Abstract
The chromogranins (chromogranin A and chromogranin B), secretogranins (secretogranin II and secretogranin III), and additional related proteins (7B2, NESP55, proSAAS, and VGF) that together comprise the granin family subserve essential roles in the regulated secretory pathway that is responsible for controlled delivery of peptides, hormones, neurotransmitters, and growth factors. Here we review the structure and function of granins and granin-derived peptides and expansive new genetic evidence, including recent single-nucleotide polymorphism mapping, genomic sequence comparisons, and analysis of transgenic and knockout mice, which together support an important and evolutionarily conserved role for these proteins in large dense-core vesicle biogenesis and regulated secretion. Recent data further indicate that their processed peptides function prominently in metabolic and glucose homeostasis, emotional behavior, pain pathways, and blood pressure modulation, suggesting future utility of granins and granin-derived peptides as novel disease biomarkers.
-
Introduction
Regulated secretion
Secretory granule biogenesis and content
-
Structural Comparison of Granins
Why consider the granins as members of a structurally and functionally related family?
The original granin proteins: CgA and CgB
Additional members of the granin family: SgII, SgIII, 7B2, NESP55, VGF, and proSAAS
-
Sorting and Granulogenesis
Biosynthesis and intracellular trafficking of granins
Mechanisms of granin sorting into regulated secretory pathway granules
Function of granins in dense core secretory granule biogenesis
Regulation of DCG biogenesis by the CgA-derived peptide serpinin
Regulation of intracellular calcium stores by granin proteins in DCG
-
Granin-Derived Peptides and Their Mechanisms of Action in Endocrine and Neuroendocrine Systems
Regulation of glucose balance: CgA peptide pancreastatin
Regulation of feeding and energy expenditure: VGF NERP and C-terminal peptides
Regulation of gastrointestinal function: VGF peptide TLQP-21
Regulation of prohormone convertase activity: 7B2 and proSAAS peptides
Regulation of hormone, neurotrophin, and/or neurotransmitter release: CgA peptide catestatin, SgII peptide secretoneurin, VGF C-terminal, and NERP peptides
Regulation of neural pathways that control pain, emotion, and sexual behavior: VGF- and CgA-derived peptides
Regulation of the immune system: CgA, SgII, and their peptides
Regulation of blood pressure, angiogenesis, and the cardiovascular system: CgA, SgII, and their peptides
-
Genetic Insights into Granin Function
CHGA and CHGB genetic variants (SNP)
Mouse models (transgenic and knockout)
Nonmammalian vertebrate and invertebrate model organisms
-
Granins as Disease Biomarkers
Endocrine and neuroendocrine tumors
Cardiovascular disease and hypertension
Inflammatory disease
Neurodegenerative and neuropsychiatric disease
Perspectives. Granin biomarkers: where do we go from here?
Future Directions: The Search for Receptors of Granin-Derived Peptides
Conclusions
I. Introduction
In this review, we discuss the advantages of considering granins as members of an extended but functionally conserved family, and detail the structure, biological activities, secretory pathway sorting, genetics, and diagnostic and prognostic utility of this unique group of secreted proteins and peptide precursors. Because we broadly review eight granin proteins and their peptides, concentrating on endocrine, neuroendocrine, and neuronal functions, several other areas of interest have not received in-depth coverage. Fortunately, a number of excellent recent reviews provide additional detail on the structures and activities of specific granins and granin-derived peptides; these have been cited throughout our review, and several are summarized in Table 1.
Table 1.
Summary of recent and highly cited reviews on the extended granin family
| Authors | Title | Ref. | Year | Content |
|---|---|---|---|---|
| ISI Web of Science top five cited reviews | ||||
| Winkler H, Fischer-Colbrie R | The Chromogranins A and B: the First 25 Years and Future Perspectives | 9 | 1992 | Commentary review of the first 25 yr after the first identification of CgA |
| Huttner WB, Gerdes HH, Rosa P | The Granin (Chromogranin/ Secretogranin) Family | 369 | 1991 | Review of chromogranins and secretogranins, addresses chemical utility of granin family, sorting, granulogenesis, and biomarker potential |
| Taupenot L, Harper KL, O'Connor DT | The Chromogranin-Secretogranin Family | 43 | 2003 | Review of the extended granin family (chromogranins, secretogranins, NESP55, 7B2, and HISL-19); primarily focuses on molecular and genetic aspects and their biomedical implications |
| Simon JP, Aunis D | Biochemistry of the Chromogranin-A Protein Family | 370 | 1989 | Biochemical properties of CgA and its derived peptides |
| Somogyi P, Hodgson AJ, DePotter RW, Fischer-Colbrie R, Schober M, Winkler H, Chubb IW | Chromogranin Immunoreactivity in the Central Nervous-System | 371 | 1984 | CNS immunoreactivity of CgA and its relation to other peptidergic and monoaminergic pathways |
| Other reviews covering the granin family of proteins | ||||
| Helle KB | The Granin Family of Uniquely Acidic Proteins of the Diffuse Neuroendocrine System: Comparative and Functional Aspects | 44 | 2004 | Review the extended granin family (CgA, CgB, SgII, SgIII, NESP55, 7B2, HISL-19, VGF and proSAAS). Focus on molecular and biochemical aspects and the functional biological role of the major derived peptides |
| Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata SK, Anouar Y | Chromogranins A and B and Secretogranin II: Evolutionary and Functional Aspects | 45 | 2008 | Focus on CgA, CgB and SgII: evolution, chemical properties and functional role as propeptide precursors and granulogenic factors |
| Zhao E, Zhang D, Basak A, Trudeau VL | New Insights into Granin-Derived Peptides: Evolution and Endocrine Roles | 372 | 2009 | Critical evaluation of the evolution of granin protein; focus on distribution and function of vasostatin, CgB1–41 and secretoneurin |
| Bartolomucci A, Pasinetti GM, Salton SR | Granins as Disease-Biomarkers: Translational Potential for Psychiatric and Neurological Disorders | 293 | 2010 | The biomarker potential and functional role in neurological and psychiatric disorders of CgA, CgB, SgII, SgIII, HISL-19, 7B2, NESP55, VGF and proSAAS fragments/peptides |
| Conlon MJ | Granin-Derived Peptides as Diagnostic and Prognostic Markers for Endocrine Tumors | 290 | 2010 | Focus on CgA, CgB, and SgII marker for endocrine tumors |
| Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M | Granins and Granin-Related Peptides in Neuroendocrine Tumours | 295 | 2010 | Focus on CgA, CgB, SgII, SgIII, HISL-19, 7B2, NESP55, VGF, and proSAAS marker for neuroendocrine tumors |
| Proceedings/special issues on the granin proteins | ||||
| Helle KB, Aunis D | Chromogranins: Functional and Clinical Aspects | 373 | 2000 | Proceedings of Session VII of the 10th International Symposium on Chromaffin Cell Biology; published in the book series Advances in Experimental Medicine and Biology, Vol. 482 |
| Hernández-Cruz A, Eiden LE (eds) | The Chromaffin Cell as a Stress Transducer | 430 | 2010 | Proceedings of the 15th International Symposium on Chromaffin Cell Biology published in Cellular and Molecular Neurobiology, issue 30 (8), 2010. (Cited Refs. include 140, 141, 310, 374) |
| Vaudry H, Metz-Boutigue M-H (eds) | GRANINS: Thirty-Five Happy Years in the Granulosome World | 431 | 2010 | Special issue published in Regulatory Peptide, 165 (1), 2011. (Cited Refs. include 238, 239, 257, 290, 295, 311, 375–385) |
The top section shows results of an ISI search conducted on March 14, 2011, using granin, chromogranin, secretogranin, VGF, proSAAS, or NESP-55 as topic search criteria appearing in title and/or abstract. Additional reviews covering the granin family, and those included in three special issues/proceedings, are also noted.
A. Regulated secretion
Hormones, growth factors, neuropeptides, processing enzymes, and catecholamines are just some of the proteins and neurotransmitters that are secreted from endocrine, neuroendocrine, and neuronal cells. Secretion can be constitutive, as it is for Ig release from B cells (1), but for many biologically active molecules, it is more likely to be highly regulated and coupled to the exposure of cells to specific secretagogues or to depolarization (2). Secretory proteins destined for the regulated secretory pathway enter the rough endoplasmic cisternae, are transported to the trans-Golgi network (TGN), and are then targeted into dense-core secretory granules (DCG), otherwise known as large dense-core vesicles (LDCV) or, in the adrenal medulla, chromaffin granules (CG). Targeting is mediated by receptors that control entry into the regulated pathway (sorting by entry) and/or by progressive condensation of regulated secretory proteins within the immature granule during maturation (sorting by retention) and the budding off of clathrin-coated vesicles that contain incorrectly sorted, constitutively secreted proteins (e.g., furin) (3–5). This generates a pool of highly concentrated cargo, crystalline in the case of insulin (6, 7). Winkler coined the term vesicular cocktail to indicate the presence of a large variety of solutes inside the CG; cargo with concentrations as high as 1.8 mm for chromogranin A (8, 9) and 0.5–1 m for catecholamines (10) have been reported. An osmolarity of approximately 1500 mOsm has been estimated when ATP, Ca2+, peptides, ascorbate, and other proteins including enzymes, hormones, and growth factors are additionally considered (11). The granule cargo is therefore protected within LDCV from forming a formidable osmotic load by its relative insolubility and condensation (12). This state of aggregation contributes to gradual dissipation of cargo, such as for prolactin (13), insulin (14, 15), and catecholamines (16), after their release from the cell. Other proteins targeted into the regulated secretory pathway granules include enzymes such as dopamine-β-hydroxylase (17), tissue plasminogen activator (18), and members of the prohormone convertase (PC) family that process precursor proteins, generating diverse, biologically active secreted peptides (19, 20). The signals that target proteins into the regulated secretory pathway and/or cause their retention in this pathway have been the subject of intense investigation (reviewed in Refs. 3, 4, 21, and 22) and extensive discussion in Section III of this review, yet generalizable sorting mechanisms for regulated protein export still remain elusive.
LDCV, which are generally 80–120 nm in diameter, are estimated to number 10,000–30,000 in a typical endocrine or chromaffin cell (23–26); a subset of these fuse to the cell's plasma membrane in response to a secretory stimulus (27, 28), sometimes releasing only a fraction of each vesicle's content through a transiently formed pore (29). Although the LDCV pool is large, and proteins can be stored for several days, mature LDCV in pancreatic β-cells containing the most recently synthesized insulin, for example, bud from the Golgi and translocate within minutes to positions closest to the plasma membrane, where they fuse and release their contents, often before the secretion of cargo from chronologically older LDCV (22).
B. Secretory granule biogenesis and content
Packaging of hormones, growth factors, enzymes, and catecholamines in LDCV requires a mechanism for secretory vesicle formation or biogenesis (discussed in Section III), that has been shown relatively recently to depend on several granin family members, chromogranin A (CgA), CgB, secretogranin II (SgII), and SgIII (30–33). Insight into secretory granule content was first obtained through the study of soluble proteins that were released from the adrenal medulla or were constituents of catecholamine-containing CG, obtained by subcellular fractionation (34–39). The most abundant protein initially identified in adrenal and later parathyroid CG (40) was CgA, representing almost 50% of the soluble protein content of the adrenal chromaffin secretory granule (41). Approximately 20 yr after the initial description of CgA (11), an immunologically and structurally related protein was identified in the CG and in brain, CgB (41), and in subsequent years, additional similar proteins have been discovered, biochemically characterized, and cloned (e.g., SgII) (42). The chromogranin and secretogranin proteins share many properties, including acidic isoelectric point (pI), binding to calcium, propensity to form aggregates, and the presence of multiple dibasic cleavage sites, all of which are discussed in Section II and have been described in several excellent reviews (11, 43–45). Indeed the presence of chromogranins in secretory vesicles, coupled with their high capacity for Ca2+ binding, are critical for Ca2+ storage and provide a sizeable intracellular reservoir of Ca2+ that can be mobilized via inositol 1,4,5-triphosphate (IP3)-receptor (IP3R)/Ca2+ channels (46, 47). Subsequent studies indicate that the extended granin family is substantially larger than the chromogranins, CgA and CgB, and secretogranins, SgII and SgIII, and now includes HISL-19 antigen (SgIV), 7B2 (SgV), neuroendocrine secretory protein of Mr 55,000 (NESP55) (SgVI), VGF (SgVII), and proSAAS (SgVIII) (1). The majority of these proteins are the precursors of biologically active peptides, modulating, for example, pain pathways, inflammatory responses, metabolic and mood disorders, and blood pressure (BP). Chromogranin-derived peptides have been previously reviewed (48–50) and are updated here in Section IV where additional peptides derived from the extended granin family are described.
Analysis of the genomic structural organization and coding sequences of individual granin proteins suggest functional conservation throughout vertebrate evolution (45, 51). Furthermore, recent single-nucleotide polymorphism (SNP) characterization of the human CHGA (CgA) (52) and SCG3 (SgIII) (53) genes is consistent with an important functional contribution of these granins to hypertension and obesity, respectively (see Section V). Moreover, the relative abundance of granins is likely responsible for their expanding utility as disease biomarkers (see Section VI).
II. Structural Comparison of Granins
Biochemical and structural features of the granin proteins CgA, CgB, SgII, SgIII, 7B2 (SgV), NESP55 (SgVI), VGF (SgVII), and proSAAS (SgVIII)1 are reviewed below and summarized in Table 2. In addition, evolutionary conservation is discussed, drawing on protein sequence data from vertebrates (e.g., zebrafish to human) and invertebrates.
Table 2.
Comparison of granin proteins
| Granin | Preprotein |
Mature protein |
Dibasic sitesh | AA/% prolineh | AA/% glutamateh | pI calch/obs | % α-Helixh | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AAh | Calculated MMh (kDa) | AAh | Calculated MMh (kDa) | Observed MM (kDa) | ||||||
| CgA | 457 | 51 | 439 | 49 | 75h | 10 | 29/6.3 | 90/19.7 | 4.5/4.9h | 38 |
| CgB | 677 | 78 | 657 | 77 | 110h | 16 | 34/5.0 | 116/17.1 | 4.8/5.2h | 26 |
| SgII | 617 | 68 | 587 | 68 | 86b | 9 | 42/6.8 | 78/12.6 | 4.5/5.0b | 40 |
| SgIII (i1) | 468 | 53 | 449 | 51 | 57m | 6 | 20/4.3 | 55/11.8 | 4.8/5.1m | 46 |
| 7B2 (i1) | 212 | 24 | 186 | 21 | 21p | 4 | 20/9.4 | 15/7.1 | 6.1/5.0p | 30 |
| NESP55 | 245 | 28 | 201 | 23 | 28m | 9 | 27/11.0 | 37/15.1 | 4.7/5.0m | 25 |
| VGF | 615 | 67 | 593 | 65 | 90h | 10 | 77/12.5 | 97/15.8 | 4.5/ND | 39 |
| ProSAAS | 260 | 27 | 227 | 24 | 27r | 6 | 34/13.1 | 18/6.9 | 5.5/ND | 42 |
Number of amino acids (AA) and calculated molecular mass (MM) of the preprotein, number of amino acids and calculated molecular mass of the mature protein, observed molecular mass of the mature protein, number of dibasic sites, number/content of proline, number/content of glutamate, calculated (calc) and observed (obs) pI, and secondary structure (percent α-helix) predicted using PSIPRED (386, 387) are shown for human (h), bovine (b), porcine (p), rat (r), or mouse (m) granin proteins. i1, Isoform 1; ND, not determined.
