Abstract
The concept of using an immunoisolation device to facilitate the transplantation of islets without the need for immunosuppression has been around for more than 50 yr. Significant progress has been made in developing suitable materials that satisfy the need for biocompatibility, durability, and permselectivity. However, the search is ongoing for a device that allows sufficient oxygen transfer while maintaining a barrier to immune cells and preventing rejection of the transplanted tissue. Separating the islets from the rich blood supply in the native pancreas takes its toll. The immunoisolated islets commonly suffer from hypoxia and necrosis, which in turn triggers a host immune response. Efforts have been made to improve the supply of nutrients by using proangiogenic factors to augment the development of a vascular supply in the transplant site, by using small islet cell aggregates to reduce the barrier to diffusion of oxygen, or by creating scaffolds that are in close proximity to a vascular network such as the omental blood supply. Even if these efforts are successful, the shortage of donor islet tissue available for transplantation remains a major problem. To this end, a search for a renewable source of insulin-producing cells is ongoing; whether these will come from adult or embryonic stem cells or xenogeneic sources remains to be seen. Herein we will review the above issues and chart the progress made with various immunoisolation devices in small and large animal models and the small number of clinical trials carried out to date.
Islet Transplantation
Limited Supply of β-Cells and Potential New Sources
Immunogenicity
Immunobarrier Protection
-
Materials
Alginate
Polycations and anions
Agarose
Nanoencapsulation
Other
Macrodevices
The search for better biomaterials
-
Successes, Failures, and Issues with Transplanted Immunoisolated Cells
Small animal models
Large animals and humans
Transplantation site
Kinetics of insulin release
-
Efforts Aimed at Improving Longevity of Transplanted Immunoisolated Tissue
Aggregates: why they work better than whole islets
Cell composition: are non-β-cells necessary?
Proangiogenic and other factors
Summary
I. Islet Transplantation
Islet transplantation is a form of insulin replacement that matches normal physiology much more closely than exogenous insulin injections. The result is a reduction in the incidence of hypoglycemia, improved glycemic control (1), and overall improvement in quality of life (2). It is important to point out that β-cell replacement therapy should also provide great benefit for some people with type 2 diabetes mellitus (T2DM), where β-cell insufficiency is a key part of the pathogenesis (3). It has, however, associated with it the burden of immunosuppression and so is reserved for selected patients with severe glycemic variability, recurrent hypoglycemia, and hypoglycemia unawareness despite intensive insulin management. An advantage over whole pancreas transplant, which is more widely available for similarly selected patients with type 1 diabetes mellitus (T1DM), is that it is a much less invasive procedure with a shorter hospital stay and lower associated morbidity. However, there are as yet no randomized controlled trials comparing the two treatments. The first significant islet transplantation in 1989 by the team of Paul Lacy (4) lasted just a few days. Since then, great progress has been made in the field, notably by Shapiro et al. (1) at Edmonton who in 2000 reported insulin independence in all of a series of seven transplanted patients. This success was likely due to several changes in the transplantation procedure: elimination of steroids and inclusion of sirolimus in the immunosuppression regimen, limited cold ischemia time of the recovered pancreases, and the large number of islets that were transplanted. The 5-yr follow-up results from the Edmonton center showed that of 65 patients rendered insulin independent, 80% remained so at 1 yr, but less than 50% were insulin free at 2 yr, and just 10% were at 5 yr. However, many who returned to insulin continued to have reduced insulin requirements and less frequent occurrence of hypoglycemia, indicating persistence of meaningful β-cell survival (5). Results approaching this level of success have been reported by other centers (6).
The disadvantages of the current approach to islet transplantation are the need for at least two donor pancreases for most recipients and graft failure, which occurs within a relatively short period of time compared with whole pancreas transplantation (7). Poor vascularization and relative hypoxia of the transplanted cells (8, 9), continuing destruction by autoimmunity and allorejection (10), and exposure to the toxic effects of immunosuppressive drugs (11) are all thought to contribute to early graft failure. For these reasons, islet transplantation remains an experimental treatment available only for carefully selected cases of T1DM. This will remain the case until these deficiencies are overcome, and even then a severe shortage of donor islets will limit the number of patients that can be treated.
II. Limited Supply of β-Cells and Potential New Sources
There is a gross mismatch between the availability of islets or β-cells for transplantation and the number needed to treat all potential candidates. A solution to this problem is being pursued vigorously by many groups (12) with recent notable successes in deriving glucose-responsive insulin-producing cells from human embryonic stem cells (hESC) (13, 14). These derived cells do not respond well to glucose in vitro, but mature and function like normal β-cells after being transplanted into mice. β-Cell expansion in vivo or in vitro is another attractive avenue. The ability of differentiated β-cells to replicate is important during normal growth (15), pregnancy (16, 17), and obesity (18). However, human β-cells have shown less potential for replication compared with rodent β-cells (19). A search for small molecules to stimulate expansion of existing β-cells or direct stem cells toward a β-cell phenotype is under way, in some cases with the use of high-throughput screens of chemical libraries (20). Isolated human pancreatic duct cells can also be stimulated to form new islet cells in vitro (21). However, whether these pancreatic sources will be able to produce a sufficient number of β-cells for clinical efficacy remains to be seen.
Porcine islets have been viewed as a potential source of β-cells for transplantation due to the close homology between porcine and human insulin and the similarities of islets from the two species. There has been concern about possible transmission of porcine endogenous retrovirus to recipients, but porcine lines apparently free of infectious porcine endogenous retrovirus have been identified. Transdifferentiation of hepatic (22), bile duct epithelial (23), and acinar (24) cells to β-like cells has led to some encouraging results. As with all of the potential sources of replacement β-cells, much work will be necessary to develop cells that have all the necessary characteristics of native β-cells and, indeed, safety in terms of maintaining a differentiated phenotype with no risk of teratogenicity, which is a concern with the use of cells derived from hESC.
III. Immunogenicity
The problem of immune rejection remains a significant challenge not only for the transplantation of islets but also for most forms of tissue transplantation. Even with the advent of new sources of β-cells, some form of immunoprotection will most likely be required. Cells differentiated from hESC retain their antigenicity and can be expected to be susceptible to both transplant rejection and autoimmunity; likewise, transdifferentiated cells and xenogeneic cells will predictably elicit an immune response unless progress is made with genetic engineering that somehow reduces antigenicity or eliminates the problem (25). There is one notable exception, induced pluripotent stem cells (26, 27) derived from somatic cells, such as fibroblasts. There has been some success in directing such cells to an insulin-secreting phenotype (28). These could be an ideal way to replenish the β-cell deficit of T2DM, because they could be derived from fibroblasts or some other cell source obtained from the patient in need, thus eliminating the need for immunoprotection. However, there is theoretical concern that the genetic predisposition to T2DM would ultimately lead to reduced number and function in these transplanted β-cells over time (29).
Activation of the innate immune system is triggered when the donor tissue contacts the recipient's blood cells in a reaction termed the instant blood-mediated inflammatory response (30). Treatment of the islets before transplantation with the anticoagulants heparin and dextran sulfate may ameliorate this inflammatory and immune-mediated response. Further loss of transplanted islets results from failure of engraftment due to ongoing immune-mediated attack in the form of allograft rejection and in some cases, reactivation or ongoing autoimmunity (31). In addition, the toxic effects of immunosuppressive drugs (32), glucotoxicity (33, 34), and islet amyloid deposition (35) have all been suggested to contribute.
Transplantation of islets as a treatment for T1DM has the added challenge of autoimmunity, along with the allogeneic or xenogeneic response of the host to the transplanted tissue. Clinical studies have shown a higher rejection rate of islets transplanted to individuals with circulating anti-glutamic acid decarboxylase and anti-insulinoma associated protein antibodies, markers of autoimmune β-cell destruction in T1DM (36). Indeed, there is also a higher rejection rate when islets are transplanted to individuals with common human leukocyte antigen class 1 alleles, indicating the involvement of autoimmune cell-mediated destruction of the donor tissue recognized as self. Indeed, autoreactive T cells have been implicated as being important in the rejection of transplanted islets (37). It has been shown that autologous islet transplants survive better than allogeneic transplants, even when a greater number of islets are transplanted (32).
Standard immunosuppression protocols for islet transplantation (and indeed whole organ transplantation) have been associated with adverse effects on the transplanted tissue. Cyclosporine and the newer calcineurin inhibitors tacrolimus and sirolimus have been implicated in inhibition of β-cell engraftment and revascularization (39) and may also have direct toxic effects on the β-cells. There is evidence that they inhibit β-cell replication (40–42) and neogenesis in the case of mycophenolate mofetil (43). They also can have negative effects on insulin sensitivity, which can be detrimental to the transplant success (44). Aside from direct effects on the transplanted tissue, progression of cardiovascular disease and nephrotoxicity has long been associated with corticosteroids and calcineurin inhibitors, respectively. The Edmonton protocol had initial success with corticosteroid-free immunosuppression, induction with daclizumab (a monoclonal antibody to IL-2 receptor), followed by high-dose sirolimus (a non-calcineurin-inhibiting anti-T-cell agent) in the first 3 months and low-dose tacrolimus (1). Contrary to the postulation that the absence of steroids was crucial was the subsequent report of prolonged graft survival in two patients who received simultaneous islet and kidney transplants under steroid and cyclosporine immunosuppression (45). Other relatively new agents in use with potentially less associated toxicity include the induction agents OKT3 and rabbit anti-thymocyte globulin (46), and TNF-α blockers infliximab and etanercept (47). The latter have been postulated to reduce periinfusion inflammation and β-cell loss. The Minnesota group used a protocol consisting of anti-thymocyte globulin and etanercept in the peritransplant period, and tacrolimus and sirolimus thereafter. All eight patients achieved insulin independence despite the relatively small number of islets received from single donors. Graft failure was associated with subtherapeutic levels of sirolimus and tacrolimus, suggesting that a key player in sustaining islet engraftment was sufficient immunosuppression (46). Longer-term insulin independence was achieved by the same group with the use of the same induction agents, and everolimus and cyclosporine thereafter (48). Along with research focused on development of immunosuppressive drugs with less harmful side effects, much work has been done on developing a means of inducing immunological tolerance (49, 50).
