Souza R. N. (2011) TWIST1 promotes the odontoblast-like differentiation of dental stem cells. Adv. Dent. Res. 23, 280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu Y., Qin C., Xie Y., Bonewald L. F., Feng J. Q. (2009) Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs 189, 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duverger O., Zah A., Isaac J., Sun H. W., Bartels A. K., Lian J. B., Berdal A., Hwang J., Morasso M. I. (2012) Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J. Biol. Chem. 287, 12230–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orsini G., Ruggeri A., Jr., Mazzoni A., Papa V., Mazzotti G., Di Lenarda R., Breschi L. (2007) Immunohistochemical identification of decorin and biglycan in human dentin. A correlative field emission scanning electron microscopy/transmission electron microscopy study. Calcif. Tissue Int. 81, 39–45 [DOI] [PubMed] [Google Scholar]
- 35. Nikdin H., Olsson M. L., Hultenby K., Sugars R. V. (2012) Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth. PLoS ONE 7, e31525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Avery J. K. (2001) Oral Development and Histology, 3rd ed., pp. 235–236, Thieme Medical Publishers, Inc., New York [Google Scholar]
- 37. Lu Y., Yuan B., Qin C., Cao Z., Xie Y., Dallas S. L., McKee M. D., Drezner M. K., Bonewald L. F., Feng J. Q. (2011) The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J. Bone Miner. Res. 26, 331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang R., Lu Y., Ye L., Yuan B., Yu S., Qin C., Xie Y., Gao T., Drezner M. K., Bonewald L. F., Feng J. Q. (2011) Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prasad M., Butler W. T., Qin C. (2010) Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 51, 404–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verdelis K., Ling Y., Sreenath T., Haruyama N., MacDougall M., van der Meulen M. C., Lukashova L., Spevak L., Kulkarni A. B., Boskey A. L. (2008) DSPP effects on in vivo bone mineralization. Bone 43, 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gibson M. P., Zhu Q., Liu Q., D'Souza R. N., Feng J. Q., Qin C. (2012) Loss of dentin sialophosphoprotein leads to periodontal diseases in mice. J. Periodontal Res. doi: 10.1111/j.1600-0765.2012.01523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang B., Cao Z., Lu Y., Janik C., Lauziere S., Xie Y., Poliard A., Qin C., Ward L. M., Feng J. Q. (2010) DMP1 C-terminal mutant mice recapture the human ARHR tooth phenotype. J. Bone Miner. Res. 25, 2155–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H., Teng P. N., Jayaraman T., Onishi S., Li J., Bannon L., Huang H., Close J., Sfeir C. (2011) Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J. Biol. Chem. 286, 29462–29469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Narayanan K., Ramachandran A., Hao J., He G., Park K. W., Cho M., George A. (2003) Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J. Biol. Chem. 278, 17500–17508 [DOI] [PubMed] [Google Scholar]
- 45. Ravindran S., Narayanan K., Eapen A. S., Hao J., Ramachandran A., Blond S., George A. (2008) Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J. Biol. Chem. 283, 29658–29670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maciejewska I., Qin D., Huang B., Sun Y., Mues G., Svoboda K., Bonewald L., Butler W. T., Feng J. Q., Qin C. (2009) Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tissues Organs. 189, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang B., Maciejewska I., Sun Y., Peng T., Qin D., Lu Y., Bonewald L., Butler W. T., Feng J., Qin C. (2008) Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif. Tissue Int. 82, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siyam A., Wang S., Qin C., Mues G., Stevens R., D'Souza R. N., Lu Y. (2012) Nuclear localization of DMP1 proteins suggests a role in intracellular signaling. Biochem. Biophys. Res. Commun. 424, 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sreenath T. L., Cho A., MacDougall M., Kulkarni A. B. (1999) Spatial and temporal activity of the dentin sialophosphoprotein gene promoter: differential regulation in odontoblasts and ameloblasts. Int. J. Dev. Biol. 43, 509–516 [PubMed] [Google Scholar]
- 50. Narayanan K., Srinivas R., Ramachandran A., Hao J., Quinn B., George A. (2001) Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Pro. Natl. Acad. Sci. U.S.A. 98, 4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]