A. Why consider the granins as members of a structurally and functionally related family?
Granins are relatively abundant, acidic proteins that are localized in secretory vesicles, where they bind to calcium, aggregate, and share a number of biochemical features that are summarized in Table 2 (see also Refs. 43 and 44). What differentiates granins from classical peptide precursors that are also found in the secretory pathway? Classification is not absolute, but generally protein size and pI do provide some guidance. The largest known classical mammalian neuropeptide precursors have a size of approximately 30 kDa [proopiomelanocortin (POMC) and proenkephalin; 267 amino acids)], whereas the remainder are smaller, usually in the 10- to 15-kDa range. The granin proteins reviewed here are all larger than 24 kDa, with the majority sized greater than 50 kDa (see Table 2). The pI values for these granins range from 4.5–6.1 with a mean of 4.9, whereas neuropeptide precursors generally have higher pI (5.1–11.4) with a mean of 7.1 (n = 15).2 Although granins and peptide precursors have multiple dibasic cleavage sites, and both groups undergo differential cell-type-specific and tissue-specific processing, cleavage of classical neuropeptide precursors at specific dibasic residues to mature peptides is usually more easily predicted and complete (20, 54–56) and in neurons may even occur locally in axon terminals and dendrites (57). Perhaps the relative resistance of granins to complete cleavage is a function of secondary structure (54, 58) and/or state of aggregation. Interestingly, calcium binding at low pH and intermolecular aggregation have been noted rarely for peptide precursors (e.g., protachykinin) (59) but are very commonly associated with granins, where they play a role in sorting into LDCV and the regulated secretory pathway. Can granins be easily differentiated from the precursors of growth factors, cytokines, and hormones that are also localized in the secretory pathway? These proteins also tend to be smaller when compared with granins (10–40 kDa) and can bind divalent cations, and although many are processed from proforms, they are rarely cleaved at multiple paired basic residues into peptides, the hallmark of granins. So although there are no absolute guidelines that define granin proteins, there are advantages to discussing these eight highly similar proteins as an extended family.
B. The original granin proteins: CgA and CgB
1. Chromogranin A
The human CHGA (CgA) gene is located on chromosome 14q32.12, which spans 12,192 bp and gives rise to a transcript of 2,041 bp that encodes a 439-amino-acid mature protein (9). There are 10 dibasic sites in human CgA, which are potential sites for proteolytic cleavage (60). The dibasic sites in CgA from other species range from a minimum of seven in mouse to a maximum of 11 in African clawed frog (61–70). In homeothermic vertebrates, CgA is an approximately 48- to 52-kDa protein with a coiled-coil structure (71). The chga genomic organization has been reported for bovine (72), human (73), and mouse (66). The human CHGA gene is organized in eight exons and seven introns (Fig. 1). Exon I encodes the 5′-untranslated region (UTR) (260 bp) of the CgA mRNA and most of the signal peptide of CgA. Exons II–V encode the vasorelaxant and cardiosuppressive peptide vasostatin (VST: hCgA1–76) (74). VST is highly conserved across vertebrates: human vs. mouse, approximately 87%; human vs. chicken, approximately 79%; human vs. marsh frog, approximately 71%; and human vs. zebrafish, approximately 59%. Exon III encodes highly conserved cysteine residues that form the disulfide loop of CgA. Exon VI contains the most variable peptide sequences across species. Exon VII encodes most of the biologically active peptides including dysglycemic hormone pancreastatin (PST: hCgA250–301) (75), catecholamine release-inhibitory and antihypertensive peptide catestatin (CST: hCgA352–372) (76), and 14 amino acid peptide with N-terminal tryptophan (W) and C-terminal glutamatic acid (E) (WE14) (hCgA324–337), which acts as an autoantigen in type 1 diabetes (77). Among CgA peptides, PST is the least conserved, having only 54% homology between human and mouse (PST homology cannot be ascertained in nonmammalian vertebrates) (Fig. 2). Unlike PST, CST is highly conserved in mammals, with approximately 86% homology between human and mouse. Human CST bears moderate homology with nonmammalian vertebrates: 38% with jungle fowl, 33% with frog, and approximately 19% with zebrafish (Fig. 3). WE14 is the most conserved CgA peptide in mammals, where it is 100% conserved except for pig (∼93%). Like VST, human WE14 is highly conserved with marsh frog (71%), but less conserved with zebrafish (21%). Exon VIII contains the C terminus of the protein, including the last dibasic amino acid pair, and the 3′-UTR (407 bp) of the CgA mRNA. Encoded by part of exons VII and VIII is a highly conserved peptide, serpinin (bCgA 403–428), that is approximately 90% homologous among human, bovine, and mouse and approximately 70% homologous with zebrafish (78).
Fig 1.
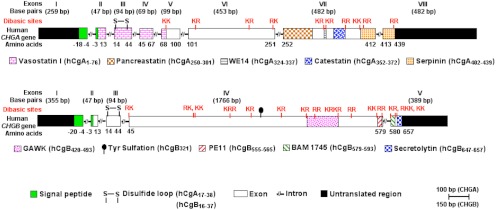
Genomic organization of human CgA and CgB. Positions of sequences encoding conserved, biologically active CgA- and CgB-derived peptides within the human CHGA and CHGB genes, respectively, are shown.
Fig. 2.
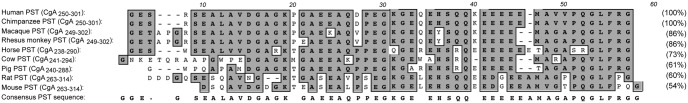
Alignment of the PST domain of CgA. PST alignment in mammalian species was performed using the ClustalW program of MacVector version 9.0, and the percentages of homology were calculated (shown in parentheses). The most conserved amino acids are highlighted in gray. PST sequences used are human (accession number NM_001275), chimpanzee (XM_510135), macaque (AB_169793), rhesus monkey (XM_001092629), horse (NM_001081814), cow (NM_181005), pig (NM_001164005), rat (NM_021655), and mouse (NM_007693).
Fig. 3.
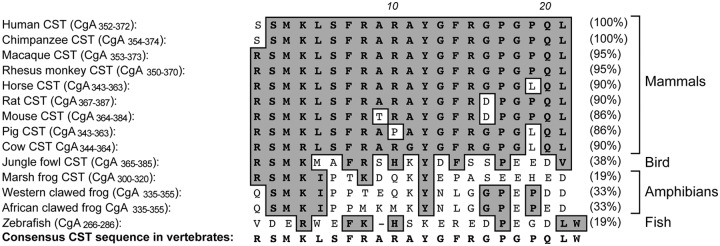
Alignment of the CST domain of CgA. CST alignment in vertebrate species was performed using the ClustalW program of MacVector version 9.0, and the percentages of homology were calculated (shown in parentheses). The most conserved amino acids are highlighted in gray. CST sequences used are human (accession number NM_001275), chimpanzee (XM_510135), macaque (AB_169793), rhesus monkey (XM_001092629), horse (NM_001081814), rat (NM_021655), mouse (NM_007693), pig (NM_001164005), cow (NM_181005), jungle fowl (XM_421330), and marsh frog (AF139924).
2. Chromogranin B
The CgB gene (CHGB) located on human chromosome 20pter-p12 comprises five exons (79). The 2666-bp mRNA transcribed from this gene encodes a preproprotein of 677 amino acids containing 16 pairs of consecutive basic amino acids (Fig. 1). CgB shares several features with CgA, including wide expression throughout the endocrine and nervous systems, acidic protein backbone, random-coil structure, and heat stability. Furthermore, two distinct sites, the Cys loop at the N terminus and a C-terminal region, share significant sequence homology (more than 40% sequence identity), and this conservation is extended to nonmammalian vertebrates (80). CgB is abundantly expressed in many neurons and peptidergic endocrine cells (9, 43, 48). After synthesis, CgB is posttranslationally O-glycosylated and sorted to large secretory vesicles. CgA and CgB represent the predominant proteins found in adrenal CG. The relative concentrations, however, vary between species; in bovine CG, more CgA than CgB is present, whereas in human and rats, more CgB than CgA is synthesized (81). Within granules, CgB is proteolytically processed at dibasic LysArg and monobasic Arg sites to several proteins of intermediate size and small peptides (82). Three small peptides, BAM-1745 (83), PE11 (84), and secretolytin (85), were characterized further. Only secretolytin, a peptide that is conserved in a number of species, was found to have biological activity as an antibacterial agent.
C. Additional members of the granin family: SgII, SgIII, 7B2, NESP55, VGF, and proSAAS
1. Secretogranin II
The SgII gene (SCG2), located on human chromosome 2q35-2q36 (79), comprises two exons. Exon 1 encodes 215 nucleotides of the 5′-UTR, and exon 2 encodes 14 nucleotides of the 5′-UTR plus the entire coding region and 3′-UTR of SgII. SgII is a 617-amino-acid preproprotein with nine pairs of consecutive basic amino acids. Endoproteolytic processing at these sites generates intermediate-sized proteins as well as several small peptides, secretoneurin (SN) (86, 87), EM66 (88), and manserin (89). The degree of processing is variable, generally higher in the nervous system although less pronounced in the adrenal medulla, where the high concentration of catecholamines significantly inhibits proteolytic enzymes (90). SN is highly conserved across evolution; SN is 90–100% identical between mammals and 84–87% identical between human and cartilage fish (91, 92). Bony fish SN is 45–67% identical to mammalian SN; in some bony fish, two SN variants with differing N-terminal sequences are found in the genome, resulting from ancestral gene duplication. In addition to SN, EM66 is another highly conserved peptide within SgII (50–70% identical between human and lower vertebrates) (93–96).
2. Secretogranin III
The SgIII gene (SCG3) located on human chromosome 15q21 comprises 12 exons. The 3366-bp SgIII mRNA encodes a 468-amino-acid acidic secretory protein with seven pairs of consecutive basic amino acids that is well conserved during evolution, from mammals to fish (Fig. 4A). SgIII is synthesized as an N-glycosylated protein and cleaved proteolytically in secretory vesicles to intermediate-sized proteins (97). No biologically active peptides derived from SgIII have been described.
Fig. 4.

Evolutionary conservation of vertebrate and invertebrate granins. Protein sequences for SgIII (SCG3), 7B2, VGF, and proSAAS were aligned using the program ClustalW2 (422), edited with Jalview version 2.5.1 (423), and displayed using the ClustalX color scheme. Amino acid numbers shown in the alignments correspond to the entire protein coding sequences for rat SCG3 (A), the mature human 7B2 protein (B), rat VGF (C), and human proSAAS (D). Three functional domains of SCG3, which bind to cholesterol, CgA, and carboxypeptidase E (CPE), each contain regions that have been highly conserved throughout vertebrate evolution (A). For 7B2, the most highly conserved regions include the cysteine-stabilized PC2 interaction domain that is conserved even in invertebrates, and the C-terminal peptide catalytic inhibitor of PC2, which is highly conserved in vertebrates (B). Three highly conserved regions of VGF, one that includes the NERP-2 peptide and another that includes the C-terminal TLQP and AQEE peptides, are each conserved in higher and lower vertebrates (C). For proSAAS, the C-terminal PC1/3 inhibitory peptide with its LLRVKRL motif is conserved in vertebrates (D). No bird orthologs of VGF or proSAAS were identified using looser BLAST search parameters (51), querying with these short conserved domains. SgIII (SCG3) sequences are human isoform 1 (NP_037375), dog (XP_535482), cow (NP_001095567), mouse (NP_0331561), rat (NP_446308), chicken (XP_413807), Xenopus (NP_001079046), and zebrafish (NP_957051). 7B2 sequences aligned are human isoform I (accession number NP_001138229), mouse (NP_033188), rat (NP_037307), cow (NP_001039463), chimpanzee (NP_001092019), zebrafish (NP_957020), Aplysia (ABF21075), C. elegans (NP_508020), and Drosophila isoform B (NP_001014608). VGF sequences aligned are human (NP_003369), rat (NP_112259), mouse (NP_001034474), cow (XP_875466), horse (XP_001916046), macaca (NC_007860), chimpanzee (XP_519275), opossum (XP_001371271), and zebrafish (XP_001343121). ProSAAS sequences included are human (NP_037403), mouse (NP_038920), rat (NP_062152), cow (NP_001077149), orangutan (XP_002831656), and zebrafish (NP_001159601).
3. 7B2 gene and protein
Human 7B2 (SCG5), located on chromosome 15q13-q14, has six exons. Two variant mRNA of 1244 and 1241 bp are transcribed, the longer encoding a protein that is one amino acid longer than that encoded by the shorter transcript, which uses an alternate in-frame splice junction. 7B2 and proSAAS, which is discussed in Section II.C.6, have the least acidic pI of the granin proteins reviewed here and exhibit functional and structural homology to one another, with each containing a C-terminal peptide inhibitor of PC catalytic activity (98, 99). 7B2 is perhaps the most evolutionarily conserved member of the granin family, particularly in vertebrates but also with orthologs identified in several invertebrate species including Aplysia, Caenorhabditis elegans, and Drosophila (Fig. 4B). Two highly conserved regions stand out, a proline-rich sequence that is critical for 7B2 function as a chaperone of PC2, the other encompassing the C-terminal peptide inhibitor of PC2 catalytic activity (reviewed in Ref. 100). Comparison of PC2 and 7B2 patterns of expression, the former a subset of the latter, and the phenotypes of 7B2- and PC2-null mice, the former developing a lethal form of Cushing's disease whereas the latter remain generally healthy, suggests that 7B2 may chaperone other proteins in addition to PC2 (101, 102).
4. NESP55 gene and protein
NESP55 is part of the extremely complex imprinted GNAS gene locus on human chromosome 20q13.2, which encodes the α-subunit of the stimulatory G protein (Gsα). By using multiple promoters and different first exons, mRNA encoding several distinct proteins including Gsα, NESP55, XLas, and 1A, and also antisense transcripts (termed nespas in mice), are transcribed from this locus (103–106). The NESP55 exon encoding the 5′-untranslated RNA plus the complete open reading frame of NESP55 is spliced onto exons 2–13 of Gsα (107). This splice pattern is found in all species analyzed so far. In addition, further splicing in the 3′-untranslated RNA leads to one prominent shorter mRNA variant (108). Any of these NESP mRNA can be genomically imprinted and transcribed only from the maternal allele. The NESP55 protein consists of 244 amino acids and has six pairs of consecutive basic amino acids in its primary sequence. At these sites, NESP55 is cleaved to smaller peptides including the C-terminal peptide GAIPIRRH (107).
5. VGF gene and protein
The human VGF gene located on 7q22.1 is comprised of two exons. The 2586-bp transcript encodes a 615-amino-acid protein, with the entire protein-coding sequence found uninterrupted on exon 2 of the VGF gene. As shown in Table 2, VGF is an acidic, proline- and glycine-rich polypeptide. Mammalian VGF orthologs have 10–11 conserved clusters of basic residues, many of which are processed in endocrine, neuroendocrine, and neuronal cells in a tissue-specific manner, generating a number of peptide fragments (109). Comparison of mammalian VGF proteins reveals several regions of high sequence conservation that are shared by a putative zebrafish VGF ortholog (Fig. 4C), whereas no closely related invertebrate VGF proteins have been identified. Sequence conservation in the zebrafish VGF ortholog may define structurally or functionally important regions of the protein; the neuroendocrine regulatory peptide (NERP) and C-terminal peptide regions that encode a number of bioactive peptides (see Section IV) are the most highly conserved (Fig. 4C).
6. ProSAAS gene and protein
The human PROSAAS (PCSK1N) gene is found on chromosome Xp11.23 and includes three exons. The 990-bp proSAAS transcript encodes a 260-amino acid precursor protein. Analogous to 7B2, the most highly conserved protein segment within proSAAS, approximately 85% identical between zebrafish and mouse, is part of the C-terminal peptide inhibitor of PC1/3 catalytic activity (ELLRVKRL; conserved sequence is underlined) (Fig. 4D). Although the processed peptides from proSAAS are conserved in higher vertebrates, this does not generally extend to lower vertebrates, suggesting that proSAAS may function as a peptide precursor only in higher vertebrates, although its endocrine and neural distribution is conserved in Xenopus and Danio rerio (51).