The development of a method of immunosuppression potent enough to inhibit rejection of transplanted islets, yet safe enough for use in patients including children who develop T1DM such that the benefit outweighs the risks, remains a daunting challenge (51). There are both practical and ethical concerns specific to undertaking the procedure in children. Children with T1DM are subject to higher risk of morbidity and mortality from complications of the disease. However, the results to date in adults, although improving, as yet do not justify the burden of current immunosuppression regimens except in those at extremely high risk from hypoglycemia, despite optimal insulin administration and monitoring. The selection of these cases is not straightforward, and estimation of risk difficult. This becomes somewhat more straightforward when a patient is already immunosuppressed for a renal, lung, or cardiac transplant. The practical concerns are whether the quantity of islets transplanted in childhood will be sufficient to meet demands as the child grows and the need for ongoing immunosuppression to preserve the graft even in pregnancy. On the other hand, a smaller mass of islets is likely to succeed in a low-weight child, so islets from one pancreas are more likely to be sufficient than in adults (52).
IV. Immunobarrier Protection
One approach to improve the outcomes of islet transplantation is immunoisolation, where islets are enclosed in a barrier device that facilitates the exchange of oxygen, nutrients, and insulin but protects the islets against the host immune response. The main focus of this review will be immunoisolation. If a sufficiently effective and safe immunoisolation device for use in islet transplantation in humans can be found, the absence of harmful immunosuppression would dramatically sway the balance of outcomes toward benefit and away from harm for many more patients with diabetes. Semipermeable microcapsules were first described by Chang (53) in the 1960s. He proposed the use of polymer membranes to contain and deliver enzymes and perhaps cells to the bloodstream to treat a deficiency or “genetic accident.” Predating this was the hypothesis that transplantation to an immune-privileged site would facilitate long-term survival of donor pancreas tissue. Browning and Resnik (54) transplanted pancreas to the anterior chamber of the eye in syngeneic and allogeneic mouse models but showed no better results with this site compared with the sc site. Other sites examined for their immunoprivileged potential were the cheek pouch in hamsters (55) and the testis (56), which resulted in limited success. The concept of immunobarrier protection for islets was first described in 1977 by Chick et al. (57). A device was implanted in diabetic rats in which islets were placed on the outside of hollow tubes made of a semipermeable acrylic copolymer, which carried blood as an arteriovenous shunt. The idea behind what was called a vascularized bioartificial pancreas was that the membrane should be permeable to small molecules like oxygen, nutrients, glucose, and insulin but impermeable enough to prevent immune destruction. The devices restored normoglycemia for a period of hours before their removal. This was followed by the use of a microencapsulation approach by Lim and Sun, who in 1980 reported successful reversal of diabetes in rat recipients of immunoisolated islets for up to 15 wk (58). This was with the use of alginate microcapsules with poly-l-lysine (PLL) and polyethylenimine coatings. It was initially thought that these coatings would provide a level of permselectivity that would not allow the entry of Ig or cytokines produced by the host's immune system. However, as will be discussed in Section V.F, the requirements for permselectivity are not yet clearly defined.
Macrodevices, which rely on diffusion rather than arteriovenous shunts, have also been used to provide immunoprotection to transplanted islets. In 1991, Lacy et al. (59) reported reversal of diabetes in rats transplanted sc and ip with acrylic hollow fibers containing islets immobilized in alginate for up to 60 d. With both micro- and macroencapsulation, inadequate oxygenation has been a significant obstacle in the way of long-term graft success (60). Central necrosis is commonly observed in islets recovered after transplantation. In preclinical studies, the biocompatibility of the materials and device design issues remain ongoing problems. If these issues can be addressed, immunoisolation provides an attractive means of protecting transplanted islets from rejection. An additional benefit is that the barrier could prevent release of potentially harmful foreign donor cells, such as those derived from hESC with teratoma-forming characteristics.
V. Materials
See Table 1 for a list of materials in use for islet immunoisolation.
Table 1.
Materials used to encapsulate islets
| Material | Characterization | Features | Refs. |
|---|---|---|---|
| Alginate | In vitro, rodent, dog, nonhuman primate, human | Naturally occurring, porous, hydrogel capsules, divalent cation, cross-linking | 57–67, 116–119, 121, 127–129, 138 |
| PLL/poly-l-ornithine coating | In vitro, rodent, human | Synthetic, permselective layer, inflammatory | 65, 67, 69, 70, 120 |
| Agarose | In vitro, rodent, dog | Naturally occurring, porous, hydrogel capsules, thermogelation | 76–81 |
| PEG | In vitro, rodent, nonhuman primate | Synthetic hydrogel, conformal coating, photo-cross-linking | 83–86, 125, 147 |
| Chitosan | In vitro, rodent | Naturally occurring, porous, hydrogel capsules, thermosensitive | 85–87, 93–96 |
| Collagen | In vitro, rodent | Naturally occurring, used in polymer blends, biodegradable | 14, 97, 98 |
| Polydiallyldimethyl ammonium chloride | In vitro, rodent | Synthetic, layer-by-layer coating, poor islet viability | 87, 98, 99, 101 |
| PLL/PEG copolymer | In vitro, rodent | Synthetic, conformal coating, good islet viability | 88, 89 |
| PVA/PEG lipid | In vitro | Synthetic, conformal coating, good islet viability | 90 |
| Host cell-PEG lipid | In vitro | Synthetic and cellular, conformal coating, good islet viability | 91, 92 |
| Silica | In vitro, rodent | Synthetic, gaseous deposition, porous matrix | 102 |
| Hydroxymethyl polysulfone | In vitro, rodent | Synthetic, porous capillary, supports vascular growth | 112 |
| N-Isopropyl-acrylamide/hemoglobin | In vitro, rodent | Synthetic and protein, improved oxygen transport, sustained islet viability | 103 |
| Poly(N,N-dimethyl acrylamide) | In vitro | Synthetic, tubular membranes, good oxygen transport | 104 |
A. Alginate
Alginate is an anionic polysaccharide produced by seaweed, whose biocompatibility and gelling properties make it useful for microencapsulation (Fig. 1D). To maximize biocompatibility and elimination of endotoxin, rigorous purification of the naturally occurring compound is required (61). Inadequate alginate purification has correlated with increased alginate immunogenicity and decreased encapsulated islet viability (62). Impure alginates can induce increased islet necrosis, splenocyte proliferation, and TNF-α secretion. The resulting purified polymers when added to water form a hydrogel when cross-linked with divalent cations. To form microcapsules, an alginate-cell suspension is extruded as droplets from a device into a cross-linking solution. Air-droplet generators have been superseded by electrostatic devices where a set voltage gradient is used to ensure generation of uniformly sized microspheres, in contrast to the larger, less regular spheres generated with air-droplet generators.
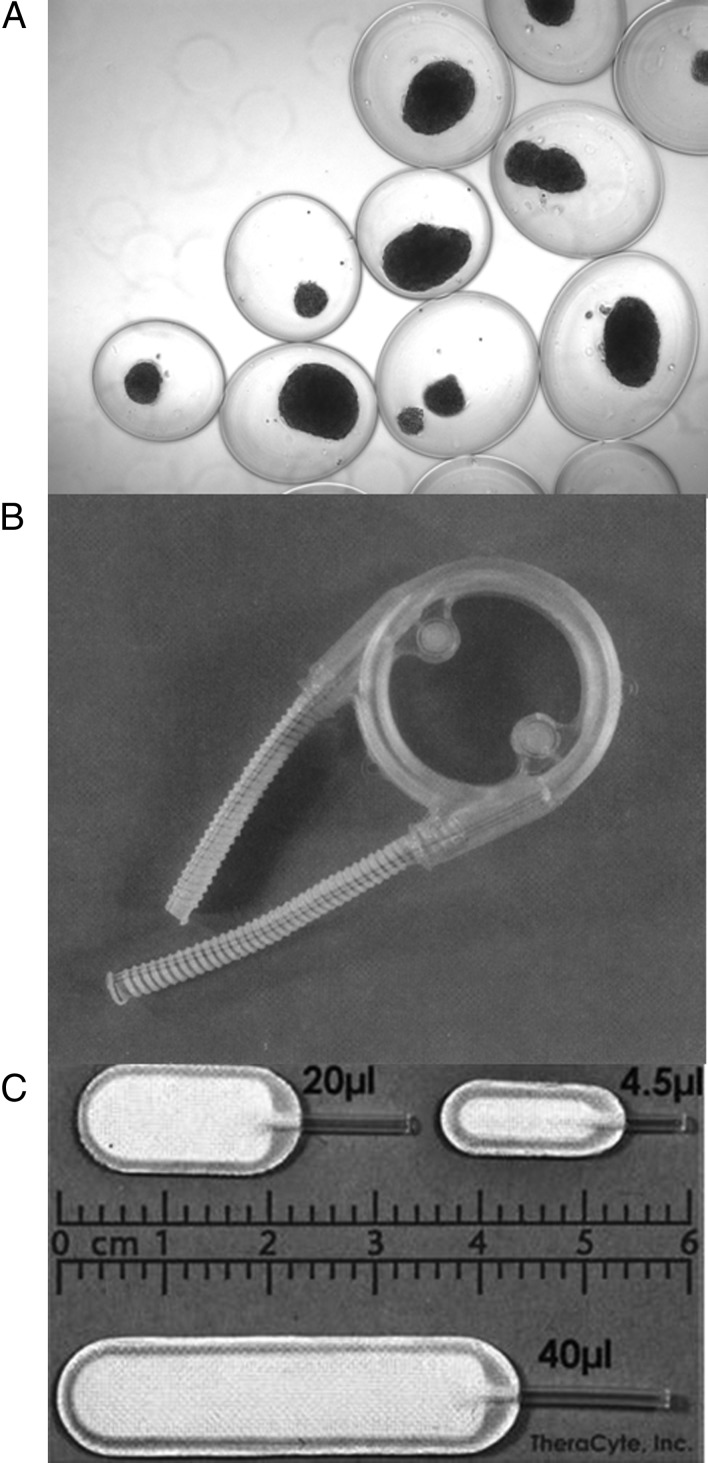
Fig. 1.
A, Alginate capsules containing islets; B, the hybrid artificial pancreas; C, the TheraCyte device.
Alginate is made up of chains of mannuronic acid (M) and guluronic acid (G), the proportions of which vary between different source species. Alginate with a higher G content (or high-G alginate) typically comes from the stems of the plant, whereas the more flexible high-M alginate originates in the leaves. These units form bonds in a sequence that has no regular pattern, but the composition and sequence of these units in an alginate determines a microcapsule's properties, function, permeability, and mechanical strength. Diffusion of molecules through the gelled polymer is affected by a number of factors; pore size is sometimes referred to, but gels do not consist of uniformly sized pores. The gel is actually a nonuniform mesh of alginate strands that serves as a barrier to the movement of molecules passing from the outside of the capsule to the tissue within or vice versa (63).
The association of the monomeric units M and G that make up alginate can be thought of as blocks, although a fixed pattern does not exist, and heterogeneity rules. Regions of consecutive mannuronic units are called M-blocks, likewise there are G-blocks, and M bound to G are MG-blocks.
Many complex variables are involved in making alginate microcapsules, as discussed in a recent paper by Mørch et al. (64). These variables include G and M composition, concentration, viscosity, gel homogeneity, and capsule size. These variables determine the strength, flexibility, durability, and permselectivity of the gel capsules. All of these can be influenced by the cross-linking cations, which include calcium, barium, and strontium. Alginates form a relatively stable gel when adjacent positively charged blocks are cross-linked by cations.
The size of the capsules and degree of swelling when immersed in a saline solution have implications for the transplant setting. Swelling of capsules results in weaker more permeable capsules that are more likely to break apart with time. Some of the swelling is thought to be due to exchange of calcium with sodium and magnesium, which do not serve as cross-linkers. High-G alginates tend to form stronger gels than high-M alginates (64), which is due to the binding of cations with greater affinity to G-blocks than to M-blocks or MG-blocks. MG-blocks have more inherent flexibility that facilitates greater interaction between G-blocks. The cation used also has implications for the characteristics of the capsule produced. When calcium (the cation used traditionally) is used, the resulting high-G alginate capsules are smaller and swell less than a high-M alginate capsule, which results in greater stability. Barium is attractive because it has a higher affinity toward alginate compared with calcium and can be used at lower concentrations of 1–20 mm. There are concerns about barium toxicity; once the gels are washed, the amount of barium released from the gels over time needs to be studied, but it may be negligible. Our group has used concentrations up to 20 mm in rodents without complications (65).
High-M alginates form weaker, more permeable gels than high-G alginates, and the use of barium instead of calcium does not significantly alter this. Higher concentrations of both the alginate and the cross-linking cation tend to produce stronger capsules (64). To produce an alginate capsule of useful strength and durability, concentrations of calcium used as a cross-linking agent are of the order of 100 mm. Strontium is another cation that has been used for cross-linking alginates, with evidence of binding affinity intermediate to that of calcium and barium when high-G alginates are used and the equivalent effect to both when high-M alginate is used (64). The amount of permselectivity required to protect encapsulated cells is poorly understood. One hypothesis is that if cells are not releasing many factors that trigger an immune or inflammatory response, such as antigens, cytokines, and chemokines, simple separation from immune cells will be sufficient. Others think that the entrance of cytokines, Ig, and complement must be restricted. For this reason, alginate capsules are often coated with a polycation layer such as PLL to obtain permselectivity. Alginate gels have some restrictive properties, which are dependent upon concentration and their negative charge. The good protection of cells found with alginate capsules without a polycation coating (66, 67) in rodent models is instructive, but the requirements for permselectivity may be different in larger animals and humans.
B. Polycations and anions
PLL has been used as a positively charged coating on alginate to confer greater permselectivity and mechanical strength. However, the resulting capsules have the disadvantage of greater bioreactivity because PLL has been shown to initiate a proinflammatory response in the transplant setting (68). To counteract this, an additional thin coating layer of alginate is usually added to serve as a barrier between the polycation and the host tissues. This is not always sufficient to completely prevent an immune reaction. ζ-Potential measurements (surface charge) are helping to provide some insight into the biocompatibility of different alginate-PLL formulations after implantation (69). An alternative polycation poly-l-ornithine has been used for the same purpose but has given no better results (70, 71). Simple alginate capsules without a polycation coating have been shown to elicit minimal cellular reaction in rodent models as shown by Duvivier-Kali et al. (67). Similar success in rats has been reported by Safley et al. (66) and with a relatively open polytetrafluoroethylene (PTFE) membrane (72). Whether a permselective polycation coating will be needed for success in large animals and humans remains an open question.
Alginates combined with an anionic polymer cellulose sulfate have also been used with some success (73). After comprehensively testing over 200 combinations of anions and cations in different capsule designs, Wang et al. (74) chose a combination of sodium alginate, cellulose sulfate, poly-methyl co-guanidine, PLL, and calcium chloride to produce microcapsules with optimal mechanical strength and permselectivity. The fusion of the polylmethyl coguanidine- cellulose sulphate (PMCG)-CS/PLL-sodium alginate (SA) membrane onto the previously tested PMCG-CS/CaCl2-SA capsule was found to improve the uniformity of the capsule “pore” distribution, thus increasing mechanical strength and permselectivity without compromising the mass transport kinetics of insulin and glucose. Alginate and cellulose sulfate have also been combined with various polyvinylamines to provide greater mechanical strength and to optimize permeability (75). In a different approach to obtain stable permselective membranes, Schneider et al. (76) adopted a layer-by-layer approach to create multiple alternating layers of polycation and polyanion polymers on alginate microcapsules. Polyethyleneimine, poly(dimethyldiallylammonium chloride), chitosan, polyacrylacid, carboxymethylcellulose, cellulose sulfate, and pectin were used to create multilayered microcapsules only 145-nm thick. This system created capsules with adjustable mechanical stability and immunoprotection by altering the layer composition. Although similar amounts of fibrosis were observed between layered and unlayered alginate capsules, less macrophage penetration was observed in the multilayered system. However, this degree of macrophage invasion was out of keeping with previous studies of alginate capsules. The question arises whether the comparison of the layered capsules to optimal single-layer capsules would show any significant advantage.
C. Agarose
Agarose is another hydrogel and a naturally occurring thermosensitive polymer found in the cell walls of seaweed that has been used for microencapsulation. Agarose with a gelling temperature of 15–30 C has been used for encapsulation of cells. The gelling temperature is dependent on the concentration of agarose used. Generally, formulations that remain gelled up to 60 C are used such that the capsules once formed will not melt in the range of temperature seen in the mammalian body. As with alginate capsules, the technique of droplet extrusion is used (or alternatively immersion in oil). This is followed by hardening with reduction in temperature. An agarose layer has been shown to be sufficient for islet immobilization, and an additional agarose coating prevents extrusion of cells and appears to be biocompatible, at least in the short term (77). Increased permselectivity can be provided by increasing the concentration of agarose (78). In one study, agarose-encapsulated islets remained viable and functional up to 67 wk in cell culture and were still able to recover normoglycemia in diabetic rats (79). Transplanted allogeneic hamster islets encapsulated in agarose have been shown to survive for up to 100 d in mouse recipients, and modified agarose capsules, in which agarose is combined with polystyrene sulfonic acid, can protect xenogeneic (rat to mouse and pig to dog) islets from rejection for weeks to months (80, 81). A recent study demonstrated reversal of diabetes in rats using cryopreserved islets encapsulated in agarose (82). Agarose has not been studied as widely as alginate for islet encapsulation but it seems unlikely to offer any great advantage over the latter.
D. Nanoencapsulation
As an alternative to microencapsulation, nanothin multilayered nanocapsules have been implemented in an attempt to increase coverage of encapsulated islets and decrease islet response time for insulin release to external stimuli. Polyethylene glycol (PEG) is a hydrogel polymer, which can be used as a conformal coating on islets (Fig. 1B). Photopolymerization is a method conventionally employed for covalently cross-linking these molecules together (83). When exposed to UV or visible light, PEG can be cross-linked and polymerize in the presence of compounds called photoinitiators. As long as the duration and intensity of exposure to light, temperature, and pH are controlled, suspensions of PEG acrylate with islet cells can be photopolymerized to provide encapsulation with limited irradiation damage to the enclosed cells (84). The conjugation of glucagon-like peptide 1 (GLP-1), a hormone that stimulates insulin gene transcription and is antiapoptotic for β-cells, to the PEG hydrogel has also been shown to mitigate islet cell death in response to photopolymerization (85). The thin conformal coating has the advantage of shorter distance for diffusion for oxygen, nutrients, and insulin and a smaller volume of material for transplantation, which is important for some transplant sites. It is not as biocompatible as the other hydrogels, and although it offers some degree of permselectivity, it cannot provide complete protection from cytokines (86). Although it is unclear how much protection from cytokines is needed, low-dose immunosuppression therapy was shown to improve outcomes in terms of reversal or diabetes and glucose tolerance when PEG-coated porcine islets were transplanted to rats (a discordant xenogeneic model) (87).
Krol et al. (88) used a layer-by-layer deposition technique on the islets directly, alternating layers of either poly-(allylamine hydrochloride) or poly-(diallyldimethylammonium chloride) as polycation and poly-(styrene sulfonate) as the polyanion. Only slightly reduced insulin release was observed with these nanocapsules, and islets were protected against islet-specific antibodies. Unexpectedly, an odd number of polyelectrolyte layers (net positive charge) yielded glucose-responsive insulin release, whereas an even number of layers yielded unregulated insulin release, presumably due to some unspecific stimulation. Chaikof and co-workers (89) found that the polyelectrolytes used by Krol et al. (88) had a negative impact on the viability of islets and therefore developed nanocapsules by layering a biotin-labeled PLL-PEG copolymer with streptavidin. This coating strategy resulted in enhanced islet viability, but implantation of coated islets into the portal vein of the liver resulted only in transient reversal of diabetes. Coated islets also did not convert a statistically significant number of mice to normoglycemia when compared with uncoated islet transplants. An expansion to this approach has been adopted incorporating GLP-1 peptide to improve glucose-stimulated insulin secretion in conformally coated islets (90).