III. Sorting and Granulogenesis
A. Biosynthesis and intracellular trafficking of granins
Granins are expressed in endocrine cells and peptidergic neurons, which in addition to having the constitutive secretory pathway that is present in all cells also have a regulated secretory pathway (110). Proteins are secreted through the constitutive pathway continuously, whereas secretion through the regulated pathway occurs only when the cell is stimulated by a secretagogue. Granins are synthesized at the rough endoplasmic reticulum (RER) and inserted into the RER cisternae via the signal peptide located at the N terminus of the molecule. They are subsequently transported from the RER to the Golgi complex via transport vesicles similar to other secretory proteins (111). Within the Golgi stacks, the granins are sorted away from constitutively secreted and lysosomal proteins. They are then packaged at the TGN into immature granules together with other granule proteins such as prohormones and their processing enzymes. Granins are partially processed to various biologically active peptides in the immature secretory granules, which then undergo maturation involving further acidification of the granule milieu and removal of the clathrin coat and synaptotagmin IV and vesicle-associated membrane protein 4 from the granule membrane (reviewed in Ref. 112). Mature secretory granules are stored, and their contents are released upon stimulation (Fig. 5).
Fig. 5.
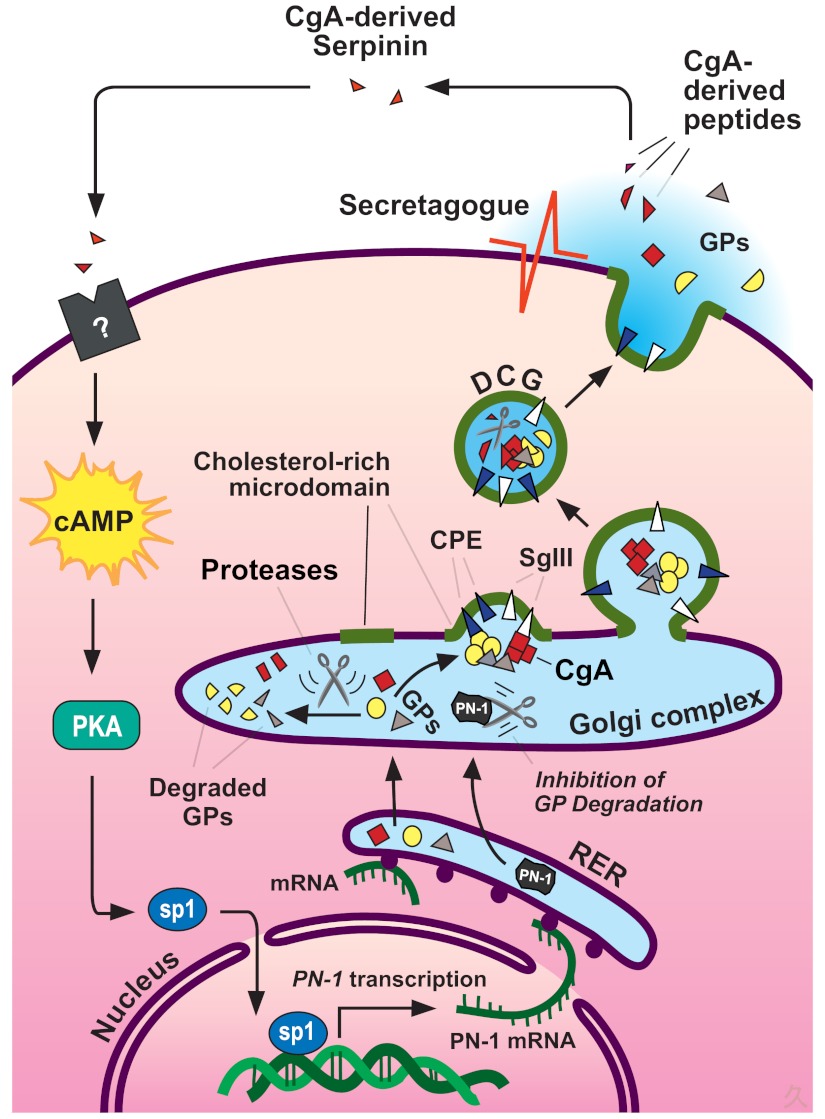
Model for intracellular trafficking of granule proteins and autocrine regulation of DCG biogenesis by the CgA-derived peptide serpinin in endocrine cells. Granule proteins (GPs, including granins and prohormones) are synthesized at the RER and then transported to the Golgi complex where they are sorted at the TGN into the regulated secretory pathway. Granins (e.g., CgA) and prohormones form aggregates, which bind to SgIII or carboxypeptidase E (CPE), sorting receptors that are anchored to cholesterol-sphingolipid-rich membrane microdomains at the TGN. These membrane domains bud under the driving force of the granin (CgA and CgB) aggregates to form immature granules. Specific proteolytic enzymes process the prohormones fully or the granins partially to yield biologically active peptides. The granins, the major protein in the granules, condense to form mature DCG. Excess granule proteins are degraded in the Golgi complex. Upon stimulation of the cell, DCG exocytose and release their contents. In cells expressing CgA, a C-terminal peptide, serpinin, is released and binds to a putative G protein-coupled receptor to increase the transcription and biosynthesis of protease nexin-1 (PN-1) via a cAMP-PKA-Sp1 signal transduction pathway. PN-1 inhibits GP degradation to increase GP levels, which in turn leads to more DCG formation to replenish the ones secreted.
B. Mechanisms of granin sorting into regulated secretory pathway granules
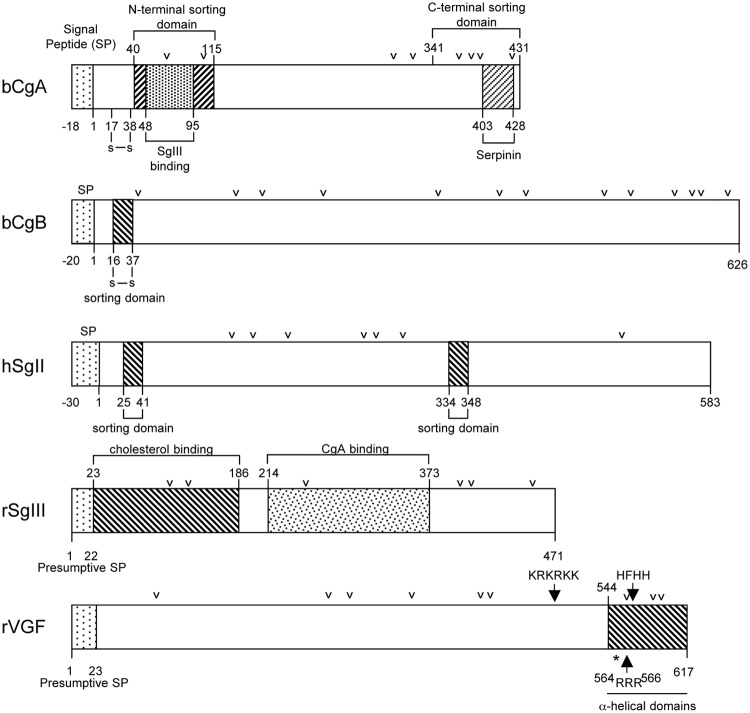
Studies on the sorting of granins into regulated secretory pathway granules have focused mainly on CgA, CgB, and SgIII, although some work has also been done on SgII and VGF but not on the other granins. Initial investigations have proposed that because granins are highly acidic proteins and tend to aggregate at low pH in the presence of calcium in the TGN, they are sorted out from other proteins and enter the budding granules in a passive manner (113, 114). However, later studies have identified sorting determinants on the granins, suggesting that active interaction with a specific binding protein (i.e., sorting receptor) or a lipid may be involved in the sorting mechanism (as detailed in Section III.B) (115–124). Furthermore, a number of studies have indicated that aggregation alone is not sufficient for sorting of CgA, CgB, and VGF (117, 120). Nevertheless, aggregation remains an important first step for concentrating the granins at the TGN before binding to membrane components for efficient sorting. Targeting signals for CgA, CgB, SgIII, SgII, and VGF (Fig. 6) have been reported, along with the interacting membrane receptors for CgA and SgIII. These are described in the following sections.
Fig. 6.
Schematic diagram showing the structures and sorting domains of bovine (b) CgA, bovine CgB, human (h) SgII, rat (r) SgIII, and rat VGF. The sorting determinants of these granins and the binding sites referred to in the text are indicated. The arrowheads represent paired or multiple basic residues that are potential PC cleavage sites. The asterisk in the VGF structure represents a noncanonical cleavage site 553RPR555 that is cleaved to generate a number of peptides.
1. Chromogranin A
Taupenot's group (121, 125) has shown that the N-terminal domain of CgA (bovine/human residues 40–115) not containing the disulfide bonded loop structure (Cys17-Cys38) is necessary for directing CgA into the secretory granules of PC12 cells. Additionally, Hosaka et al. (119) have shown that the N-terminal domain (rat/bovine residues 48–111/48–95) of CgA was essential for binding to SgIII, the proposed sorting receptor for CgA. This domain of CgA binds strongly to a SgIII domain comprising residues 214–373 at pH 5.5 in the presence of 10 nm calcium. SgIII itself is anchored to cholesterol-rich membranes in secretory granules, which are derived from the TGN. These investigators also demonstrated that the CgA-SgIII interaction was necessary for targeting CgA to granules of the regulated secretory pathway in AtT-20 cells, a pituitary cell line; PC12 cells, a neuroendocrine cell line; and pancreatic β-cells (119, 123). However, Cowley et al. (126) showed that a 90-amino acid evolutionarily conserved C-terminal domain, not the N-terminal domain, was critical for sorting CgA to the regulated secretory pathway in GH4C1 cells but played no role in PC12 cell sorting. It is unknown whether this C-terminal domain binds SgIII or other membrane components. In another study, transfection of N- or C-terminal truncated frog CgA into COS-7 cells resulted in retention of these mutant forms in the Golgi complex, whereas the full-length form induced secretory granule biogenesis (124). Hence, both the N and C termini may have targeting information, but there is cell specificity in terms of which sorting determinant is used to sort CgA (11, 116, 119, 124–129).
2. Chromogranin B
Gerdes and co-workers (115) have identified an N-terminal disulfide bonded loop within the first 37 amino acids of CgB (Fig. 6) that was essential for sorting this granin into the regulated secretory pathway in PC12 cells. They found that this signal, when fused to an α1-antitrypsin reporter, a constitutively secreted protein, was sufficient to direct this protein to the regulated secretory pathway (130). More importantly, this signal functions at the level of the TGN by binding to membrane components that give rise to secretory granules. However, the membrane components have not been identified. Interestingly, the sorting efficiency of the α1-antitrypsin reporter protein was increased 5-fold when two loops were present. Multiples of these loop-sorting determinants present on the surface of aggregates of CgB could lead to enhanced binding to membranes, thereby increasing sorting efficiency at the TGN. However, this loop structure is not required for sorting CgB into the regulated secretory pathway in GH4C1 cells, again showing cell-type specificity in the sorting determinants used.
3. Secretogranin II
Truncation analyses of SgII revealed targeting signals in both the C and N termini. Courel et al. (122) found that two putative α-helix-containing domains, hSgII25–41 and hSgII334–348, can act independently, and each is sufficient for sorting SgII into regulated secretory pathway granules in PC12 cells. However, it is unclear whether these sorting domains interact with membrane components at the TGN to mediate sorting. Interestingly, it has been shown in a yeast two-hybrid system that SgII interacts with SgIII (131). This raises the possibility that in cells such as those in the sympathoadrenal system that contain both SgII and SgIII, SgII could be targeted to the secretory granules by binding to TGN membrane-anchored SgIII.
4. Secretogranin III
SgIII is sorted into secretory granules by binding to cholesterol-rich membranes at the TGN in AtT-20 cells (132). Structure-function analysis has identified an N-terminal domain comprised of residues 23–186 (rat) of SgIII that specifically binds to cholesterol (132). Cholesterol, an integral part of the TGN membrane, plays a critical role in curvature formation for granule biogenesis (112, 133). Thus, SgIII, by binding to cholesterol in TGN membrane domains, which are destined to be budded off to become the secretory granule membrane, facilitates its own targeting to the regulated secretory pathway. Concomitantly, SgIII anchored to these cholesterol-rich domains in the TGN membrane is poised to act as receptor for sorting CgA and other prohormones into the regulated secretory pathway (33).
5. VGF sorting
Studies on VGF sorting have identified a C-terminal 73-amino acid fragment (rat 545–617) containing two predicted α-helix domains and four PC cleavage sites (Fig. 6), which was sufficient for targeting this granin to the regulated secretory pathway in PC12 and INS cells (120). Mutation studies indicate that although the helical domains are not necessary, the 564RRR566 PC cleavage site and adjacent HFHH domain, and PC catalytic activity, each contribute to VGF sorting and release. As yet another example of cell type-specific differences in sorting, expression of VGF and multiple deletion mutants in rat FRT thyroid cells resulted, in all cases, in regulated secretion from apical domain LDCV (134).
C. Function of granins in dense core secretory granule biogenesis
Several granins, including CgA, CgB, SgII, and SgIII, have been demonstrated to be granulogenic proteins. CgA or CgB when overexpressed in fibroblasts induced granule-like structures with a dense core, which were capable of releasing their contents in a regulated manner (30, 31, 135). Down-regulation of CgA expression in PC12 cells or in a transgenic mouse by antisense CgA resulted in decreased DCG biogenesis in chromaffin cells (136). Additionally, a CgA knockout (KO) mouse (CgA-KO) exhibited decreased DCG in the adrenal medulla (137). On the other hand, Hendy et al. (138) reported normal DCG biogenesis in the adrenal medulla of the CgA-KO they generated, but other granin levels were increased, which could have compensated for the lack of CgA. Likewise, a CgB-KO showed normal DCG biogenesis and morphology due to compensatory granin biosynthesis (139). Depletion of SgII expression by SgII small interfering RNA in PC12 cells led to a decrease in both number and size of DCG (32). These in vivo and in vitro studies taken together support the function of granins in DCG formation.
Granins aggregate into large complexes at low pH in the presence of calcium at the TGN (113). A granulogenic N-terminal determinant in CgA, located within b/hCgA40–115 of the mature protein (Fig. 6), has been shown to facilitate aggregation. These granin complexes then interact directly or indirectly with cholesterol-sphingolipid-rich membranes, providing the driving force to induce budding at the TGN to form DCG. For a review on membrane lipids involved in granule biogenesis, see Kim et al. (112). Because SgIII is anchored to cholesterol-rich domains in the TGN membrane, it could facilitate DCG biogenesis by providing a platform for recruitment of the granin complexes (Fig. 5). Within the DCG, a coiled-coil structure of CgA has been suggested to play a role in granule core condensation (71). Thus, granin proteins function in granule biogenesis and the sorting and secretion of other proteins including peptide hormones from the regulated secretory pathway, as further detailed in several excellent recent reviews (78, 140, 141).