An analogous maleimide PEG-lipid approach was used by Teramura et al. (91) to facilitate multilayered nanothin coatings of polyvinyl alcohol (PVA) on the islet surface. In this case, PEG conjugated lipids anchor into the islet membranes and the exposed maleimide allows covalent attachment of thiol-modified PVA. Islet function was not impaired by this process in vitro, but the biocompatibility of this coating in vivo was not investigated. However, in two separate extensions of this concept, Teramura et al. (92, 93) explored encapsulation of islets using living cells. Encapsulation of donor islets with host cells could be an effective strategy to achieve biocompatibility. Islets modified with either biotin-PEG-lipid or polyDNA-PEG-lipid were encapsulated with similarly modified HEK293 cells. In the case of biotin-PEG-lipid, streptavidin was used to adhere the 293 cells to the islet surface, whereas cell surfaces modified with polyDNA-PEG-lipid require only cDNA sequences for adhesion. The polyDNA-PEG-lipid approach was developed as a streptavidin-free alternative to avoid known xenogeneic responses to the protein. Both strategies result in functional islet preparations but have yet to be tested in vivo.
E. Other
Chitosan is derived from naturally occurring chitin found in the shells of crustaceans and insects. Its biocompatibility and good handling properties make it useful in a range of applications such as a vehicle for the delivery of pharmaceuticals, as a filler in orthopedic surgery and periodontics, and as wound dressings to promote healing (94). When cross-linked with glutaraldehyde, it forms a hydrogel. It can also be combined with glycerol-2-phosphate disodium salt hydrate to make a thermosensitive material that is liquid at room temperature and gels at 37 C. Chitosan microcapsules have been reported to protect rat islets transplanted to diabetic mice for up to 4 wk (95). It has also been used in combination with other polymers such as alginate and hydroxyapatite to make scaffolds on which cells are seeded (96, 97) (osteoblasts and hepatocytes, respectively) in tissue engineering applications.
Collagen can also be used in various ways for islet transplantation because, as a key component of connective tissues in bone, cartilage, tendon, ligament, and skin, it becomes an obvious choice for use in tissue engineering. Because it is biocompatible and biodegradable, it is an attractive material for creating scaffolds for cell transplantation (98). It has not proved useful as an immunoisolation material in itself but may provide benefits when prevascularized to promote survival of transplanted islets (99). Microcapsules made from a core of sodium cellulose sulfate with poly-(diallyldimethylammonium chloride) as a surrounding solid membrane have been found to have favorable properties in terms of ease of production, mechanical strength, and lack of bioreactivity. When used to contain a human adenocarcinoma cell line with cytochrome p450 activity to deliver activated chemotherapy to pancreatic tumors in mice, capsules showed no evidence of an immune response or fibrotic reaction after several months (99, 100). [Success has also been reported in phase I/II clinical trials for the treatment of inoperable pancreatic cancer (101).] When used to encapsulate hamster pancreatic β-cell line HIT-T15 cells, there was no difference in viability, glucose uptake, and insulin release compared with unencapsulated cells (102).
Silica encapsulation of islets has also proven to be an effective immunobarrier (103). Deposition of gaseous silicon alkoxides occurs under inert conditions to form a porous siliceous matrix around the islets, with greater exposure time leading to greater thickness. This siliceous membrane is a fairly bio-inert surface that displays good transport properties and could serve as a reasonable alginate substitute. Most islet cells appear to be viable after this deposition process and show glucose-dependent insulin secretion, but there is still concern over the toxicity of alcohol by-products associated with the process. Transplantation of silica-encapsulated rat islets showed normoglycemia in diabetic rat models for up to 12 wk.
Oxygen transport across the microcapsule barrier is crucial for encapsulated islet survival and prevention of necrosis (104). In an effort to enhance oxygen transport, capsules for islets were made from a blend of poly (N-isopropylacrylamide-co-acrylic acid) and cross-linked hemoglobin. The resulting encapsulated islets displayed increased viability and insulin secretion in cell culture as well as sustained normoglycemia for a longer period than microcapsules without hemoglobin.
Finally, hydrophilic poly(N,N-dimethyl acrylamide) cross-linked with hydrophobic di-, tri-, and octa-methacrylate telechelic polyisobutylene stars generated hydrogels with good oxygen transport properties, membrane permeability, and controlled pore size (105). Encapsulation of islets with small tubular membranes formed by rotational cross-linking of the polymer displayed viability over a period of 1.5 months and secreted insulin in response to a glucose challenge assay. Although this hydrogel is also known to have good biocompatibility, in vivo experiments with encapsulated islets have not been performed.
Despite evaluation of an extensive range of candidate materials for islet immunoisolation, there is as yet no convincing evidence that the newer materials are superior to the more established alginate and PEG. This may well change as studies move from preclinical phase in small animal models to nonhuman primates and clinical trials. The latter represent much more discerning transplant models with far greater challenges in terms of evading the host's immune response.
F. Macrodevices
An alternative to microcapsules is a larger device designed to contain a greater amount of tissue. An attractive aspect of some macrodevices is that they may be retrievable and/or reloadable. Various designs and materials consisting of polymer membranes have been used including planar devices that could be discs or sheets, between which tissue resides as a thin layer (106). Hollow fibers have also been used (59). The macrodevice that was used to transplant porcine islets sc in an unsuccessful clinical trial in Mexico was made of a biocompatible stainless steel tube, with PTFE stoppers on either end (107).
The issues about biocompatibility and porosity are similar to those considered for microencapsulation, but macrodevices typically have greater mechanical strength. Lacy et al. (59) found that immobilizing islets in alginate before inserting them into a macrodevice reduced necrosis of the islets. This maneuver limits clumping of islets, which is problematic because clumping increases the distance oxygen must diffuse to supply the innermost cells, leading to central necrosis. Suzuki et al. (106) also used alginate to immobilize islets in combination with a planar device. A downside of adding the mechanical strength of a macrodevice to the permselectivity of a microcapsule is the extra space it occupies. The ratio of device surface area to contained tissue volume is a critical issue because it may be that a macrodevice would be so large that it would be impractical for transplantation (108). This means that packing density must be maximized so as to minimize the size of the device. Through bioengineering and the use of proangiogenic factors, there may be ways to promote development of a vascular network or indeed prevascularize macrodevices before insertion of the islets to be transplanted. This would help to obtain better packing density (109). For example, the TheraCyte device (Fig. 1C) is composed of a double membrane of PTFE, an inner permselective membrane for immunoisolation and an outer less selective membrane to facilitate angiogenesis in close proximity to the material contained within (110), which in turn can be promoted by infusion of vascular endothelial growth factor (VEGF) transcutaneously into the device (111, 112). Lembert et al. (113) compared the effect of smooth and rough surfaces of porous hydroxymethylated polysulfone capillaries used to encapsulate islets. In this system, rat islet allografts displayed not only sustained normoglycemia in a diabetic rat model but also extensive vascularization of rough, but not smooth, capillary surfaces after explantation. Encapsulated porcine xenografts, however, displayed extensive fibrotic overgrowth after explantation in addition to vascularization in the same rat model. In vitro studies revealed that polysulfone-encapsulated islets release VEGF, and the rough capillary surface helps support vascular growth on the implant. Although preliminary studies of vascularized macrodevices have shown promise, there is a long way to go before such devices could be trialed in humans, and much fine-tuning would be necessary to ensure safety.
G. The search for better biomaterials
Although countless materials in various configurations have been tested, it remains to be seen whether any of these will be suitable for islet transplantation in humans. The available materials are not perfectly biocompatible; some degree of tissue reaction in the host is almost always evident, especially in larger animals. New approaches include the conjugation of biomolecules to the biomaterials for encapsulation that specifically inhibit activation of the complement and coagulation cascades. Such biomolecules include heparin, which can effectively reduce the instant blood-mediated inflammatory response, and regulators of complement activation molecules such as factor H that reduce the amount of C3b and iC3b (components of the complement system) deposited on a biomaterial surface and activation of complement on contact with blood (114). The geometry and diffusion properties of encapsulation devices are also critical for optimizing encapsulated tissue oxygenation. Polypropylene mesh was used by Dang et al. (115) to produce alginate capsules of varying shapes and sizes in contrast to the strictly spherical capsules produced using the traditional electrostatic droplet generator. This new method also facilitates surface modification of the capsules to manipulate their charge and orientation on transplantation.
VI. Successes, Failures, and Issues with Transplanted Immunoisolated Cells
A. Small animal models
It is clear that successful transplantation of encapsulated islet cells has been easier to achieve in smaller animal models such as mice and rats than in larger animals. This is evidenced by the large number of publications relating to the former and a dearth of publications describing the latter (Table 2). As an example of very early success in rodents, in 1980, Lim and Sun (58) succeeded in reversing diabetes for almost 3 wk in an allogeneic rat islet transplant model using alginate capsules. In 2001, Duvivier-Kali et al. (67) reported on the use of simple barium alginate capsules to reverse diabetes for up to 350 d in syngeneic and allogeneic BALB/c and NOD mice. These capsules had very little permselectivity and therefore could not have acted as an efficient barrier to Ig or cytokines, but could have acted as a barrier to cells. However, immune rejection of syngeneic and allogeneic tissue is largely dependent on direct contact of T cells, and this simple capsule proved to be a sufficient barrier to protect transplanted islets in this setting. This is probably true for all cellular components of all of the immune responses that need to be considered, allogeneic, autogenic, and xenogeneic. Antibody mediators of the humoral immune response may be more of a problem. This level of permselectivity is not sufficient to protect against indirect contact, escaped donor tissue debris presented to host T cells by antigen-presenting cells, and the subsequent up-regulation of mediators of the humoral immune response. This pathway becomes an issue particularly when donor tissue is necrotic and larger amounts of antigenic debris are shed. The low level of permselectivity provided by simple alginate capsules is more likely to be sufficient if the donor tissue remains healthy, especially in lower animals, which have a less discriminating immune system compared with higher animals. Preformed IgM antibodies that recognize the Gal epitope expressed on the surface of endothelial cells contribute to the hyperacute rejection of whole organ xenografts. Xenogeneic islet cell transplants, in particular adult pig islet cells, have little Gal epitope (116), so they are not as prone to hyperacute rejection. Rejection secondary to a cell-mediated response does occur in unprotected xenografts of islets or cells, but Omer et al. (117) had success using simple barium alginate capsules for transplantation of porcine neonatal cell clusters to immunocompetent diabetic mice, and long-term protection was also provided to adult porcine islets (66, 118).
Table 2.
Transplantation sites used in clinical and preclinical studies
| Site | Advantages | Disadvantages | References |
|---|---|---|---|
| Peritoneal cavity | No limitation on space, minimally invasive transplantation | Lack of association with vascular network, pooling and clumping in gravity-dependent areas | 56, 58, 63–65, 68, 72, 75, 77–79, 115–120, 127, 128, 136 |
| Intraportal vein | Delivery of insulin physiologically to the portal circulation | Increased risk of complications during transplantation, limitation of space, possible toxic effect on liver and steatosis | 1, 4–8, 10, 11, 31, 32, 36, 37 44–46, 48 |
| Kidney capsule | Association with a vascular network | Limited space | 9, 33, 35, 39, 93, 96, 110, 129, 143 |
| Subcutaneous tissue/epididymal fat pad | Proximity to vasculature, ease of retrieval | Large surface area may be required, scarring | 53, 54, 57, 58, 93, 96, 99, 104–111, 122, 123–125 |
| Omentum | Large amount of space, proximity to vasculature and portal system | Invasive transplant procedure | 9, 54, 130, 131 |
| Arteriovenous | Close as possible to blood supply for oxygen supply and insulin delivery | High risk of bleeding or thrombosis | 55, 121, 122 |
| Muscle | Proximity to rich blood supply | Limited space | 132 |
| Intraocular | Easy access, ease of monitoring graft status | Not practical for clinical use | 52 |
B. Large animals and humans
An especially difficult challenge has been to achieve success in allogeneic or xenogeneic transplants to large animals such as nonhuman primates. In 1993, Soon-Shiong et al. (119) reported cure of a diabetic dog for 6 months with encapsulated syngeneic islets. This was followed by report of a patient transplanted ip with encapsulated allogeneic islets with a claim of insulin independence lasting 9 months (120). There is general skepticism about the validity of the data provided in this paper. Another controversial paper was from Sun et al. (121), who reported transplants of encapsulated adult pig islets to cynomolgus monkeys with reversal of diabetes lasting in one case 804 d. This result has not been reproduced; however, Elliott et al. (122) managed to reduce insulin requirements for 6 months with encapsulated neonatal porcine islet cell clusters in cynomolgus monkey recipients. Greater success was achieved by Monaco et al. (123) with the use of a hybrid artificial pancreas (Fig. 1B), following on from the vascularized bioartificial pancreas concept developed by Chick et al. (57) in the 1970s. A hollow fiber membrane device was anastomosed to the common iliac artery and vein in dogs, with an acrylic housing containing islets separated from the circulation only by the semipermeable membrane. Insulin requirements were reduced by 50% for up to 284 d in the allogeneic recipients and up to 106 d in a smaller number of the xenogeneic recipients (124). Follow-on studies using modified devices to increase both the hollow fiber membrane length and size of the islet chamber gave better results in porcine to canine transplants (123). Between 114,000 and 341,000 IE was sufficient to treat pancreatectomized dogs along with just 4–16 IU insulin per day for 57–366 d in half of the recipients. Thrombosis was the cause of failure in most recipients, and there was an absence of a cellular infiltrate on histological analysis, suggesting that adequate immune protection was provided by the semipermeable membrane. The use of warfarin rather than aspirin was suggested as a means of reducing the thrombosis rate, but the associated risks of thrombosis or hemorrhage are barriers to a clinical trial of such a device.
Recently, Wang et al. (73) reported reversal of diabetes in a dog model using encapsulated allogeneic islets. David Scharp of Novocell Inc. demonstrated protection of a small number of human islets in hollow fiber macrodevices transplanted sc in nine normal, type 1 and type 2 diabetic recipients, from both allogeneic and autoimmune attack; greater than 90% of the islets remained viable after 2 wk (125). The thin coating facilitated transplantation of the large number of islets needed. In 2006, Calafiore et al. (127) reported results from two patients in a clinical trial of allogeneic islets encapsulated in alginate with a poly-l-ornithine coating, transplanted ip, providing evidence for reduced insulin requirement and fewer incidents of severe hypoglycemia. Simple barium alginate-encapsulated islets have also been shown to be safe in a small clinical trial, although prolonged detection of C-peptide was evident in only one of four patients with diabetes transplanted with the capsules ip (128). Elliott et al. (129) reported a case of xenotransplantation of encapsulated porcine islets to a 41-yr-old male resulting in reduced insulin requirements and detectable porcine C-peptide up to 11 months later. On retrieval of the capsules 9.5 yr later, fluorescence staining indicated the presence of live islet cells (129).
C. Transplantation site
The choice of transplantation site is a very important issue. In clinical practice, naked islets are transplanted into the liver via the portal vein. A potential advantage is that this allows delivery of insulin to the portal circulation as occurs with normal physiology. The portal vein is accessed under radiological guidance or with a laparotomy. Complications include portal vein thrombosis and hemorrhage, but these now occur infrequently. For transplantation of encapsulated islets, the ip space has been used most often. There is less restriction on the volume of material that can be transplanted compared with the intraportal site, and the procedure is even less invasive. Disadvantages include lack of close contact with a vascular network; capsules typically float in the cavity or adhere to the omentum. Moreover, there is likely to be difficulty retrieving the capsules if needed. Another concern is clumping of the capsules on the pelvic floor, a gravity-dependent effect in biped humans and nonhuman primates.
Other sites that have been used are the sc site and the kidney subcapsular space. Clearly, space is limited in the kidney capsule, although success has been reported by Dufrane et al. (130) in the form of detectable porcine C-peptide after 60 d in two of seven cynomolgus monkeys that received 15,000 IE/kg of porcine islets encapsulated in simple alginate capsules under the kidney capsule. However, these results are yet to be reproduced. Alternative sites for transplantation have been suggested, such as an omental pouch. An omental pouch created surgically has the advantage of unlimited space for encapsulated islets, relatively easier retrievability of the transplanted tissue, delivery of insulin to the portal circulation, and improved proximity to vasculature for both oxygen and nutrient supply and insulin release. Studies of both encapsulated rat islets (131) and unencapsulated nonhuman primate islets (132) on a biodegradable scaffold transplanted to an omental pouch have shown reversal of diabetes to be at least as effective as transplants to the kidney capsule and intrahepatic sites. The failure to demonstrate superiority to the more established transplant sites is disappointing, but the omental pouch warrants further evaluation. A novel site recently reported to show success is the skeletal muscle (133).
D. Kinetics of insulin release
An issue for any encapsulation system in any location is the kinetics of insulin release into the circulation. This question has received very little study. Islets in the pancreas are richly vascularized with fenestrated capillaries, and β-cells can turn insulin secretion on and off within seconds, which means that insulin secretion into the portal vein can be very dynamic to accommodate the demands of meals and exercise. There is no way that increases of secretion can be delivered as rapidly as insulin diffuses through the hydrogel or some other membrane to reach the circulation. Certainly there is a time lag before insulin secreted into the peritoneal space is absorbed to the venous system (134). The situation may be even more serious when there is a need to shut off secretion after meals or with exercise. It may be that the individuals with encapsulated islets will need to take precautions to prevent hypoglycemia (135).
VII. Efforts Aimed at Improving Longevity of Transplanted Immunoisolated Tissue
A. Aggregates: why they work better than whole islets
Isolated islets, removed from their normal blood supply, face a fundamental problem with oxygen delivery. The proportion of islets that are destroyed shortly after infusion into the recipient has been estimated to be as great as 50–80% (9). Various imaging modalities have been developed to help quantify this loss (136). Much of this early loss is thought to be due to ischemia of islets before revascularization could occur (9, 33).
The use of islet cell aggregates of controlled size can be predicted to provide better transplant outcomes for encapsulated islet cell transplantation (137). When designed to be smaller than whole islets, they are more easily provided with oxygen and nutrients, giving them a greater chance at long-term viability. The advantages of islet cell aggregates over whole islets in low-oxygen conditions in terms of viability and function were first studied by Dionne (138), who exposed islets and aggregates to solutions with reduced oxygen concentration to determine differences of insulin secretion. It was found that the oxygen tension at which insulin secretion was reduced was much lower with small cell aggregates of about 45 μm in diameter than with intact islets. The same group went on to show with mathematical modeling that the viability and function of aggregates in microcapsules would remain much greater at low oxygen tensions (such as that of the transplant environment) compared with whole islets in microcapsules (137). These results were reproduced experimentally comparing encapsulated islet cell aggregates (40–50 μm in diameter) in vitro and in vivo to encapsulated whole islets (100–200 μm in diameter), demonstrating their superior viability and function in low-oxygen environments (139). We examined the use of islet cell aggregates smaller than whole islets in capsules as a means of overcoming this oxygen diffusion limitation (Fig. 2). Oxygen consumption rates, insulin to DNA ratios, and insulin secretion were used as measures of the viability and function of encapsulated islets and islet cell aggregates after culture in low-oxygen conditions. Each measure proved superiority for the encapsulated islet cell aggregates compared with the islets in their ability to withstand the hypoxic conditions that are present in the transplant setting. We also found that transplanted encapsulated islet cell aggregates fared better than encapsulated islets in terms of histological evidence of necrosis on retrieval and cure rates in diabetic recipients. A concordant xenogeneic rat to mouse model was used for these experiments, but it is likely the advantages demonstrated are applicable to all islet transplant situations requiring encapsulation.
Fig. 2.

A, An encapsulated islet shows an area of central necrosis; B, encapsulated aggregates are stained a darker color indicating no or minimal necrosis.