D. Regulation of DCG biogenesis by the CgA-derived peptide serpinin
Initial studies by Kim et al. (30, 142) demonstrated that CgA, but not CgB, played an important role in regulating DCG biogenesis in PC12 cells and pituitary 6T3 cells, a mutant cell line of AtT-20 cells that lacks DCG. Recent studies in pituitary AtT-20 cells have provided evidence for an autocrine mechanism that up-regulates DCG biogenesis to replenish these granules after stimulated exocytosis (Fig. 5). The autocrine signal was identified as serpinin, a novel 26-amino acid peptide that is cleaved from the C-terminal region of CgA (Fig. 6) (78). Serpinin was first isolated from AtT-20 cell-conditioned medium and is released in an activity-dependent manner from DCG. Subsequently, serpinin was found to increase cAMP levels in the cell, presumably through binding to a putative G protein-coupled receptor and activation of adenyl cyclase. This then led to an increase in transcription of a protease inhibitor, protease nexin 1 (PN-1). A protein kinase A (PKA) inhibitor blocked the increase in PN-1 mRNA in serpinin-treated AtT-20 cells. PN-1 was shown to inhibit degradation of granule proteins, including granins in the Golgi complex, stabilizing those proteins and increasing their levels, which then significantly enhanced DCG formation and granule numbers (78, 112). The up-regulation of transcription of PN-1 mRNA was found to be mediated by the transcription factor Sp1, which upon serpinin treatment of the AtT-20 cells, translocated from the cytoplasm to the nucleus. The PN-1 promoter contains several Sp1-binding sites, and mithramycin A, an inhibitor of Sp1 binding to DNA, blocked the up-regulation of PN-1 transcription in the presence of serpinin. Additionally, a luciferase-reporter assay demonstrated that mutation of the Sp1 promoter inhibited serpinin-induced up-regulation of PN-1 (143). Thus, as shown in the model developed from studies of AtT-20 cells (Fig. 5), serpinin acts in an autocrine/paracrine fashion to enhance granule biogenesis by up-regulating PN-1 transcription via a cAMP-PKA-Sp1-mediated pathway. Treatment of PC12 cells with serpinin also led to an increase in PN-1 transcription, suggesting that this mechanism of regulation of DCG biogenesis may also extend to other (neuro)endocrine cells (78). Such a mechanism might seem wasteful, but regulation at the posttranslational level may in fact be quite efficient to rapidly replenish small numbers of DCG released at any one time, because it is possible to increase the levels of many granule proteins through one step, inhibition of degradation. Interestingly, biogenesis of insulin DCG in pancreatic β-cells is regulated in an autocrine manner involving a posttranscriptional mechanism to transiently stabilize mRNA encoding granule proteins upon glucose stimulation to release insulin (144). Thus, it would appear that autocrine/paracrine signaling to regulate DCG biogenesis at the posttranscriptional/translational level may be used by various endocrine cells to replenish released granules.
E. Regulation of intracellular calcium stores by granin proteins in DCG
Endocrine, neuroendocrine, and neuronal cells secrete a variety of peptides and hormones via calcium-dependent release. The number of DCG in an endocrine cell (∼10,000), and the high Ca2+ binding capacity of resident granin proteins and their abundance (∼2–4 mm), together constitute a recently recognized, major intracellular calcium reservoir (46, 47). Coupled with the abundance of IP3R in secretory granule membranes and the direct modulatory interactions demonstrated between either CgA or CgB and IP3R/Ca2+ channels, a mechanism has been established for the regulation of cytosolic calcium stores and granule exocytosis in secretory cells. This crucial role of DCG and granin proteins CgA and CgB in calcium homeostasis has been elegantly reviewed elsewhere (46, 47).
IV. Granin-Derived Peptides and Their Mechanisms of Action in Endocrine and Neuroendocrine Systems
Diverse biological activities of specific granin-derived peptides are reviewed below, with greater attention devoted to the endocrine, neuroendocrine, cardiovascular, inflammatory, and neural contributions of peptides derived from CgA, CgB, SgII, 7B2, VGF, and proSAAS. These include roles as PC inhibitors or regulators of PC folding/sorting, and contributions to hormone release (insulin, PTH, and vasopressin), glucose homeostasis, catecholamine release, neuronal excitability, autoimmunity, and smooth muscle and vascular contractility. As a consequence of these activities, granin-derived peptides critically modulate pain pathways, metabolic and mood disorders, and BP. Below, we review the physiological contributions of various granin-derived peptides, organizing the discussion by physiological system, and provide a summary of peptide biological activities, organized by granin (Table 3).
Table 3.
Biological activities of granins and granin-derived peptides
| Peptide (aa mature protein) or mature protein (region) | Biological activities |
|---|---|
| CgAH | |
| VST I (1-76) | Vasodilator, antimicrobial, inhibits PTH secretion, promotes cell adhesion, proapoptotic, inhibits endothelial cell proliferation/migration |
| VST II (1-115) | Antimicrobial, vasodilator |
| Chromacin (176-197) | Antimicrobial |
| PST (250-301) | Inhibits insulin release (β-cells), glucose uptake, PTH release, and glycogenolysis; stimulates glucagon and histamine release |
| CST (352-372) | Inhibits nAchR and catecholamine release, vasodilator, induces endothelial cell proliferation/migration, reduces cardiac contractility |
| Serpinin (402-439) | DCG biogenesis; inhibitor of cell death |
| PST (357-428) | Inhibits PTH release |
| CgA (1-439) | DCG biogenesis, CgB interactor, SgIII interactor, IP3R interactor; [regulator of DCG biogenesis, BP, blood glucose] |
| CgBH | |
| CgB1-41 | Inhibits PTH secretion |
| Secretolytin (647-657) | Antimicrobial |
| CgB (1-657) | DCG biogenesis, hormone secretion, catecholamine secretion and vesicle exocytosis, IP3R interactor, CgA interactor; [regulator of DCG biogenesis, BP] |
| SgIIR | |
| SN (154-186) | Stimulates LH release, stimulates neurotransmitter release (DA, GABA, glutamate), stimulates monocyte and endothelial cell migration |
| SgII (1-586) | DCG biogenesis |
| SgIIIR | |
| SgIII (192-351) | CgA interaction domain |
| SgIII (1-164) | Cholesterol interaction domain |
| SgIII (351-449) | CPE interaction domain |
| SgIII (1-449) | DCG biogenesis |
| 7B2H | |
| C-terminal (CT) (155-185) | PC2 inhibitor |
| 7B2 (1-185; 1-186) | PC2 chaperone; [regulator of PC2 maturation, pituitary hormone secretion] |
| NESP55B | |
| LSAL (159-162) | 5-HT1b receptor antagonist |
| NESP55H mRNA | NESP55 transcript of complex imprinted GNAS gene locus encoding Gsα is involved in pseudohypoparathyroidism |
| VGFR | |
| NERP-1 (262-286) | Suppresses vasopressin secretion |
| NERP-2 (290-327) | Suppresses vasopressin secretion; stimulates feeding, locomotor activity, body temperature, oxygen consumption |
| TLQP-62 (533-594) | Increases feeding, antidepressant, increases neuronal electrical excitability, causes mechanical and cold allodynia |
| TLQP-21 (533-553) | Increases energy expenditure, modulates inflammatory pain and gastric contractility; inhibits feeding in hamsters |
| AQEE-30 (565-594) | Antidepressant, induces thermal hyperalgesia and penile erection |
| LQEQ-19 (576-594) | Induces thermal hyperalgesia and penile erection |
| VGF (1-594) | [Regulator of energy balance, memory, depression, reproduction] |
| ProSAASR | |
| Big PEN-LEN (188-227) | PC1 inhibitor |
| Little PEN-LEN (188-221) | PC1 inhibitor |
| ProSAAS (1-227) | PC1 inhibitor; [regulator of prenatal neuropeptide processing, body weight, locomotion] |
Biological and biochemical activities of the mature granins and granin-derived peptides are listed. Amino acid (aa) sequence numbering of the individual peptides is based on that of the mature human (H), bovine (B), or rat (R) granin proteins. Please note that the amino acid sequence numbers for VGF, proSAAS, SgIII, and their respective peptides that are shown in this table correspond to the mature proteins, while those referred to elsewhere in this article correspond to the preprotein sequences. Shown in square brackets are functions assigned based on the analysis of KO or transgenic mice, so could be due to activities of individual peptides and/or the mature granin. CPE, Carboxypeptidase E; 5-HT, serotonin; GABA, γ-aminobutyric acid; nAchR, nicotinic acetylcholine receptor; DA, dopamine.
A. Regulation of glucose balance: CgA peptide pancreastatin
The first granin-derived peptide to be discovered was the CgA peptide PST, which was initially identified in porcine pancreas as a C-terminally amidated 49-mer peptide (pCgA240–288), which strongly inhibited glucose-induced insulin release from the isolated perfused pancreas (75). Subsequently, PST was found to be a CgA peptide, forming the basis of the prohormone concept for CgA (145). In human plasma, the major form detected was a 52-amino acid PST (hCgA250–301). Although it shares five Glu with the gastrin sequence and the carboxyl-terminal sequence Arg-Gly-amide with vasopressin, PST is not homologous to any family of peptides (75) and is found only in mammals, where the homology is relatively low (∼54%) (Fig. 2). After proteolytic cleavage from CgA, PST requires C-terminal amidation by the peptide α-amidating monooxygenase (PAM) for activation. PST exerts multiple, potentially dysglycemic actions, including inhibition of glucose-stimulated insulin release from pancreatic β-cells (75), inhibition of glucose uptake in adipocytes and hepatocytes (146), and induction of glycogenolysis (147, 148). The dysglycemic actions of PST are also achieved in experimental animals in vivo. PST stimulates glucagon release in vivo in mice (149) and rats (150) and in vitro in the pig (151) and inhibits secretion of pancreatic polypeptide (152) and PTH (153) as well as pancreatic exocrine secretion (154). In humans, PST decreases glucose uptake (by ∼50%) and increases spillover of free fatty acids (by 4.5- to 6.4-fold) (155). The lack of change in forearm plasma flow indicates a metabolic, rather than hemodynamic, mechanism of action of PST (155). Although PST is elevated in type 2 diabetes mellitus (by ∼3.7-fold), it is not significantly elevated in the more modestly insulin-resistant state of obesity and does not change during substantial (∼7 kg) weight loss (155), which speaks for pathophysiological changes of PST in diabetes rather than as a response to insulin resistance. Consistent with the antagonistic effect of PST on insulin action, CgA-KO mice display increased insulin sensitivity that is reversed by PST administration (156) (see Section V.B). In wild-type mice, plasma PST levels increase with age, indicating that the rise in PST levels parallels reduced insulin tolerance and hence insulin action. These observations establish a role for PST in human intermediary metabolism and disease and suggest that qualitative hereditary alterations in the primary structure of PST may give rise to inter-individual differences in glucose disposition.
B. Regulation of feeding and energy expenditure: VGF NERP and C-terminal peptides
Several lines of evidence demonstrate an important role for VGF and VGF-derived peptides in the regulation of feeding and energy expenditure, stimulated by the initial observation that germline deletion of the Vgf gene in mice results in a hypermetabolic, lean, and obesity-resistant phenotype (157–160) (see Section V.B). Consistent with these gene KO studies demonstrating VGF function in energy balance, VGF mRNA levels are up-regulated by fasting in the arcuate nucleus (ARC), with leptin treatment limiting the fasting-induced increase (157). Under ad lib feeding conditions, VGF colocalizes in the ARC with POMC and modestly with NPY-expressing neurons, which form a critical hypothalamic circuit to regulate food intake and energy expenditure (161), whereas colocalization increases with NPY and decreases with POMC in the fasted state (157). Finally, VGF expression in the ARC nuclei of Siberian hamsters precedes hibernation-induced metabolic and body weight changes (162).
As noted in Section II, a number of VGF-derived peptides have been identified, including the peptide designated TLQP-21, which regulates energy balance (163) and is itself modulated by feeding in gastric neuroendocrine cells (164). Central TLQP-21 delivery does not affect feeding in mice but exerts an anorexigenic effect in Siberian hamsters (165) and predominantly stimulatory effects on the male hypothalamic-pituitary-gonadal axis (166). Chronic intracerebroventricular (icv) administration of TLQP-21 in mice increases energy expenditure and rectal temperature, an effect accompanied by increased serum epinephrine and decreased norepinephrine levels, but with no measurable changes in locomotor activity or free T3 and free T4 serum levels (163, 167). In addition, TLQP-21 treatment increased catabolic markers such as peroxisome proliferator-activated receptor δ, β-3 adrenergic receptor, and uncoupling protein 1 mRNA in white adipose tissue but had no effect on hypothalamic mRNA encoding metabolically active hypothalamic peptides (163, 168). Central delivery of TLQP-21 also prevented high-fat diet-induced obesity without any effect on food intake (163, 167). Jethwa et al. (165) confirmed a catabolic role for TLQP-21 in hamster, where central delivery exerts an anorectic effect, decreasing body weight and adipose fat mass. Surprisingly, the metabolic profiles of TLQP-21-treated mice and hamsters closely match the phenotype of VGF-KO mice (157, 158). An intriguing hypothesis to reconcile this apparent discrepancy has been proposed (168): one or more VGF-derived peptides have an anabolic role, increasing energy storage and opposing TLQP-21 effects, thus accounting for the phenotype of the germline VGF-KO mice. Data have been reported that support this model: VGF peptides TLQP-62, HHPD-41 (168), and NERP-2 (169) exert an orexigenic effect when injected icv. The VGF-derived amidated peptide, NERP-2, administered icv to rats and mice, rapidly stimulated food intake and increased body temperature, oxygen consumption, and locomotor activity via an orexin-dependent mechanism (170, 171). Treatment with anti-NERP-2 IgG decreased food intake, whereas NERP-2-induced effects were abrogated by administration of anti-orexin IgG or orexin receptor antagonists (171). Finally, NERP-2 did not induce food intake or locomotor activity in orexin-deficient mice (171).
C. Regulation of gastrointestinal function: VGF peptide TLQP-21
Anatomical, molecular, and pharmacological evidence has established that the VGF peptide TLQP-21 plays a prominent role in gastroenteric function (reviewed in Ref. 172). In situ hybridization studies showed that VGF mRNA is highly expressed in the myenteric plexus, with clear evidence of expression in the glandular portion of the stomach (173, 174). More directed characterization of the gastrointestinal tract demonstrated VGF immunoreactivity in nerve fibers of the peripheral system, in a subpopulation of neurons of the enteric plexus, and in enterochromaffin-like and somatostatin cells in rat stomach (164, 175, 176). Brancia et al. (164) also determined the abundance (162 ± 11 pmol/g) of TLQP peptides by ELISA and described a decreased (∼50%) concentration of these peptides in rat stomach after prolonged fasting.
In vitro and ex vivo studies showed that TLQP-21 peptide dose-dependently induces contraction of gastric fundus strips but fails to induce contraction of stomach antrum or more distal gut portions such as jejunum and ileum (176). TLQP-21 also induced prostaglandin (PG)-E2 and PGF(2α) release from the mucosal layer, whereas fundus strip contraction was completely abolished by pretreatment with the cyclooxygenase (COX) inhibitors indomethacin or naproxen as well as PG antagonists (176).
Classical studies established that experimental ulcerative lesions, induced by sc administration of cysteamine, increased VGF mRNA in both sensory neurons of the nucleus tractus solitarius and in neurons of the dorsal motor nucleus of the vagus that directly project to the stomach (177, 178). In line with a potential involvement of TLQP-21 in the outflow pathway from central nervous system (CNS) to gastrointestinal tract, acute icv but not ip or iv administration of TLQP-21 inhibited gastric emptying in a time- and dose-dependent manner (176) and reduced ethanol-induced gastric lesions in rats (179). The TLQP-21 gastroprotective effect against ethanol injury was accompanied by a significant increase in gastric PGE2 and COX-1 expression. The nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (70 mg/kg, sc), the nonselective COX inhibitor indomethacin (10 mg/kg, orally), and capsaicin denervation removed TLQP-21 gastroprotection. Central TLQP-21 injection also inhibited gastric acid secretion (180), and both inhibition of gastric emptying (176) and gastric acid secretion (180) were prevented by indomethacin pretreatment. Overall, recent data therefore suggest that TLQP-21 centrally mediates gastroenteric functions by inducing the synthesis of PG. Additional studies should establish whether the biological activity of TLQP-21 that has been established in vitro, in isolated organ contraction assays, is paralleled by a similar functional role in vivo.
D. Regulation of prohormone convertase activity: 7B2 and proSAAS peptides
Regulation of PC catalytic activity is a mechanism shared by two granins, 7B2 and proSAAS, that has the potential to affect the relative levels of the protein substrates of PC2 and PC1/3, respectively, and the resultant peptides generated by precursor protein cleavage in the regulated secretory pathway (56, 181).
1. 7B2 protein and peptides
The granin 7B2 functions as a chaperone to regulate PC2 catalytic activity. First isolated in 1982 from pig anterior pituitary (182), the 7B2 protein sequence has been highly conserved evolutionarily, and the purified protein has an acidic pI of 4.9 and is processed into peptides (100). Interaction of pro-7B2 with pro-PC2 was subsequently demonstrated and possibly blocks premature activation of the PC2 zymogen in the secretory pathway (100, 183). Recent studies further demonstrate that 7B2 prevents PC2 unfolding and aggregation in the secretory vesicle, perhaps through a chaperone-like mechanism that may generalize to additional proteins in the regulated pathway because 7B2 is expressed more widely than PC2 (184). Processing of 7B2 at a site composed of five basic residues (R151–R155 of pro-7B2) releases a C-terminal peptide, 7B2CP, which like 7B2 functions as a potent inhibitor of PC2 catalytic activity in vitro, in the nanomolar range (98, 185, 186). Structure function analysis of this 31-amino acid peptide indicates that a C-terminal Lys-Lys pair is required for its initial binding to PC2 and its inhibitory activity. Once hydrolyzed at this site, the C-terminal inhibitory peptide is inactivated and dissociates from the catalytic site, and PC2 catalytic activity increases (187–189). However, it is unclear whether the 7B2CP peptide has similar PC2 inhibitory activity in vivo, in the secretory pathway, and if so, in which secretory compartment this peptide interferes with pro-PC2 conversion to PC2.
2. ProSAAS protein and peptides
Functional characterization of the neuroendocrine secreted protein proSAAS, identified in a screen of C-terminally extended peptide intermediates in the brains of Cpefat/Cpefat mice, revealed potent PC1/3 inhibitory activity in vitro, in the nanomolar range (190, 191). Structural comparison with 7B2 (99) and combinatorial peptide library screens (192) further identified a PC1-inhibitory hexapeptide, LLRVKR, in proSAAS that was located at the C-terminal end of the processing intermediate peptide designated PEN (99, 193, 194). Similar to 7B2, the precise functional roles of PEN (proSAAS221–242) and PEN-LEN (proSAAS221–260) in vivo, in the secretory pathways of neural, endocrine, and neuroendocrine cells, have until relatively recently remained unclear. Analysis of PEN-LEN expression in embryonic and adult brain, showing accumulation in embryonic d 15.5 whole-brain extracts at a developmental age when prodynorphin processing by PC1/3 does not occur, and undetectable levels in the adult brain when prodynorphin is processed by PC1/3 are consistent with in vivo functionality (195). Recent targeted ablation of the ProSAAS gene in mice by homologous recombination has demonstrated a role for proSAAS in fetal neuropeptide processing in vivo, and in adult body weight regulation and locomotion (195) (see also Section V.B). Adult proSAAS-KO mice, however, have normal hypothalamic peptide levels detected by quantitative peptidomics approaches and normal pituitary ACTH by gel filtration and RIA analysis, suggesting that PC1/3 activity in the adult is not affected by lack of proSAAS, nor were levels of PC1/3 or PC2 protein altered in whole brain or pituitary (195).
E. Regulation of hormone, neurotrophin, and/or neurotransmitter release: CgA peptide catestatin, SgII peptide secretoneurin, VGF C-terminal, and NERP peptides
1. CgA-derived peptide CST
The peptide CST was initially identified as the most potent endogenous antagonist to nicotinic cholinergic receptor that inhibits nicotine-evoked catecholamine secretion in an autocrine/paracrine fashion (76, 196). Subsequently, CST was found to act as a potent vasodilator in vivo in rat by stimulating the release of histamine (197). Such release of histamine by CST was also demonstrated in vitro from mast cells (198). CST also inhibits desensitization of catecholamine release induced by nicotine (199). The naturally occurring human variants of CST (Gly364Ser, Pro370Leu, and Arg374Gln) displayed differential potencies toward inhibition of nicotinic cholinergic agonist-evoked catecholamine secretion from sympathochromaffin cells in vitro in the following rank order of potency: Pro370Leu more than wild-type more than Gly364Ser more than Arg374Gln (196). In vivo, human carriers of the Gly364Ser allele had profound alterations in autonomic activity, in both the parasympathetic and sympathetic branches, and may be protected against the development of hypertension, especially in males (200). The impact that these CST-driven alterations in catecholamine secretion and autonomic activity have on cardiac physiology and BP is discussed in Section IV.H.
2. SgII-derived peptide SN
In vitro studies demonstrated that SN induces dopamine release from rat striatal slices in a dose- and calcium-dependent manner (201). These results were corroborated in vivo by microdialysis experiments (202). You et al. (203) extended these studies to the substantia nigra and neostriatum where treatment with SN increased the release of dynorphin B and classical transmitters like dopamine, glutamate, and γ-aminobutyric acid. In the endocrine system, a regulatory action of SN on the pituitary was observed; SN stimulated LH release and synthesis in goldfish gonadotrophs (204) and the mammalian LβT2 cell line (205). In contrast to GnRH, treatment with SN in the low nanomolar range specifically induced LHβ but not FSH release from LβT2 cells. Additional effects of SN on immune, endothelial smooth, and muscle cells are discussed below (see Section IV.G).
3. VGF C-terminal and NERP peptides
The VGF-derived NERP-1 and NERP-2 were initially identified in a screen for biologically active, C-terminally amidated peptides from human medullary thyroid carcinoma TT cells (169). NERP are highly abundant in rat hypothalamus and colocalize with vasopressin in storage granules, and consistent with a role in the regulation of water balance, VGF mRNA levels in both paraventricular nucleus of the hypothalamus (PVN) and supraoptic nucleus are regulated by water deprivation, and NERP-1 suppresses angiotensin II-induced vasopressin secretion from PVN and supraoptic nucleus in hypothalamic explants (169). As noted in Section IV.B, Nakazato and colleagues (171) have shown that NERP-2 administered into rats or mice increases food intake via an orexin-dependent mechanism, suggesting that this VGF-derived peptide also functions by selectively stimulating the release of the neuropeptide orexin in specific hypothalamic circuits.
The C-terminal VGF-derived peptides TLQP-62 and AQEE-30 have been demonstrated to increase synaptic activity in cultured hippocampal neurons (206), whereas TLQP-62 stimulates electrical potentiation in hippocampal slices (207) and dorsal horn neuron excitability in spinal cord slices (208). In hippocampal slices, TLQP-62-induced electrical potentiation was selectively blocked by the brain-derived neurotrophic factor (BDNF) scavenger TrkB-Fc, Trk tyrosine kinase inhibitor K252a, and tissue plasminogen activator STOP, which inhibits tissue plasminogen activator, an enzyme involved in pro-BDNF cleavage to BDNF (207). These data suggest that TLQP-62 may function in part by selectively stimulating release of BDNF in the hippocampus, and perhaps in other regions of the CNS. Consistent with this model, AQEE-30 and the shorter C-terminal peptide LQEQ-19 activate microglia and stimulate phosphorylation of MAPK p38 (209), critical steps in nociceptive signaling that could induce BDNF release from microglia after injury, which in turn has been shown to mediate changes in dorsal horn neuronal excitability (210).
F. Regulation of neural pathways that control pain, emotion, and sexual behavior: VGF- and CgA-derived peptides
1. Pain
VGF is abundantly expressed in neurons of both sympathetic and spinal sensory ganglia (175). Positive immunostaining for VGF is observed in the spinal cord, particularly in the superficial dorsal horn and in the region surrounding the central canal and in many neuronal cell bodies of the spinal ganglia (175). Recently, increased VGF mRNA and protein levels have been observed in dorsal root ganglia and spinal cord after sciatic nerve transection or in other neuropathic pain models (208, 209, 211–213). In dorsal root ganglia sensory neurons or in dorsal horn, VGF colocalizes with substance P, calcitonin gene-related peptide (CGRP), TrkA, and P2X3 (209, 214). Intraplantar or intrathecal delivery of C-terminal VGF-derived peptides (TLQP-21, TLQP-62, AQEE-30, or LQEQ-19) consistently induces hyperalgesia or hypersensitivity in different models of pain (208, 209, 214). One of these studies showed that icv delivery of TLQP-21 exerts an analgesic effect in the forepaw-injected formalin test (214). Riedl et al. (209) further established that thermal hyperalgesia mediated by AQEE-30 and LQEQ-19 was dependent upon the activation of microglial p38 MAPK.
Intraperitoneal delivery of the CgA4–16 peptide does not directly modulate pain but rather increases the number of abdominal constrictions induced by ip acetic acid administration (215). The pronociceptive effect induced by CgA4–16 was blocked by pretreatment with L-type calcium channel antagonist diltiazem or the COX-2 inhibitor indomethacin. In addition, CgA4–16 potentiated CGRP- and capsaicin-induced abdominal writhing but not that evoked by substance P. Similar investigation of CgA47–66 inflammatory activity revealed dose-dependent effects; below 0.5 mg/kg ip, administration produced antinociceptive effects, whereas at 2 mg/kg, it produced a pronociceptive effect on acetic acid-induced abdominal pain in rats (216). Pronociceptive effects of CgA47–66 were also blocked by diltiazem and indomethacin, and writhing evoked by CGRP or capsaicin was abolished by CgA47–66 (216).
2. Emotional behavior and psychiatric disease
In humans, schizophrenia has been associated with lower levels or no change in CgA, CgB, and SgII in cerebrospinal fluid (CSF) (217–221). VGF mRNA levels are also reduced in peripheral leukocytes of drug-free depressed patients (222) and in discrete areas of postmortem brain obtained from patients with bipolar disorder (223) but are unchanged in postmortem brain from patients who suffered from major depression or schizophrenia (223). In contrast, increased CSF content of VGF23–62 was reported in first-onset drug-naive schizophrenic patients (224, 225).
Of the granin proteins, VGF has been perhaps the most extensively investigated for its role in emotional behavior and psychiatric disease. Preclinical studies in rodents demonstrate that VGF levels are up-regulated by antidepressant drugs and voluntary exercise and are reduced in animal models of depression and antidepressant efficacy, including in learned helplessness and the forced swim test (222, 226, 227). VGF-derived peptides TLQP-62 and AQEE-30, administered icv or into the hippocampus in rodents, exert an antidepressant-like effect in the forced swim test, tail suspension test, and novelty-induced hypophagia test (226, 227), whereas neither peptide affects anxiety- or novelty-induced locomotor activity (226, 227). Although the mechanisms of these antidepressant actions remain to be fully elucidated, C-terminal VGF peptides such as TLQP-62 and AQEE-30 have been shown to enhance hippocampal synaptic plasticity as well as neurogenesis in the dentate gyrus (206, 207, 227). Consistent with the antidepressant efficacy of VGF peptides, VGF-deficient mice have increased immobility in the forced swim and tail suspension tests while showing impairments in both contextual fear-conditioned learning and spatial learning in the Morris water maze (207, 226).
Currently, there are few data to support a role for other granin proteins in emotional behavior. The icv administration of SgII-derived peptides GE-19, GAIPIRRH, and SN exerted no or minimal effect on anxiety- and novelty-induced activity in rats and mice (228) despite inducing release of dopamine from rat striatal neurons (201, 202).
3. Sexual behavior
Injection of C-terminal VGF-derived peptides AQEE-30 and LQEQ-19 into the PVN stimulates nitric oxide production in the PVN and facilitates penile erection in rats (229, 230). VGF-induced penile erection is partially inhibited by pretreatment with the nitric oxide-synthase inhibitor l-NG-nitro-l-arginine methyl ester, the oxytocin receptor antagonist d-[CH (2)](5)Tyr(Me)-Orn(8)-vasotocin, morphine, and muscimol (229, 230). VGF-derived peptides therefore modulate male erectile behavior and gonadotropin responses of the male hypothalamic-pituitary-gonadal axis (TLQP-21) (166), consistent with infertility observed in male VGF-KO mice (158).
G. Regulation of the immune system: CgA, SgII, and their peptides
Relevant to the immune and cardiovascular systems, a potent chemotactic activity of SN toward monocytes, eosinophils, and endothelial cells was established (87). Like SN, CST also stimulated chemotaxis of human peripheral blood monocytes, exhibiting its maximal effect at 1 nm, which was comparable to the established chemoattractant formylated peptide Met-Leu-Phe (fMLP) (231). This finding indicated that CST could be considered an inflammatory cytokine. Recent studies further demonstrate that both CST and the antimicrobial peptide chromofungin, comprising the third amphipathic helix of the CgA-derived peptide VST-1, induce calcium entry into human neutrophils (232). These studies establish a pathway by which endocrine and immune systems could communicate. In addition, CST was found to induce chemotaxis in human umbilical vein endothelial cells with a maximal effect at 1 nm, comparable to angiogenic vascular endothelial growth factor (VEGF) or SN (233). Moreover, the local presence of SN within the rat CNS influenced the topographical distribution of inflammatory cell infiltrates in acute T-cell-mediated encephalomyelitis (234). Clustering of macrophages, but not of T lymphocytes, was observed at sites of SN immunoreactivity in all stages of experimental autoimmune encephalomyelitis, suggesting a proinflammatory role for SN in vivo. In contrast to SN, which activates inflammatory cell migration and extravasation, the CgA-derived peptide VST-1 prevents vascular leakage and VEGF-induced endothelial cell migration (235–237). In addition to regulating immune cells, a number of granin-derived peptides, including chromofungin (CgA47–66) and CST, directly inhibit growth of fungi, yeast, and bacteria. Contributions of granins and granin-derived peptides to inflammatory conditions and innate immunity have recently been reviewed (238, 239).
H. Regulation of blood pressure, angiogenesis, and the cardiovascular system: CgA, SgII, and their peptides
1. Hypertension
Because excess sympathetic activity is implicated as a cause of hypertension, and basal plasma CgA concentration is correlated with sympathetic tone (240, 241), one would expect that mechanisms involving CgA and the CgA peptide CST might be altered in hypertension or in individuals at risk for the development of hypertension. Compared with age-matched normotensive controls, patients with essential hypertension have increased plasma CgA (242). It has also been shown that plasma CST is diminished not only in established cases of essential (hereditary) hypertension but also in the still-normotensive offspring of patients with hypertension (243), indicating that an early deficiency in CST might play a pathogenic role in the subsequent development of hypertension. Subjects with such a family history demonstrate increased epinephrine secretion in addition to diminished CST (243), indicating that CST exerts an inhibitory effect on chromaffin cells in vivo. Taken together, these findings suggest a complex relationship between BP and the expression of CgA and its peptides, CST and PST (see also Section VI.B). Recent studies indicate an inverse relationship between circulating levels of CgA and CST (244). Consistent with findings in humans, targeted ablation in mice of the Chga gene resulted in high BP that was rescued by treatment with CST (137) (see Section V.B).
Later studies revealed a more complicated biphasic (or U-shaped) dose-response curve for Chga gene copy number and basal BP, and parallel changes in adrenal catecholamine storage and release (245). High or low CgA expression in mice and humans was associated with maximal BP and maximal BP responses to cold stress, respectively (245). Thus, these studies indicate that CgA plays a critical role in the regulation of BP, but the relationship between CgA levels and BP is not strictly linear and is also dependent on the extent of CgA processing into its bioactive peptides. Moreover, a number of variants of the human CgA peptide CST have been characterized (see Section V.A), that differentially affect catecholamine release and hypertension risk (200). In addition to inhibiting catecholamine secretion with different potencies, these variant CST peptides are processed from CgA with different efficiencies (246). Lastly, reduced conversion of CgA to CST has been measured in hypertensive patients, and independent genetic loci have been identified in twin studies that influence BP through diminished CgA processing to CST (244). One of these encodes a subunit of the vesicular H(+)-translocating ATPase, a protein that regulates secretory vesicle acidification, and CgA sorting, secretion, and processing (244). A minor SNP variant of this ATPase is associated with increased systolic BP (244).