B. Cell composition: are non-β-cells necessary?
The structure of islets and the relationship between β- and non-β-cells within an islet have been studied extensively over the years. Several groups have shown that clusters of β-cells secrete insulin more effectively compared with single cells, indicating that some communication between the cells should be preserved in transplant situations (140–144). Studies of the microvasculature and physiology of islets have shown that non-β-cells are downstream from β-cells; that is, the inner core of β-cells is supplied first and non-β-cells thereafter (145, 146). This would imply that glucagon and somatostatin produced by non-β-cells have a limited influence on the function of β-cells. Indeed, when aggregates composed of almost entirely β-cells with less than 5% non-β-cells were transplanted under the kidney capsule, cure rates were comparable to recipients receiving a corresponding volume of whole islets, indicating that the presence of a normal number of non-β-cells is not necessary for successful transplantation; indeed, they may not be necessary at all (144). These findings support the use of islet cell aggregates that may differ in terms of cell composition and structure for transplantation, which will be an important consideration especially when new sources of β-cells or β-like cells become available for transplantation. However, these preclinical studies cannot be interpreted as indicative of outcomes in the clinical setting. Food intake and exercise behaviors are clearly quite different in humans compared with rodents. The possibility of hypoglycemia in the absence of functioning α-cells is of concern. In clinical nonencapsulated islet transplantation, there is a reduced incidence of hypoglycemia. Restoration of the glucagon secretion from α-cells in response to hypoglycemia has been demonstrated (147) and may play a significant role in this reduction in hypoglycemia.
C. Proangiogenic and other factors
Encapsulated islets cannot become directly revascularized, but efforts have been aimed at encouraging revascularization on the outer coating of the devices with the use of proangiogenic factors such as VEGF. This has been tried in a planar membrane diffusion device (111) and a stainless steel mesh (109), both devices that were transplanted sc. PEG modified with IL-1 receptor inhibitor peptide was found to provide superior protection to MIN6 cells when exposed to activated cytotoxic T cells, IL-1, and TNF-α (148). Another group showed higher viability when mouse islets were encapsulated in GLP-1 C-immobilized PEG. GLP-1 C is an analog of GLP-1 (85). GLP-1 analogs have also been incorporated into alginate capsules but with more chemical modification of GLP-1, resulting in lower available concentrations of bioactive GLP-1 (149). GLP-1 has been used to treat nonimmunoisolated islets before transplantation (38, 150) and has been administered to recipients after transplant with some success (126).
VIII. Summary
Transplantation of encapsulated islets has the potential to restore glucose homeostasis in diabetes without the need for immunosuppression. But several major obstacles need to be overcome first: the development of a renewable source of β-cells, reduced activation of a destructive host immune response, and improved survival of β-cells when transplanted.
There are extensive research efforts focused on these challenges and many promising results as presented here. Once identified and combined, the optimal β-like cell, biomaterial, or device, with or without proangiogenic, antiimmune, or antiapoptotic molecules, will hopefully provide a clinically useful means of β-cell replacement. In parallel, many groups are working on other therapeutic options such as a mechanical closed loop, employing continuous glucose monitoring coupled with insulin pumps. Only time will tell how and when these new therapies unfold, but the outcome will be reduced morbidity and mortality and the potential for a more normal lifestyle for the countless people diagnosed with diabetes every year.
Acknowledgments
This work was supported by Grant RO1 DK 50657 from the National Institutes of Health, the Juvenile Diabetes Research Foundation, and the Diabetes Research and Wellness Foundation. E.S.O. was the recipient of a traveling studentship from the National University of Ireland.
Disclosure Summary: GCW has equity interests in and is on the Scientific Advisory Board of Beta O2 of Israel. ESO, AV, and EGA have nothing to disclose.
Footnotes
- G
- Guluronic acid
- GLP-1
- glucagon-like peptide 1
- hESC
- human embryonic stem cells
- M
- mannuronic acid
- PEG
- polyethylene glycol
- PLL
- poly-l-lysine
- PTFE
- polytetrafluoroethylene
- PVA
- polyvinyl alcohol
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- VEGF
- vascular endothelial growth factor.
References
- 1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 2. Tharavanij T, Betancourt A, Messinger S, Cure P, Leitao CB, Baidal DA, Froud T, Ricordi C, Alejandro R. 2008. Improved long-term health-related quality of life after islet transplantation. Transplantation 86:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn SE, Zraika S, Utzschneider KM, Hull RL. 2009. The β-cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scharp D, Lacy P, Ricordi C, Boyle P, Santiago J, Cryer P, Gingerick R, Jaffe A, Anderson C, Flye W. 1989. Human islet transplantation in patients with type I diabetes. Transplant Proc 21:2744–2745 [PubMed] [Google Scholar]
- 5. Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. 2005. Five-year follow-up after clinical islet transplantation. Diabetes 54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 6. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. 2006. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 7. Frank A, Deng S, Huang X, Velidedeoglu E, Bae YS, Liu C, Abt P, Stephenson R, Mohiuddin M, Thambipillai T, Markmann E, Palanjian M, Sellers M, Naji A, Barker CF, Markmann JF. 2004. Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg 240:631–640; discussion 640–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritz-Laser B, Oberholzer J, Toso C, Brulhart MC, Zakrzewska K, Ris F, Bucher P, Morel P, Philippe J. 2002. Molecular detection of circulating β-cells after islet transplantation. Diabetes 51:557–561 [DOI] [PubMed] [Google Scholar]
- 9. Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. 1995. A selective decrease in the β-cell mass of human islets transplanted into diabetic nude mice. Transplantation 59:817–820 [PubMed] [Google Scholar]
- 10. Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, Freeman ML, Beilman GJ, Sutherland DE. 2007. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 87:1477–1501, x [DOI] [PubMed] [Google Scholar]
- 11. Alejandro R, Barton FB, Hering BJ, Wease S. 2008. Update from the Collaborative Islet Transplant Registry. Transplantation 86:1783–1788 [DOI] [PubMed] [Google Scholar]
- 12. Bonner-Weir S, Weir GC. 2005. New sources of pancreatic β-cells. Nat Biotechnol 23:857–861 [DOI] [PubMed] [Google Scholar]
- 13. D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 14. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- 15. Matveyenko AV, Veldhuis JD, Butler PC. 2008. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and β-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab 295:E832–E841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Assche FA, Aerts L, De Prins F. 1978. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 85:818–820 [DOI] [PubMed] [Google Scholar]
- 17. Sorenson RL, Brelje TC. 1997. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29:301–307 [DOI] [PubMed] [Google Scholar]
- 18. de Koning EJ, Bodkin NL, Hansen BC, Clark A. 1993. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in β-cell population. Diabetologia 36:378–384 [DOI] [PubMed] [Google Scholar]
- 19. Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. 2010. Adaptive changes in pancreatic β-cell fractional area and β-cell turnover in human pregnancy. Diabetologia 53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. 2009. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5:258–265 [DOI] [PubMed] [Google Scholar]
- 21. Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. 2000. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 97:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. 2009. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem 284:33509–33520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. 2009. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol 201:37–47 [DOI] [PubMed] [Google Scholar]
- 24. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. 2008. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. d'Apice AJ, Cowan PJ. 2008. Gene-modified pigs. Xenotransplantation 15:87–90 [DOI] [PubMed] [Google Scholar]
- 26. Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. 2007. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318:1920–1923 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 28. Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. 2008. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 283:31601–31607 [DOI] [PubMed] [Google Scholar]
- 29. Halban PA, German MS, Kahn SE, Weir GC. 2010. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab 95:1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. 2007. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 14:288–297 [DOI] [PubMed] [Google Scholar]
- 31. Harlan DM, Kenyon NS, Korsgren O, Roep BO. 2009. Current advances and travails in islet transplantation. Diabetes 58:2175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bellin MD, Sutherland DE, Beilman GJ, Hong-McAtee I, Balamurugan AN, Hering BJ, Moran A. 2011. Similar islet function in islet allotransplant and autotransplant recipients, despite lower islet mass in autotransplants. Transplantation 91:367–372 [DOI] [PubMed] [Google Scholar]
- 33. Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. 2002. β-Cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51:66–72 [DOI] [PubMed] [Google Scholar]
- 34. Montaña E, Bonner-Weir S, Weir GC. 1993. β-Cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. 2010. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci USA 107:4305–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jaeger C, Brendel MD, Hering BJ, Eckhard M, Bretzel RG. 1997. Progressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allografts. Diabetes 46:1907–1910 [DOI] [PubMed] [Google Scholar]
- 37. Hilbrands R, Huurman VA, Gillard P, Velthuis JH, De Waele M, Mathieu C, Kaufman L, Pipeleers-Marichal M, Ling Z, Movahedi B, Jacobs-Tulleneers-Thevissen D, Monbaliu D, Ysebaert D, Gorus FK, Roep BO, Pipeleers DG, Keymeulen B. 2009. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes 58:2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toso C, McCall M, Emamaullee J, Merani S, Davis J, Edgar R, Pawlick R, Kin T, Knudsen LB, Shapiro AM. 2010. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int 23:259–265 [DOI] [PubMed] [Google Scholar]
- 39. Mineo D, Sageshima J, Burke GW, Ricordi C. 2009. Minimization and withdrawal of steroids in pancreas and islet transplantation. Transpl Int 22:20–37 [DOI] [PubMed] [Google Scholar]
- 40. Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH. 2006. Sirolimus is associated with reduced islet engraftment and impaired β-cell function. Diabetes 55:2429–2436 [DOI] [PubMed] [Google Scholar]
- 41. Zahr E, Molano RD, Pileggi A, Ichii H, Jose SS, Bocca N, An W, Gonzalez-Quintana J, Fraker C, Ricordi C, Inverardi L. 2007. Rapamycin impairs in vivo proliferation of islet β-cells. Transplantation 84:1576–1583 [DOI] [PubMed] [Google Scholar]
- 42. Nir T, Melton DA, Dor Y. 2007. Recovery from diabetes in mice by β-cell regeneration. J Clin Invest 117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao R, Ustinov J, Korsgren O, Otonkoski T. 2007. Effects of immunosuppressive drugs on in vitro neogenesis of human islets: mycophenolate mofetil inhibits the proliferation of ductal cells. Am J Transplant 7:1021–1026 [DOI] [PubMed] [Google Scholar]
- 44. Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G. 2008. mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57:945–957 [DOI] [PubMed] [Google Scholar]
- 45. Lakey JR, Kin T, Warnock GL, Shapiro AM, Tsapogas P, Imes S, Korbutt GS, Kneteman NM, Rajotte RV, Ryan EA. 2007. Long-term graft function after allogeneic islet transplantation. Cell Transplant 16:441–446 [PubMed] [Google Scholar]
- 46. Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. 2005. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 293:830–835 [DOI] [PubMed] [Google Scholar]
- 47. Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R. 2005. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 5:2037–2046 [DOI] [PubMed] [Google Scholar]
- 48. Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN, Papas KK, Papas K, Sutherland DE, Moran A, Hering BJ. 2008. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant 8:2463–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fiorina P, Shapiro AM, Ricordi C, Secchi A. 2008. The clinical impact of islet transplantation. Am J Transplant 8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 50. Lynch RJ, Platt JL. 2009. Escaping from rejection. Transplantation 88:1233–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daneman D. 2002. Islet cell transplantation and other new technologies for treating type 1 diabetes: a paediatric view. Horm Res 57(Suppl 1): 54–59 [DOI] [PubMed] [Google Scholar]
- 52. Hathout E, Lakey J, Shapiro J. 2003. Islet transplant: an option for childhood diabetes? Arch Dis Child 88:591–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang TM. 1964. Semipermeable microcapsules. Science 146:524–525 [DOI] [PubMed] [Google Scholar]
- 54. Browning H, Resnik P. 1951. Homologous and heterologous transplantation of pancreatic tissue in normal and diabetic mice. Yale J Biol Med 24:140–152 [PMC free article] [PubMed] [Google Scholar]
- 55. House EL, Burton C, Cooper H, Anderson E. 1958. The implantation of neonatal pancreas into the cheek pouch of the alloxan diabetic hamster. Endocrinology 63:389–391 [DOI] [PubMed] [Google Scholar]
- 56. Ferguson J, Scothorne RJ, Johnston ID. 1973. Proceedings: the survival of transplanted isolated pancreatic islets in the omentum and testis. Br J Surg 60:907. [PubMed] [Google Scholar]
- 57. Chick WL, Perna JJ, Lauris V, Low D, Galletti PM, Panol G, Whittemore AD, Like AA, Colton CK, Lysaght MJ. 1977. Artificial pancreas using living β-cells: effects on glucose homeostasis in diabetic rats. Science 197:780–682 [DOI] [PubMed] [Google Scholar]
- 58. Lim F, Sun AM. 1980. Microencapsulated islets as bioartificial endocrine pancreas. Science 210:908–910 [DOI] [PubMed] [Google Scholar]
- 59. Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE. 1991. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science 254:1782–1784 [DOI] [PubMed] [Google Scholar]
- 60. Colton CK. 1995. Implantable biohybrid artificial organs. Cell Transplant 4:415–436 [DOI] [PubMed] [Google Scholar]
- 61. Martinsen A, Skjåk-Braek G, Smidsrød O. 1989. Alginate as immobilization material. I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol Bioeng 33:79–89 [DOI] [PubMed] [Google Scholar]
- 62. De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. 1997. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 40:262–270 [DOI] [PubMed] [Google Scholar]
- 63. de Vos P, Bucko M, Gemeiner P, Navrátil M, Svitel J, Faas M, Strand BL, Skjak-Braek G, Morch YA, Vikartovská A, Lacík I, Kolláriková G, Orive G, Poncelet D, Pedraz JL, Ansorge-Schumacher MB. 2009. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 30:2559–2570 [DOI] [PubMed] [Google Scholar]
- 64. Mørch YA, Donati I, Strand BL, Skjåk-Braek G. 2006. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7:1471–1480 [DOI] [PubMed] [Google Scholar]
- 65. Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. 2005. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 79:52–58 [DOI] [PubMed] [Google Scholar]
- 66. Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ. 2008. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-l-lysine microcapsules versus barium alginate microcapsules without poly-l-lysine. J Diabetes Sci Technol 2:760–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Duvivier-Kali VF, Omer A, Parent RJ, O'Neil JJ, Weir GC. 2001. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 50:1698–1705 [DOI] [PubMed] [Google Scholar]
- 68. Strand BL, Ryan TL, In't Veld P, Kulseng B, Rokstad AM, Skjak-Brek G, Espevik T. 2001. Poly-l-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant 10:263–275 [PubMed] [Google Scholar]
- 69. de Vos P, de Haan BJ, Kamps JA, Faas MM, Kitano T. 2007. ζ-Potentials of alginate-PLL capsules: a predictive measure for biocompatibility? J Biomed Mater Res A 80:813–819 [DOI] [PubMed] [Google Scholar]
- 70. Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Bufalari A, Cassarani MP, Giustozzi GM, Brunetti P. 1999. Transplantation of pancreatic islets contained in minimal volume microcapsules in diabetic high mammalians. Ann NY Acad Sci 875:219–232 [DOI] [PubMed] [Google Scholar]
- 71. Calafiore R, Basta G, Luca G, Lemmi A, Racanicchi L, Mancuso F, Montanucci MP, Brunetti P. 2006. Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transplant Proc 38:1156–1157 [DOI] [PubMed] [Google Scholar]
- 72. Loudovaris T, Jacobs S, Young S, Maryanov D, Brauker J, Johnson RC. 1999. Correction of diabetic nod mice with insulinomas implanted within Baxter immunoisolation devices. J Mol Med 77:219–222 [DOI] [PubMed] [Google Scholar]
- 73. Wang T, Adcock J, Kühtreiber W, Qiang D, Salleng KJ, Trenary I, Williams P. 2008. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 85:331–337 [DOI] [PubMed] [Google Scholar]
- 74. Wang T, Lacík I, Brissová M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC. 1997. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol 15:358–362 [DOI] [PubMed] [Google Scholar]
- 75. Renken A, Hunkeler D. 2007. Polyvinylamine-based capsules: a mechanistic study of the formation using alginate and cellulose sulphate. J Microencapsul 24:323–336 [DOI] [PubMed] [Google Scholar]
- 76. Schneider S, Feilen PJ, Slotty V, Kampfner D, Preuss S, Berger S, Beyer J, Pommersheim R. 2001. Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials 22:1961–1970 [DOI] [PubMed] [Google Scholar]
- 77. Wong H, Chang TM. 1991. A novel two step procedure for immobilizing living cells in microcapsules for improving xenograft survival. Biomater Artif Cells Immobilization Biotechnol 19:687–697 [DOI] [PubMed] [Google Scholar]
- 78. Iwata H, Kobayashi K, Takagi T, Oka T, Yang H, Amemiya H, Tsuji T, Ito F. 1994. Feasibility of agarose microbeads with xenogeneic islets as a bioartificial pancreas. J Biomed Mater Res 28:1003–1011 [DOI] [PubMed] [Google Scholar]
- 79. Gazda LS, Vinerean HV, Laramore MA, Diehl CH, Hall RD, Rubin AL, Smith BH. 2007. Encapsulation of porcine islets permits extended culture time and insulin independence in spontaneously diabetic BB rats. Cell Transplant 16:609–620 [DOI] [PubMed] [Google Scholar]
- 80. Iwata H, Takagi T, Kobayashi K, Oka T, Tsuji T, Ito F. 1994. Strategy for developing microbeads applicable to islet xenotransplantation into a spontaneous diabetic NOD mouse. J Biomed Mater Res 28:1201–1207 [DOI] [PubMed] [Google Scholar]
- 81. Kin T, Iwata H, Aomatsu Y, Ohyama T, Kanehiro H, Hisanaga M, Nakajima Y. 2002. Xenotransplantation of pig islets in diabetic dogs with use of a microcapsule composed of agarose and polystyrene sulfonic acid mixed gel. Pancreas 25:94–100 [DOI] [PubMed] [Google Scholar]
- 82. Nishigaki T, Teramura Y, Suemori H, Iwata H. 2010. Cryopreservation of primate embryonic stem cells with chemically-defined solution without Me2SO. Cryobiology 60:159–164 [DOI] [PubMed] [Google Scholar]
- 83. Shen F, Li AA, Cornelius RM, Cirone P, Childs RF, Brash JL, Chang PL. 2005. Biological properties of photocrosslinked alginate microcapsules. J Biomed Mater Res B Appl Biomater 75:425–434 [DOI] [PubMed] [Google Scholar]
- 84. Cruise GM, Scharp DS, Hubbell JA. 1998. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 19:1287–1294 [DOI] [PubMed] [Google Scholar]
- 85. Lin CC, Anseth KS. 2009. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic β-cells. Biomacromolecules 10:2460–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee DY, Nam JH, Byun Y. 2004. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J Biomater Sci Polym Ed 15:753–766 [DOI] [PubMed] [Google Scholar]
- 87. Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, Hegre OD, Scharp DW. 1997. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann NY Acad Sci 831:332–343 [DOI] [PubMed] [Google Scholar]
- 88. Krol S, del Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. 2006. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett 6:1933–1939 [DOI] [PubMed] [Google Scholar]
- 89. Wilson JT, Cui W, Chaikof EL. 2008. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett 8:1940–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kizilel S, Scavone A, Liu X, Nothias JM, Ostrega D, Witkowski P, Millis M. 2010. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A 16:2217–2228 [DOI] [PubMed] [Google Scholar]
- 91. Teramura Y, Kaneda Y, Iwata H. 2007. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials 28:4818–4825 [DOI] [PubMed] [Google Scholar]
- 92. Teramura Y, Iwata H. 2009. Islet encapsulation with living cells for improvement of biocompatibility. Biomaterials 30:2270–2275 [DOI] [PubMed] [Google Scholar]
- 93. Teramura Y, Minh LN, Kawamoto T, Iwata H. 2010. Microencapsulation of islets with living cells using PolyDNA-PEG-lipid conjugate. Bioconjug Chem 21:792–796 [DOI] [PubMed] [Google Scholar]
- 94. Khor E, Lim LY. 2003. Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349 [DOI] [PubMed] [Google Scholar]