2. Angiogenesis
The CgA-derived peptides CST and VST-1 and the SgII-derived peptide SN have been linked to vasculogenesis and remodeling, also a potential contributor to hypertension. CST induces migration and proliferation of endothelial cells and stimulates chemotaxis in vascular smooth muscle cells or endothelial progenitor cells in vitro. Pronounced angiogenic and vasculogenic activities of SN and CST, comparable to that of VEGF, were identified in vivo in the mouse cornea system and in vitro in a Matrigel tube formation assay (233, 247–249). CST also exhibits vasorelaxant and antihypertensive effects through its interaction with histamine receptors (197). Delivery of SN via an expression vector or im injection of CST resulted in greatly improved clinical outcomes in mice with hind-limb ischemia induced by surgical ligation of the femoral artery (233, 250). SN also stimulated proliferation and exerted antiapoptotic effects on endothelial cells via activation of the ERK and Akt signaling pathways (233, 247). Furthermore, in an in vivo stroke model, iv administration of SN after occlusion of the right middle cerebral artery of rats led to reduced infarct volume and improved motor performance (251). In contrast to CST and SN, VST-1 inhibits VEGF-induced endothelial cell proliferation and migration and the formation of capillary-like structures (237). However, similar to CST, VST-1 has vasorelaxant properties (252).
3. Cardiac contractility
Another pathophysiological mechanism that could impact BP is the direct regulation of cardiac muscle contractility. In addition to its synthesis and secretion from the adrenal medulla, CgA and its peptides are expressed in the human heart (253). Importantly, in addition to its vasorelaxant properties, VST-1 has negative inotropic and lusitropic effects on the heart, which have been proposed to stabilize the cardiovascular system under conditions of stress, including sympathetic β-adrenergic overstimulation and cardiac injury. Interestingly, CST also has direct myocardial and coronary effects, reducing contractility (254). In the isolated working frog heart, CST acts as an important cardioinhibitor, reducing both stroke volume and stroke work (255). In fish heart, CST modulates myocardial function (negative inotropism), both under basal and increased preload conditions, and is able to counteract β-adrenergic-mediated positive inotropism (256). Due to space constraints, these important cardiovascular effects, investigated in a variety of vertebrate species, cannot be detailed here but have been beautifully described in several recent reviews (257, 258).
V. Genetic Insights into Granin Function
Studies of SNP in granin genes and their disease implications (Section V.A), transgenic and KO mouse models (Section V.B), and the potential for simpler genetic model organisms to provide substantive new insights into granin protein function (Section V.C), are reviewed below.
A. CHGA and CHGB genetic variants (SNP)
1. CHGA and CHGB coding sequence variants
Using systematic polymorphism discovery at the human CHGA (CgA) locus in 180 subjects from diverse biogeographic ancestries, three human variants of CST were identified that showed differential potencies toward inhibition of catecholamine secretion (Pro370Leu > wild-type CST > Gly364Ser > Arg374Gln) (196, 259). In the Langendorff perfused rat heart preparation, the Gly364Ser variant was found to abolish isoproterenol-induced positive inotropism and lusitropism (254). In humans, Gly/Ser heterozygotes displayed increased baroreceptor slope during upward deflections (by ∼47%) and downward deflections (by ∼44%), increased cardiac parasympathetic index (by ∼2.4-fold), and decreased cardiac sympathetic index (by ∼26%) (200). The Gly364Ser variant was associated with lower diastolic BP in two independent/confirmatory groups of patients with hypertension; genotype groups differed by approximately 5–6 mm Hg, and the polymorphism accounted for approximately 1.8% of population diastolic BP variance. The CST Gly364Ser variant therefore causes profound changes in human autonomic activity, both parasympathetic and sympathetic, and seems to reduce risk of developing hypertension, especially in men.
In the Chinese population, the Arg533Gln variant in the CHGB (CgB) gene was found to be associated with schizophrenia (260). However, in the Japanese population, both the nonsynonymous (Arg353Gly) and synonymous (Glu368Glu) CHGB variants were reported to be associated with schizophrenia (261).
2. CHGA and CHGB variants in the promoter region
Five tightly linked common promoter variants (at positions C-1014T, G-988T, G-462A, C-415T, and A-89C) were investigated in a study of 112 phenotyped twin pairs, and haplotypes were inferred with the HAP algorithm (262). The three most common haplotypes, TTGTC (Hap-A, 56.9%), CGATA (Hap-B, 23.0%), and TTGCC (Hap-C, 16.5%), accounted for more than 95% of all haplotypes. Diploid haplotypic variation in the region markedly affected both the change in diastolic BP and the final diastolic BP during cold stress (263). Homozygosity for Hap-B (CGATA/CGATA) seemed to blunt both change in diastolic BP and final diastolic BP, whereas Hap-A/Hap-B heterozygosity (TTGTC/CGATA) was associated with the greatest values of both change in diastolic BP and final diastolic BP. The more extreme trait values for heterozygotes (compared with either homozygote class) suggested the phenomenon of molecular heterosis (264). There are two common SNP in the proximal promoter of human CHGB: A-296C and A-261T. Like CHGA, CHGB promoter variant (A-261T) was found to be associated with hypertension especially in men (265). In addition, CHGB promoter polymorphisms A-296C/A-261T interact nonadditively to influence systolic BP and diastolic BP (265).
3. CHGA variant in 3′-UTR region
A common (∼27% frequency) genetic variant in the CHGA 3′-UTR (C+87T) is found to be strongly associated with human essential hypertension, accounting for up to approximately 12/9 mm Hg of BP variation within the population (266). This association is substantially more impressive in men than in women, effectively confining the effect of C+87T to men. The 3′-UTR variant also predicts environmental stress-induced increments in BP, suggesting a mechanism for early effects of the gene on a pathogenic series of events eventuating in sustained BP elevation (266).
B. Mouse models (transgenic and knockout)
1. CgA, CgB, and SgII
Chromogranins/secretogranins constitute the major soluble proteins in the vesicular matrix of catecholamine storage vesicles or CG and LDCV (9). These proteins are costored and coreleased with neurotransmitters. To gain better insight into the functions of chromogranins and secretogranins, including intracellular roles regulating catecholamine storage in the CG/LDCV and in granule biogenesis and extracellular functions mediated by a number of bioactive peptides, CgA (137) and CgB (139) KO mice have been generated.
Patch amperometry, which monitors vesicle size (capacitance) and the release of catecholamines from the same vesicle (amperometry), on primary chromaffin cells from CgA-KO mice revealed an approximately 40% drop in vesicular concentration of catecholamines (267). These findings indicate that CG from chromaffin cells lacking CgA possesses dramatically weaker capacity to accumulate catecholamines. Using amperometry as the method of quantification, it was shown that CG from chromaffin cells of CgA-KO mice release 40% less catecholamine than those from wild-type mice after a depolarizing stimulus with comparable spike pattern. Catecholamine release per quantum was reduced (by 34%) in CgA-KO mice. The kinetic analysis of secretory spikes showed that exocytosis occurred faster in CgA-KO cells, affecting mainly the last part of the spikes. These findings indicate that the matrix of LDCV without CgA is less capable of concentrating and retaining catecholamines, causing exocytosis to occur faster, eventuating in higher plasma (137) or urinary (138) catecholamines. Similarly, CG from chromaffin cells of CgB-KO mice also show decreased catecholamine release (by 33%) coinciding with the amount released per quantum (268). These findings indicate that chromogranins/secretogranins are required to concentrate catecholamines inside CG.
a. Chromogranins/secretogranins as regulators of CG biogenesis and sorting of secretory proteins.
In 2001, Loh's group provided evidence that CgA plays a crucial role in biogenesis of secretory granules (see Section III.C). Impairment of CgA expression by antisense RNA depleted secretory granules, inhibited regulated secretion of a prohormone, and reduced secretory granule protein in cells (30). Consistent with in vitro studies, targeted ablation of the Chga gene resulted in the loss of CG (137). CgA antisense transgenic mice also showed a significant reduction in CG in adrenal chromaffin cells caused by down-regulation of CgA (136). The number of CG formed was directly proportional to the amount of CgA present in adrenal medulla. These findings confirm and extend earlier reports and clearly demonstrate a critical role for CgA in the biogenesis of CG (see also Section III.C).
b. Chromogranins/secretogranins as regulators of cardiac homeostasis and hypertension.
Targeted ablation of the Chga gene in KO mice resulted in high systolic BP, elevated plasma catecholamines (137), increased reactive oxygen species, and decreased nitric oxide (269). Rescue of elevated BP to normalcy was achieved by either exogenous replacement or humanization of CgA-KO mice (137, 270). Loss of the physiological brake CST in CgA-KO mice coupled with dysregulation of transmitter storage and release may act in concert to alter autonomic control of the circulation in vivo, eventuating in hypertension (137). So although elevated plasma CgA levels are associated with hypertension, germline ablation experiments in mice suggest that the predominant physiological role of CgA is to reduce BP, most likely via CST. Consistent with this model, CST replacement rescued CgA-KO mice from their high resting BP and normalized plasma catecholamine levels. CST replacement also selectively diminished stress-induced increments in BP and heart rate in CgA-KO mice, implicating CST as an antihypertensive peptide even in stressful conditions.
A related result was found by experimental disruption of CgB expression. Targeted ablation of the Chgb locus in CgB-KO mice elevated systolic and diastolic BP by 20 and 18 mm Hg, respectively (265). This is consistent with the inverse relationship between circulating CgB levels and catecholamine secretion (see Section VI.B.2).
c. Chromogranins/secretogranins as regulators of insulin sensitivity.
PST acts as an antiinsulin peptide (see also Section IV.A), inhibiting most of the actions of insulin, so one would expect CgA-KO mice to be more sensitive to insulin owing to loss of this CgA-derived peptide. Thus, CgA-KO mice are euglycemic despite low plasma insulin and high plasma catecholamine levels, because of increased liver insulin sensitivity. Moreover, PST replacement in these mice increased blood glucose by stimulating phosphoenolpyruvate carboxykinase and glucose-6-phosphatase mRNA abundance, reducing the suppressive effect of insulin on hepatic gluconeogenesis (156). Although both CgA and CgB are conserved at the N- and C-terminal ends, CgB-KO mice show marked resistance to insulin in contrast to increased insulin sensitivity in CgA-KO mice. Identification of an insulin-sensitive CgB peptide would explain the findings in CgB-KO mice.
d. Chromogranins/secretogranins as regulators of type 1 diabetes.
Although many laboratories have identified CD4+ T cell clones in nonobese diabetic (NOD) mice that are reactive to pancreatic antigens in vitro and that also cause or accelerate diabetes in various types of in vivo experiments, the antigenic targets of other highly pathogenic CD4+ T cell clones have remained elusive. The most extensively studied of these are the BDC clones (BDC is a clonal designation for islet-specific T-cell clones) (271), which were isolated from the spleens and lymph nodes of diabetic NOD mice and include BDC-2.5. Initially, CgA was identified as the source of the antigen for BDC-2.5 and two other clones, because it was missing from CgA-KO pancreatic islet cells. Further in-depth studies led to the identification of the CgA peptide WE14 as the autoantigen for type 1 diabetes (77). It is thus clear that CgA-KO mice have provided significant insights into the function of PST and WE14 as regulators of type 1 and 2 diabetes.
2. 7B2 knockout mouse model
Using biochemical and cell biological approaches, 7B2 was found to be a critical chaperone for PC2 and regulator of its catalytic activity (100). To test protein function in vivo, exon 3 was disrupted by random integration of a transposon into the 7B2 gene in mice (101); analysis of this line supported a key role for 7B2 in PC2 activation, although also revealing additional important contributions to the regulation of pituitary hormone secretion. Homozygous 7B2-KO mice lack PC2 activity and, in addition, die before 9 wk of age from a severe Cushing's syndrome that results from pituitary ACTH hypersecretion (101), a consequence of interrupted PC2 processing. The 7B2-KO mice are hypoglycemic, hyperproinsulinemic, and hypoglucagonemic, with a number of metabolic and endocrine abnormalities (101, 272) that are largely reversed by adrenalectomy (102) or adenoviral-mediated expression of 7B2 in the pituitary (272). 7B2- and PC2-KO mice share similarities in glucose homeostasis and have depressed enkephalin and glucagon levels and increased pituitary ACTH, but the phenotypes differ in that 7B2-null mice develop severe pituitary Cushing's disease, adrenal cortical hyperplasia, and increased circulating ACTH (101), the latter likely a result of decreased dopamine levels and inhibitory control in the intermediate lobe of the pituitary (102). Loss of 7B2 therefore has an overlapping yet more substantial effect on the function of the regulated secretory pathway in the pituitary than does PC2 ablation, suggesting potentially novel roles for 7B2 beyond PC2 catalytic regulation.
3. VGF knockout mouse model
VGF was identified on the basis of its rapid, robust transcriptional regulation by neurotrophic growth factors (273–275) and is secreted from neuronal, neuroendocrine, and endocrine cells (109, 276, 277). Unlike other granins, initial elucidation of VGF function relied largely on the development of a KO mouse model (158). Although indistinguishable from littermates at birth, adult homozygous germline VGF-KO mice are lean and hypermetabolic, 50–70% the size of wild-type mice, and resistant to diet- or lesion-induced and specific forms of genetically induced obesity and diabetes (157, 158, 160). Food intake is similar in absolute terms although increased per gram of body weight in VGF-KO mice, which is consistent with endocrine changes such as increased corticosterone levels and increased insulin sensitivity (158, 160). In VGF-KO mice, hypothalamic levels of NPY and AGRP mRNA are elevated by 600 and 800%, respectively, although POMC mRNA is reduced by 75% in comparison with controls, compatible with a fasting state (158). Importantly, VGF-KO mice are fully resistant to obesity induced by diet, gold thioglucose treatment (a hypothalamic lesion-induced form of obesity), and ectopic overexpression of agouti (Ay/a mice) or targeted deletion of the melanocortin 4 receptor (melanocortin 4 receptor KO mice) (157, 158, 160). VGF-KO mice are partially resistant to obesity induced by leptin deficiency (ob/ob mice) although remaining vulnerable to obesity induced by monosodium glutamate administration to neonatal mice (which damages the hypothalamus and sympathetic nervous system) (157, 160). Overall, these data suggest that VGF has a functional role in projections of the sympathetic nervous system that innervate metabolic tissues (109, 157). Recent studies further demonstrate a Vgf gene-dosage-dependent effect on lipid storage and adipocyte cell size and increased mitochondrial number, cristae number, and uncoupling protein expression in VGF-KO brown adipose tissue, providing a possible mechanism for their higher metabolic rate (159). As noted in Section IV.B, the phenotype of germline VGF-KO mice is not totally consistent with the effects of certain icv-administered VGF peptides on energy balance. Future conditional ablation of the Vgf gene in the adult nervous and endocrine systems may help to clarify distinct roles for this granin during development and in the adult.
Perhaps more in line with the cloning of VGF as a neurotrophin-inducible gene product, and the role that BDNF plays in the regulation of synaptic plasticity and behavior, heterozygous and homozygous VGF-KO mice have deficits in memory and emotional behavior (207, 226). In this case, the antidepressant/anxiolytic efficacy of VGF-derived C-terminal peptides AQEE-30 and TLQP-62 (223, 226, 227) is consistent with the prodepressive phenotype of VGF heterozygous KO mice (226) and their resistance to the antidepressant effect of voluntary exercise in the forced swim and tail suspension tests (226). VGF is also required for some of the behavioral effects and signal transduction pathway activation that is stimulated by LiCl treatment (223). Potentially relevant to antidepressant efficacy, TLQP-62 regulates hippocampal progenitor proliferation (227) and stimulates electrical potentiation in hippocampal slices via a BDNF-dependent mechanism (207), although both TLQP-62 and AQEE-30 increase electrical excitability of dissociated hippocampal neurons (206). Future experimentation to better delineate VGF function in emotional behavior, memory, and energy metabolism will likely blend in vivo and in vitro peptide administration studies with the analysis of germline and conditional KO mice.
4. ProSAAS knockout mouse models
Similar to 7B2, proSAAS was initially functionally characterized as a potent PC1/3 inhibitor in vitro, using biochemical and cell-based assays (see Section IV.D.2). However, the physiological impact of proSAAS in vivo was clarified only recently through the analysis of transgenic (278) and KO (279) mouse models. Approximately 2-fold overexpression of proSAAS mRNA, driven by the β-actin promoter in transgenic mice, led to a delayed increase in body weight and fat mass and slightly elevated fasting blood glucose levels in adult 10- to 12-wk-old mice, which did not appear to be due to hyperphagia (278). Because the phenotypes of PC1-KO mice (280) and proSAAS transgenic mice are quite distinct and pituitary peptide processing in proSAAS transgenic mice appeared normal, overexpression of proSAAS in this transgenic line was felt to be insufficient to substantially impair PC1 activity in the pituitary. As a result, it is likely that proSAAS and/or peptides cleaved from this precursor have biological activities that account for the moderate, late-onset obesity in transgenic mice and that these are independent of an effect on PC1 activity (278). To further assess proSAAS function in vivo, a germline KO line was subsequently generated and analyzed (279). Fetal proSAAS-KO mice exhibit complete, adult-like processing of prodynorphin in the prenatal brain, rather than incomplete processing, the result of inhibitory proSAAS intermediates affecting PC1 activity, which is seen in brains of wild-type fetal mice. In adult mice lacking proSAAS, PC1/3 activity does not appear to be affected, but these mice do exhibit decreased locomotion and a male-specific 10–15% decrease in body weight, again arguing that proSAAS and/or proSAAS-derived peptides have bioactivities that are independent of this protein's inhibition of PC1/3 catalytic activity (279).
C. Nonmammalian vertebrate and invertebrate model organisms
Analysis of granins in lower vertebrates has proven incredibly useful in defining evolutionary conservation of granin structure and function, as discussed in Section II and comprehensively reviewed (45). Simple genetic models such as zebrafish and C. elegans offer the added advantage of more tractable genetic manipulation, coupled to the analysis of functional outcomes. Biochemical analysis of Xenopus and zebrafish (D. rerio) proSAAS proteins indicates that these nonmammalian molecules inhibit mouse PC1/3 with nanomolar inhibition constants and exhibit neural and endocrine distributions, suggesting functional conservation (51). Similar studies have demonstrated conservation of 7B2 function in C. elegans (281, 282) and Drosophila (283), in addition to higher vertebrate organisms (100), all of which express PC2. Compared with 7B2 and proSAAS, it has been difficult to biochemically test phylogenetic conservation of CgA, CgB, and SgII function; however, expression and distribution of chromogranin transcripts, proteins, and chromogranin-derived peptides have been examined. Using one- and two-dimensional electrophoresis coupled with immunoblotting (284), or immunohistochemistry (285–287), investigators were able to demonstrate CgA-like, SgII-like, and/or CgB-like proteins in mammals, birds, amphibians, fish, and arthropods and CgA mRNA in zebrafish (288). Future studies in some of these simpler genetic model organisms, including zebrafish and C. elegans, which are amenable to RNA knockdown experimental strategies, should provide complementary functional data to that obtained using KO mouse models.
VI. Granins as Disease Biomarkers
Biological markers (i.e., biomarkers) are characteristics that can be objectively measured and evaluated as an indicator or surrogate endpoint of a biological process, pathophysiological process, or a response to pharmacotherapeutic intervention (289). The identification of clinically useful biomarkers can therefore have a profound impact on disease diagnosis and treatment, our understanding of disease pathogenesis, and the development of new drugs. The widespread utility of granin-derived peptides in serum and CSF as biomarkers of specific diseases, including hypertension, neurodegenerative disease [e.g., amyotrophic lateral sclerosis (ALS) and Alzheimer's disease], neuropsychiatric disease (e.g., depression, schizophrenia, and bipolar disease), and cancer, and more recently to establish therapeutic efficacy during drug development, is reviewed below (see also Refs. 48, 52, and 290–293). The diversity of granin biomarkers is evident in Table 4, where therapeutic utility in endocrine and neuroendocrine tumor diagnosis and prognosis are summarized, and in Fig. 7 and accompanying Table 5, where specific contributions of granin biomarkers to neuropsychiatric, neurological, and cardiovascular disease are explored.
Table 4.
Studies relating to granins as disease biomarkers for endocrine and neuroendocrine tumors
| CgA | CgB | SgII | SgIII | 7B2 | NESP-55 | VGF | ProSAAS | |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | 307, 308, 389 | 307, 308, 389 | N/A | N/A | N/A | N/A | 302, 416 | N/A |
| Gastroenteric tumors | 290, 295, 306, 390, 391 | 290, 295, 306, 403, 404 | 407–409, 376 | 376 | N/A | 300, 301 | 417 | N/A |
| Insulinoma | 295, 391, 392 | 391, 392 | 298, 376 | 376 | 412 | 299 | 417, 418 | N/A |
| Liver neoplasia | 393 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lung tumors | 394 | 388 | 410 | 303 | 413 | 300, 301 | 419 | N/A |
| Medullary thyroid carcinoma | 295, 395 | 404 | N/A | 376 | N/A | N/A | 417 | N/A |
| Medulloblastoma | N/A | N/A | N/A | N/A | 304 | N/A | N/A | N/A |
| MEN and von Hippel-Lindau syndrome | 396, 397 | 405 | N/A | N/A | 414 | N/A | N/A | N/A |
| Neuroblastoma and ganglioneuroma | 398, 399 | N/A | 398 | N/A | N/A | 294, 299 | 420 | N/A |
| Parathyroid adenoma | 295 | N/A | N/A | N/A | N/A | N/A | 417 | N/A |
| Pheochromocytoma | 295, 311, 361 | 311, 406 | 311, 296, 297 | N/A | 311, 412 | 294, 299, 300, 311 | 311, 417 | 311, 421 |
| Pituitary adenoma/carcinoma | 290, 400, 401 | N/A | N/A | N/A | 415 | N/A | 417 | N/A |
| Prostate cancer | 305, 402 | 402 | 402, 411 | N/A | N/A | N/A | N/A | N/A |
MEN, Multiple endocrine neoplasia; N/A, no published study available.
Fig. 7.
Utility of granin biomarkers in psychiatric, neurodegenerative, cardiovascular, and inflammatory disease. Rectangles, representing peptide fragments of CgA (panel A), CgB (panel B), SgII (panel C), and VGF (panel D) that have been used as biomarkers, are color-coded for disease: psychiatric and neurodegenerative disease (green), hypertension and cardiovascular disease (red), and inflammatory disease (yellow). Granin regions that are recognized by antibodies (labeled ā) used in biomarker studies are denoted by the similarly colored ellipses. Note that ellipses outnumber rectangles, representing the current reliance on antibody-based detection methods and that the application of these granin biomarkers to neurodegenerative and psychiatric disease is comparatively more common. There are fewer biomarker reports for NESP55 (301), proSAAS (354), SgIII (424), and 7B2 (425), so these granins are not included in the illustration. The accompanying Table 5 provides the diseases that these diagnostic biomarkers have been used for and the references describing their application. S-S represents the N-terminal domain containing the disulfide bonded Cys-Cys loop structure.
Table 5.
Utility of granin biomarkers in psychiatric, neurodegenerative, cardiovascular, and inflammatory disease
| Biomarker: peptide (aa mature protein) or antibody (region of protein recognized) | Disease and direction of change in biomarker expression (Ref.) |
|---|---|
| CgA | |
| VST II (1-115) | ↓ AD (353) |
| CgA (193-213) | ↑ MS (357, 358) |
| PST (250-301) | ↑ HTN (315, 316) |
| CST (352-372) | ↓ HTN (243) |
| CgA | ↓ AD (426) |
| Anti-CgA | ⇆ SCZ (217); ⇆ AD (351); ↓ AD (350, 426); ↑ SIRS (335); ↑ HF (253, 317–322, 325); ⇆ cardiac hypertrophy (326) |
| Anti-CgA (68-70) | ↑ RA (427) |
| Anti-CgA (210-439) | ↓ SCZ (219); ↑ MS (357); ↑ IBD (365); ⇆ HF (323) |
| Anti-PST (250-301) | ↓ SCZ (219) |
| Anti-CST (352-372) | ↑ Brain Pick's (345); ↑ brain AD (345); ↓ entorhinal cortex AD (348); ↓ Motor neuron ALS (337, 338); ↑ saliva ALS (339) |
| Anti-GE25 (b367-391) | ↑ SCZ (220); ⇆ MS (428); ⇆ AD 428); ⇆ PD (428) |
| CgB | |
| CgB (306-365) | ↓ MS (356) |
| CgB (441-493) | ↓ MS (356); ↓ FTD (355) |
| Anti-CgB (312-331) | ↓ MS (356); ↓ SCZ (219); ⇆ thalamus SCZ (218) |
| Anti-CgB (439-451) | ↓ MS (356); ↓ HTN (265); ↑ HF (330); ↑ cardiac muscle HF (330) |
| Anti-PE11 (555-565) | ↓ motor neuron ALS (338); ↓ CA4 and CA3 hippocampus SCZ (221); ⇆ CA1 hippocampus SCZ (221); ↓ cerebral cortex AD (348); ⇆ MS (428); ⇆ AD (428); ⇆ PD (428) |
| SgII | |
| SN (152-184) | ↓ MS (356) |
| SgII (499-536) | ↓ MDD (224) |
| Anti-SN (154-186) | ↓ motor neuron ALS (338); ↓ cerebral cortex AD (348); ⇆ SCZ (220); ↑ nasal lavage AR (429); ⇆ MS (428); ⇆ AD (428); ⇆ PD (428) |
| Anti-SgII (154-165) & (172-186) | ↓ MS (356) |
| Anti-SgII (223-249) | ⇆ SCZ (219); ⇆ thalamus SCZ (218) |
| VGF | |
| VGF (1-40) | ↓ FTD (355) |
| VGF (4-40) | ↑ SCZ (224, 225); ↑ MDD (224) |
| VGF (356-375) | ↓ AD (352, 353) |
| VGF (376-389) | ↓ ALS (342, 343) |
| Anti-VGF (C terminus) | ↓ ALS (342, 343) |
The peptide and antibody biomarkers illustrated in Fig. 7 are described further here; amino acid (aa) numbers for peptide fragments, shown in parentheses, correspond to the mature human granin sequences, as do the regions recognized by antisera (except anti-GE25; b indicates bovine). References for the biomarkers are noted adjacent to the diseases for which they have been employed diagnostically or prognostically. Arrows positioned to the left of the disease indicate increased (↑), decreased (↓), or unchanged (⇆) biomarker expression. Neurodegenerative and psychiatric biomarkers were measured in samples of CSF, and the cardiovascular and inflammatory biomarkers were in plasma or serum, unless otherwise noted. Other biomarker measurements were made in human spinal cord motor neurons, brain, nasal lavage, saliva, and cardiac muscle. AD, Alzheimer's disease; AR, allergic rhinitis; FTD, fronto-temporal dementia; HF, heart failure; HTN, hypertension; IBD, inflammatory bowel disease; MDD, major depressive disorder; MS, multiple sclerosis; PD, Parkinson's disease; SCZ, schizophrenia.
A. Endocrine and neuroendocrine tumors
Chromogranin and secretogranin proteins, and their proteolytically processed peptides, are often used to identify specific tumors with a secretory phenotype (e.g., carcinoid tumors) (294, 295), allowing one to assess a tumor's degree of malignancy and metastatic potential, and to distinguish primary from metastatic lesions. For example, the SgII-derived peptide EM66 selectively discriminates benign from malignant pheochromocytoma (296, 297), although the SgII-derived peptide SN is a specific marker for endocrine pancreatic tumors (298). NESP55 staining has utility in predicting the primary lesion site of metastatic disease, distinguishing pancreatic tumors and pheochromocytomas (299) from other neuroendocrine carcinoid tumors that are derived from gastrointestinal tract or lung (300, 301).
Sometimes the loss of negative regulators or tumor suppressors (302–304) can reactivate an endo-exosecretory phenotype leading to an increase in secreted peptides. Increased circulating peptide levels parallel tumor mass and metastasis development, a negative prognostic indicator in prostate carcinoma (305) or gastrointestinal tumors (306). Also, for gastroenteropancreatic neuroendocrine tumors expressing somatostatin receptors, decreased plasma CgA is a reliable marker of patients who are most likely to respond to treatment (306). In other cases, the secreted granin peptides are diagnostic biomarkers of differentiated neuroendocrine tumors, for example in breast carcinomas (307, 308) or pheochromocytomas (309), where they are indicative of a better prognosis. Table 4 summarizes the application of granin biomarkers to endocrine and neuroendocrine tumor diagnosis, which has been the subject of a number of excellent recent reviews (290–292, 294, 295, 310, 311).
B. Cardiovascular disease and hypertension
1. CgA biomarkers
The sympathoadrenal system exerts minute-to-minute control over cardiac output and vascular tone. Basal plasma CgA concentration is correlated with sympathetic tone (240, 241), and studies in twins indicate that the basal level is highly heritable (242). As compared with age-matched normotensive controls, patients with essential hypertension have increased plasma CgA and an increased release of stored CgA in response to adrenal medullary stimulation by insulin-evoked hypoglycemia (242). Consistent with these findings in humans, expression of the Chga gene was observed to be significantly higher in adrenal glands of rat and mouse models of genetic hypertension (312–314), supporting the phenotypic association between elevated CgA and essential hypertension. The dysglycemic CgA fragment PST is also elevated in patients with essential hypertension with or without obesity (315, 316); its actions may therefore contribute to the insulin resistance that often accompanies this condition. In contrast, CST is decreased in patients with essential hypertension and even in normotensive subjects with a family history of hypertension (243). Low CST levels also predict augmented adrenergic response to stressors, suggesting that reduction in CST increases risk of hypertension (243).
Several recent clinical studies have extended the potential use of CgA as a disease biomarker to a broad range of cardiovascular diseases ranging from myocardial infarction to heart failure. Specifically, plasma CgA showed a robust association with mortality risk after myocardial infarction or acute coronary syndrome (317–319) as well as heart failure (318, 320–322). Similar data were also obtained in a mouse model of chronic heart failure (323), and in a recent preclinical study using isolated perfused rat hearts, CST was found to reduce myocardial infarct size and postischemic rise of diastolic left ventricular pressure and to significantly improve postischemic recovery. Furthermore, in isolated cardiomyocytes, CST increased the cell viability rate by about 65% after simulated ischemia/reperfusion (324).
These observations suggest a clinical consensus for the prognostic value of CgA in heart disease (320, 325). It should be highlighted, however, that contrasting findings have been reported (323, 326). However, from a biomarker and physiological viewpoint, the association between CgA and cardiovascular disease likely reflects the contributions of sympathetic function to the disease process.
2. CgB biomarkers
Expression of CgB may mark the action of still poorly characterized trans-quantitative trait loci influencing exocytotic sympathoadrenal activity (327, 328). CgB is overexpressed in rodent models of genetic (313, 314) as well as acquired (329) hypertension, suggesting augmented sympathoadrenal activity in the pathogenesis of these syndromes. Recent twin studies have demonstrated that specific genetic variants (e.g., the CgB A-261T proximal promoter SNP) predict elevated BP and/or BP responses to environmental stress and that these are associated with reduced circulating CgB levels and exaggerated catecholamine secretion (265). Similarly, in women, increased plasma CgB is associated with decreased catecholamine secretion and reduced BP responses to environmental stress. Because of this inverse relationship between CgB expression and either BP or catecholamine secretion, it was hypothesized that experimental disruption of CgB expression would elevate BP. Indeed, targeted ablation of the Chgb locus resulted in substantial elevations in both systolic BP (by ∼20 mm Hg) and diastolic BP (by ∼18 mm Hg) in CgB-KO mice (265), thereby linking CgB and the regulation of BP.