- 95. Yang KC, Qi Z, Wu CC, Shirouza Y, Lin FH, Yanai G, Sumi S. 2010. The cytoprotection of chitosan based hydrogels in xenogeneic islet transplantation: an in vivo study in streptozotocin-induced diabetic mouse. Biochem Biophys Res Commun 393:818–823 [DOI] [PubMed] [Google Scholar]
- 96. Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, Zhang M, Yao K. 2002. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 23:3227–3234 [DOI] [PubMed] [Google Scholar]
- 97. Chung TW, Yang J, Akaike T, Cho KY, Nah JW, Kim SI, Cho CS. 2002. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 23:2827–2834 [DOI] [PubMed] [Google Scholar]
- 98. Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, Korbutt GS. 2005. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng 11:1323–1331 [DOI] [PubMed] [Google Scholar]
- 99. Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. 2008. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A 14:433–440 [DOI] [PubMed] [Google Scholar]
- 100. Dautzenberg H, Schuldt U, Grasnick G, Karle P, Müller P, Löhr M, Pelegrin M, Piechaczyk M, Rombs KV, Günzburg WH, Salmons B, Saller RM. 1999. Development of cellulose sulfate-based polyelectrolyte complex microcapsules for medical applications. Ann NY Acad Sci 875:46–63 [DOI] [PubMed] [Google Scholar]
- 101. Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, Knöfel WT, Liebe S, Müller P, Nizze H, Renner M, Saller RM, Wagner T, Hauenstein K, Günzburg WH, Salmons B. 2001. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet 357:1591–1592 [DOI] [PubMed] [Google Scholar]
- 102. Stadlbauer V, Stiegler PB, Schaffellner S, Hauser O, Halwachs G, Iberer F, Tscheliessnigg KH, Lackner C. 2006. Morphological and functional characterization of a pancreatic β-cell line microencapsulated in sodium cellulose sulfate/poly(diallyldimethylammonium chloride). Xenotransplantation 13:337–344 [DOI] [PubMed] [Google Scholar]
- 103. Boninsegna S, Bosetti P, Carturan G, Dellagiacoma G, Dal Monte R, Rossi M. 2003. Encapsulation of individual pancreatic islets by sol-gel SiO2: a novel procedure for perspective cellular grafts. J Biotechnol 100:277–286 [DOI] [PubMed] [Google Scholar]
- 104. Kim YY, Chae SY, Kim S, Byun Y, Bae YH. 2005. Improved phenotype of rat islets in a macrocapsule by co-encapsulation with cross-linked Hb. J Biomater Sci Polym Ed 16:1521–1535 [DOI] [PubMed] [Google Scholar]
- 105. Isayeva IS, Kasibhatla BT, Rosenthal KS, Kennedy JP. 2003. Characterization and performance of membranes designed for macroencapsulation/implantation of pancreatic islet cells. Biomaterials 24:3483–3491 [DOI] [PubMed] [Google Scholar]
- 106. Suzuki K, Bonner-Weir S, Hollister-Lock J, Colton CK, Weir GC. 1998. Number and volume of islets transplanted in immunobarrier devices. Cell Transplant 7:47–52 [DOI] [PubMed] [Google Scholar]
- 107. Check E. 2002. Diabetes trial stirs debate on safety of xenotransplants. Nature 419:5. [DOI] [PubMed] [Google Scholar]
- 108. Juang JH, Bonner-Weir S, Ogawa Y, Vacanti JP, Weir GC. 1996. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 61:1557–1561 [DOI] [PubMed] [Google Scholar]
- 109. Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. 2006. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 81:1318–1324 [DOI] [PubMed] [Google Scholar]
- 110. Suzuki K, Bonner-Weir S, Trivedi N, Yoon KH, Hollister-Lock J, Colton CK, Weir GC. 1998. Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation 66:21–28 [DOI] [PubMed] [Google Scholar]
- 111. Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC. 2000. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant 9:115–124 [DOI] [PubMed] [Google Scholar]
- 112. Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. 2009. Human β-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation 87:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lembert N, Wesche J, Petersen P, Doser M, Zschocke P, Becker HD, Ammon HP. 2005. Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant 14:97–108 [PubMed] [Google Scholar]
- 114. Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. 2010. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol 31:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dang TT, Xu Q, Bratlie KM, O'Sullivan ES, Chen XY, Langer R, Anderson DG. 2009. Microfabrication of homogenous, asymmetric cell-laden hydrogel capsules. Biomaterials 30:6896–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McKenzie IF, Xing PX, Vaughan HA, Prenzoska J, Dabkowski PL, Sandrin MS. 1994. Distribution of the major xenoantigen (gal (α1–3)gal) for pig to human xenografts. Transpl Immunol 2:81–86 [DOI] [PubMed] [Google Scholar]
- 117. Omer A, Duvivier-Kali VF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC. 2003. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes 52:69–75 [DOI] [PubMed] [Google Scholar]
- 118. Duvivier-Kali VF, Omer A, Lopez-Avalos MD, O'Neil JJ, Weir GC. 2004. Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am J Transplant 4:1991–2000 [DOI] [PubMed] [Google Scholar]
- 119. Soon-Shiong P, Feldman E, Nelson R, Heintz R, Yao Q, Yao Z, Zheng T, Merideth N, Skjak-Braek G, Espevik T. 1993. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci USA 90:5843–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M. 1994. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343:950–951 [DOI] [PubMed] [Google Scholar]
- 121. Sun Y, Ma X, Zhou D, Vacek I, Sun AM. 1996. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest 98:1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, Vasconcellos AV, Emerich DF, Thanos C, Bambra C. 2005. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc 37:3505–3508 [DOI] [PubMed] [Google Scholar]
- 123. Maki T, Otsu I, O'Neil JJ, Dunleavy K, Mullon CJ, Solomon BA, Monaco AP. 1996. Treatment of diabetes by xenogeneic islets without immunosuppression. Use of a vascularized bioartificial pancreas. Diabetes 45:342–347 [DOI] [PubMed] [Google Scholar]
- 124. Monaco AP, Maki T, Ozato H, Carretta M, Sullivan SJ, Borland KM, Mahoney MD, Chick WL, Muller TE, Wolfrum J. 1991. Transplantation of islet allografts and xenografts in totally pancreatectomized diabetic dogs using the hybrid artificial pancreas. Ann Surg 214:339–360; discussion 361–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Scharp DW, Swanson CJ, Olack BJ, Latta PP, Hegre OD, Doherty EJ, Gentile FT, Flavin KS, Ansara MF, Lacy PE. 1994. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes 43:1167–1170 [DOI] [PubMed] [Google Scholar]
- 126. Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM. 2007. Effect of exenatide on β-cell function after islet transplantation in type 1 diabetes. Transplantation 83:24–28 [DOI] [PubMed] [Google Scholar]
- 127. Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. 2006. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care 29:137–138 [DOI] [PubMed] [Google Scholar]
- 128. Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. 2009. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32:1887–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. 2007. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14:157–161 [DOI] [PubMed] [Google Scholar]
- 130. Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. 2006. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81:1345–1353 [DOI] [PubMed] [Google Scholar]
- 131. Kin T, Korbutt GS, Rajotte RV. 2003. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant 3:281–285 [DOI] [PubMed] [Google Scholar]
- 132. Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. 2009. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 9:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Christoffersson G, Henriksnäs J, Johansson L, Rolny C, Ahlström H, Caballero-Corbalan J, Segersvärd R, Permert J, Korsgren O, Carlsson PO, Phillipson M. 2010. Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets. Diabetes 59:2569–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. De Vos P, Vegter D, De Haan BJ, Strubbe JH, Bruggink JE, Van Schilfgaarde R. 1996. Kinetics of intraperitoneally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes 45:1102–1107 [DOI] [PubMed] [Google Scholar]
- 135. Omer A, Duvivier-Kali VF, Aschenbach W, Tchipashvili V, Goodyear LJ, Weir GC. 2004. Exercise induces hypoglycemia in rats with islet transplantation. Diabetes 53:360–365 [DOI] [PubMed] [Google Scholar]
- 136. Medarova Z, Evgenov NV, Dai G, Bonner-Weir S, Moore A. 2006. In vivo multimodal imaging of transplanted pancreatic islets. Nat Protoc 1:429–435 [DOI] [PubMed] [Google Scholar]
- 137. Lewis AS. 2008. Eliminating oxygen supply limitations for transplanted microencapsulated islets in the treatment of type 1 diabetes. Massachusetts Institute of Technology: Cambridge: p 233 [Google Scholar]
- 138. Dionne KE. 1990. Effect of hypoxia on insulin secretion and viability of pancreatic islet tissue. In: Chemical engineering. Cambridge, MA: Massachusetts Institute of Technology [Google Scholar]
- 139. O'Sullivan ES, Johnson AS, Omer A, Hollister-Lock J, Bonner-Weir S, Colton CK, Weir GC. 2010. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia 53:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chertow BS, Baranetsky NG, Sivitz WI, Meda P, Webb MD, Shih JC. 1983. Cellular mechanisms of insulin release. Effects of retinoids on rat islet cell-to-cell adhesion, reaggregation, and insulin release. Diabetes 32:568–574 [DOI] [PubMed] [Google Scholar]
- 141. Bosco D, Orci L, Meda P. 1989. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res 184:72–80 [DOI] [PubMed] [Google Scholar]
- 142. Pipeleers DG, Schuit FC, Van Schravendijk CF, Van de Winkel M. 1985. Interplay of nutrients and hormones in the regulatin of glucagon release. Endocrinology 117:817–823 [DOI] [PubMed] [Google Scholar]
- 143. Halban PA, Powers SL, George KL, Bonner-Weir S. 1987. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes 36:783–790 [DOI] [PubMed] [Google Scholar]
- 144. King AJ, Fernandes JR, Hollister-Lock J, Nienaber CE, Bonner-Weir S, Weir GC. 2007. Normal relationship of β- and non-β-cells not needed for successful islet transplantation. Diabetes 56:2312–2318 [DOI] [PubMed] [Google Scholar]
- 145. Bonner-Weir S, Orci L. 1982. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883–889 [DOI] [PubMed] [Google Scholar]
- 146. Stagner JI, Samols E, Bonner-Weir S. 1988. β—α—δ pancreatic islet cellular perfusion in dogs. Diabetes 37:1715–1721 [DOI] [PubMed] [Google Scholar]
- 147. Rickels MR, Schutta MH, Mueller R, Markmann JF, Barker CF, Naji A, Teff KL. 2005. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes 54:3205–3211 [DOI] [PubMed] [Google Scholar]
- 148. Su J, Hu BH, Lowe WL, Jr, Kaufman DB, Messersmith PB. 2010. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials 31:308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim S, Wan Kim S, Bae YH. 2005. Synthesis, bioactivity and specificity of glucagon-like peptide-1 (7–37)/polymer conjugate to isolated rat islets. Biomaterials 26:3597–3606 [DOI] [PubMed] [Google Scholar]
- 150. King A, Lock J, Xu G, Bonner-Weir S, Weir GC. 2005. Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia 48:2074–2079 [DOI] [PubMed] [Google Scholar]