Recent studies further support the utility of circulating CgB levels as a biomarker of post-myocardial infarct cardiac failure in human patients, whereas in animal models, CgB mRNA and protein levels are also increased in infarcted and noninfarcted left ventricular myocardium, which is associated with increased levels of circulating CgB (330).
3. SgII biomarkers
Of potential future utility as a biomarker for cardiovascular disease, the SgII-derived peptide SN stimulates migration and proliferation of vascular smooth muscle cells (331) and acts as an endothelial cytokine to promote angiogenesis and vasculogenesis (248, 314). In addition, an association between SCG2 alleles and hypertension has recently been reported, linking the risk-associated ancestral allele to quantitative regulation of SgII expression through a previously undescribed Phox2-responsive intronic enhancer (332).
C. Inflammatory disease
Granins have been investigated as inflammatory biomarkers, building on the cytokine-like effects of granin peptides on angiogenesis, their antimicrobial activities, and their effects on human neutrophils. Because SN is chemotactic for mononuclear cells, SgII RNA and protein levels in synovium of patients with rheumatoid arthritis (RA) and osteoarthritis (OA) were examined (333). SGII mRNA was expressed in RA and OA synovial fibroblasts, and immunohistochemical staining differences in SGII between RA and OA were noted (333). Processing of CgA and CgB and SgII in the vitreous of patients with diabetic retinopathy was recently shown to decrease in diabetic retinopathy, resulting in lower levels of a number of granin-derived peptides that function to regulate inflammation (334). Lastly, serum CgA has been used as an early biomarker of disease severity in patients admitted with systemic inflammatory response syndrome (SIRS) (335). Compared with healthy controls, CgA levels were increased in SIRS patients, which correlated with other inflammatory markers and with patient mortality and were elevated to an even greater extent when SIRS was associated with infection (335).
D. Neurodegenerative and neuropsychiatric disease
1. Amyotrophic lateral sclerosis
Low CgA, CgB, and SgII levels and an association with superoxide dismutase 1 (SOD1)-positive intracellular aggregates have been reported in motor neurons of ALS patients (336–338). In addition, salivary CgA is increased in ALS patients, which positively correlates with emotional functioning (339). A common polymorphism (P413L) in the CgB gene of ALS patients has recently been identified (340). Individuals having the P413L gene variant had up to 3.5-fold relative risk to develop ALS. A potential mechanism for the increased risk conferred by P413L variant came from a preclinical study that showed that in mice, CgA and CgB interact with and determine the secretion of mutant but not wild-type SOD1 protein, which in turn may trigger microgliosis and neuronal death (341).
A decrease in VGF398–411 has also recently been identified as a potential diagnostic biomarker in ALS patients (342). This decrease in CSF VGF progressed with the clinical severity of ALS in both humans and a mouse model (343). Lower VGF immunoreactivity has been identified in lumbar anterior horn from postmortem spinal cord samples of sporadic ALS patients when compared with control patients with other diseases (344). In SOD1 G93A transgenic mice, decreased VGF protein levels in CSF, serum, and spinal cord preceded onset of clinical symptoms, whereas overexpression of full-length VGF in cultured mouse spinal cord neurons protected them against excitotoxic injury (343). In agreement, SUN N8075, a small molecule that exerts a protective effect on ER stress-induced cell death, and potently induces VGF expression, slowed ALS progression and prolonged survival in mutant SOD1 transgenic mouse and rat models (344). The protective effect exerted by SUN N8075 was fully dependent upon its up-regulation of VGF in the spinal cord of ALS mice and rats. Overall, these data suggest that VGF plays a critical role in motor neuron survival and may be a potential new target for drug discovery and development projects aimed at healing this devastating neurological disorder.
2. Dementia
Alzheimer's disease, Pick's disease, and frontotemporal dementia are neurodegenerative disorders that have in common aggregates of straight filaments composed of hyperphosphorylated tau proteins (i.e., tauopathies). Several studies identified increased CgA, SgII, and proSAAS in postmortem filament aggregates in tauopathies (345–349). Decreased CgA levels were measured in the CSF of patients with early-onset Alzheimer's disease (<65 yr old) but not in patients with senile dementia (>65 yr old) (350), although a previous study found no changes in patients' CSF CgA levels (351). More recently, several groups have used proteomics to investigate the potential utility of granins as diagnostic biomarkers for Alzheimer's disease and frontotemporal dementia: 1) VGF378–397 fragment is decreased in the CSF of Alzheimer's patients (352, 353); and 2) proSAAS (354), VGF26–62 fragment (355), and CgB441–493 fragment (355) are decreased in the CSF of patients affected by frontotemporal dementia.
3. Multiple sclerosis
Mattsson et al. (356) identified lower levels of two CgB fragments, CgB441–493 and CgB306–365, and the SgII peptide SN in the CSF of multiple sclerosis patients compared with healthy siblings and nonrelated controls. In contrast, the CgA194–213 fragment was increased in the CSF of multiple sclerosis patients when compared with patients suffering from other neurological diseases that lack an inflammatory component (357, 358). Lastly, the 7B2125–142 fragment was also increased in patients affected by neurological diseases of inflammatory origin compared with clinically isolated syndromes of demyelination (358).
4. Schizophrenia and depression
Schizophrenia is thus far the only psychiatric disease for which CgA- and CgB-encoding genes have been identified as susceptibility loci (261, 359, 360). Despite these genetic association studies, proteomic assays of CSF or postmortem tissue from schizophrenic patients have failed to identify a clear involvement of granin proteins in schizophrenia; 1) increased CgA to SN ratio (220), 2) decreased CgA and CgB but not SgII (219), 3) decreased CgB immunoreactivity in postmortem hippocampi (221), and lastly 4) a negative correlation between CgA, negative symptoms and ventricle to brain ratio in schizophrenic patients (217) have been noted. The future utility of chromogranins as schizophrenia biomarkers will therefore depend on additional investigation.
In contrast, a recent study showed a robust increase in the content of the VGF23–62 fragment in CSF samples from first-onset drug-naive schizophrenic patients (224, 225). This important study, having a large sample size, cross-validation groups, and disease-specificity analysis (depression, obsessive-compulsive disorder, and Alzheimer's disease), demonstrated a diagnostic sensitivity of 88% and a specificity of 95% for schizophrenia. The same study also established an increase in the same VGF23–62 fragment and a selective decrease in SgII529–566 fragment in a small cohort of depressed patients (224). Therefore, it is conceivable that VGF23–62 might be associated with an underlying mechanism of both schizophrenia and depression. In contrast, VGF mRNA levels were recently found to be reduced in peripheral leukocytes of drug-free depressed patients as compared with controls and were modulated in response to antidepressant treatment (222).
E. Perspectives. Granin biomarkers: where do we go from here?
Immunostaining of biopsied tissue, RIA, peptide ELISA, unbiased mass spectrometry, and the assessment of gene expression have all been used to identify granins as disease biomarkers (298, 361, 362). However, the diversity of immunoreactive granin-derived peptides that can be obtained from neuroendocrine tumors presents a major diagnostic problem, complicating the use of antigranin antisera in biomarker studies. Antisera can be highly specific for individual peptide epitopes, but these antigenic determinants are likely shared by the full-length granin and a number of processed granin fragments. Nevertheless, commercially available antisera for some of these peptides are widely used as markers for endocrine and neuroendocrine tumors (363, 364). A second issue complicating the utility of granin biomarkers is the specificity of a given biomarker for a particular disease. As discussed in Section VI.C, circulating CgA levels are a useful inflammatory biomarker. Recent studies have noted increased plasma CgA levels in inflammatory bowel disease (365), which could complicate the utility of CgA-based assays in the diagnosis of carcinoid or other neuroendocrine tumors in patients with ulcerative colitis or Crohn's disease. Future application of HPLC- and proteomic-based technologies that are able to identify and quantify fragments of granin proteins in plasma, much as is being done in CSF, will likely improve the specificity and overall utility of circulating granins and granin-derived peptides as tumor and disease biomarkers. At least for CSF analysis, combinatorial proteomic assays of multiple biomarkers could soon offer practical diagnostic and prognostic information for degenerative neurological diseases such as ALS. In fact, recent studies suggest that decreased CSF levels of VGF, in combination with other peptide biomarkers, are valuable for ALS diagnosis and perhaps also represent a prognostic biomarker, with lower levels noted before overt neurological disease in mouse ALS models (342, 343). As with other biomarkers, it will be interesting to see whether these encouraging findings translate into medical advances in the diagnosis and treatment of neurodegenerative disease, much as they have in neuroendocrine and endocrine tumor diagnosis and treatment.
VII. Future Directions: The Search for Receptors of Granin-Derived Peptides
As noted in Section IV, biological activities have been discovered for a number of granin-derived peptides, but the precise mechanisms of action and cognate receptors remain in most cases elusive. Similarly, genetic evidence is strongly supportive of critical granin functions, but again, the mechanisms by which specific signaling pathways are triggered by granin-derived peptides require further elucidation. This is perhaps the greatest future challenge confronting investigators in the granin field. Studies indicate that specific granin proteins function in granule biogenesis (CgA, CgB, SgII, and SgIII). In some cases, binding interactions with other granins and/or vesicular cholesterol-rich lipids have been clearly defined, and analysis of KO mice has provided support for granin roles in secretory vesicle formation. The same mechanistic progress has not been as easily obtained for granin-derived peptides. As detailed in Section IV.E, one CgA-derived peptide, CST, has been demonstrated to function as a noncompetitive nicotinic cholinergic receptor antagonist (76), inhibiting catecholamine release from adrenal chromaffin cells and sympathetic neurons. Findings are compatible with the binding of CST to a site within the nicotinic acetylcholine receptor channel and to a low-affinity site outside the channel (366). Although widely hypothesized to interact with classical G protein-coupled receptors, no published data have directly shown binding of any granin-derived peptides to a known or orphan G protein-coupled receptor. However, in many cases, downstream pathways often associated with G protein signaling are activated in response to peptide treatment. For example, the CgA-derived peptide serpinin increases cAMP levels and adenylate cyclase activity (143), whereas the VGF-derived peptides AQEE-30, TLQP-21, and LQEQ-19 activate MAPK and Erk1/2 kinases (209, 367). In addition, noncompetitive interaction between granin-derived peptides and G protein-coupled receptors, at sites distinct from those that interact with known ligands, is an alternative mechanism. Recently, nontraditional, receptor-independent triggering of signal transduction pathways by granin-derived peptides has been proposed as a possible mechanism of action (368). The CgA-derived peptide VST-1 has membrane-penetrating properties that suggest the potential for receptor-independent, pertussis toxin-sensitive signaling via interaction with the Gαi/o subunit (368). A related model has been proposed for CST interaction with heterotrimeric G protein in mast cell membranes to modulate histamine release (368). Major future challenges in the granin field will be the identification of receptors or physiologically important binding proteins for granin-derived peptides and further characterization of receptor-dependent and receptor-independent signaling pathways.
VIII. Conclusions
The granin proteins reviewed here share many structural and biochemical features and manifest striking evolutionary conservation. Several members contribute to very diverse functions within the regulated secretory pathway of endocrine and neuronal cells, including granulogenesis and the regulation of peptide processing. After their regulated secretion, granin-derived peptides provide autocrine, paracrine, and endocrine signals, with a range of bioactivities extending from feedback stimulation of LDCV formation to the regulation of hormone and growth factor release. Characterization of KO mouse models in preclinical studies and human genetic analyses suggests important, new functional roles and specific disease associations of granin peptides. Lastly, relatively abundant and selective expression of these secreted proteins in the nervous system and in endocrine and neuroendocrine tissues has led to their increased utility as biomarkers of disease and therapeutic efficacy. The main challenges moving forward will be to identify receptors for granin-derived peptides, elucidate their cellular signaling mechanisms, and apply increasingly powerful proteomic methods, ultimately improving the specificity and sensitivity of granin biomarker measurements in blood and CSF.
Acknowledgments
We thank Drs. Niamh Cawley and Hisatsugu Koshimizu (Eunice Kennedy Shriver National Institute of Child Health and Human Development) for critical reading of the manuscript and providing Figs. 5 and 6.
Research in the authors' laboratories was supported in part by University of Minnesota Medical School (to A.B.); Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH) (to Y.P.L.); NIH (to S.K.M. and S.R.J.S.); and National Alliance for Research on Schizophrenia and Depression, Hope for Depression Foundation, and Diabetes Action Research and Education Foundation (to S.R.J.S.).
Disclosure Summary: The authors have nothing to disclose.
Throughout the text, we refer to the granin proteins by their published names. However, we also note here the secretogranin nomenclature (SgX) introduced by Helle in 2004 (44) that conveys the notion that granin proteins are structurally and functionally related.
Mean pI was calculated from the following human mature neuropeptide precursors: agout-related protein, cocaine- and amphetamine-regulated transcript, cholecystokinin, galanin, ghrelin, GnRH, neurotensin, neuromedin U, neuropeptide W, neuropeptide Y, POMC, proenkephalin-A, protachykin β, somatostatin, and vasoactive intestinal polypeptide.
- ALS
- Amyotrophic lateral sclerosis
- ARC
- arcuate nucleus
- BDNF
- brain-derived neurotrophic factor
- BP
- blood pressure
- CG
- chromaffin granule
- CgA
- chromogranin A
- CGRP
- calcitonin gene-related peptide
- CNS
- central nervous system
- COX
- cyclooxygenase
- CSF
- cerebrospinal fluid
- CST
- catestatin
- DCG
- dense-core secretory granule
- Gsα
- α-subunit of the stimulatory G protein
- icv
- intracerebroventricular
- IP3
- inositol 1,4,5-triphosphate
- IP3R
- IP3 receptor
- KO
- knockout
- LDCV
- large dense-core vesicle
- NERP
- neuroendocrine regulatory peptide
- NESP55
- neuroendocrine secretory protein of Mr 55,000
- NPY
- neuropeptide Y
- OA
- osteoarthritis
- PC
- prohormone convertase
- PG
- prostaglandin
- pI
- isoelectric point
- PKA
- protein kinase A
- PN-1
- protease nexin 1
- POMC
- proopiomelanocortin
- PST
- pancreastatin
- PVN
- paraventricular nucleus of the hypothalamus
- RA
- rheumatoid arthritis
- RER
- rough endoplasmic reticulum
- SgII
- secretogranin II
- SIRS
- systemic inflammatory response syndrome
- SN
- secretoneurin
- SNP
- single-nucleotide polymorphism
- SOD1
- superoxide dismutase 1
- TGN
- trans-Golgi network
- UTR
- untranslated region
- VEGF
- vascular endothelial growth factor
- VST
- vasostatin
- WE14
- 14 amino acid peptide with N-terminal tryptophan (W) and C-terminal glutamatic acid (E).
References
- 1. Burgess TL, Kelly RB. 1987. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol 3:243–293 [DOI] [PubMed] [Google Scholar]
- 2. Kelly RB. 1985. Pathways of protein secretion in eukaryotes. Science 230:25–32 [DOI] [PubMed] [Google Scholar]
- 3. Arvan P, Castle D. 1998. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J 332:593–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dikeakos JD, Reudelhuber TL. 2007. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol 177:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JJ, Loh YP. 2008. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol 22:2583–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greider MH, Howell SL, Lacy PE. 1969. Isolation and properties of secretory granules from rat islets of Langerhans. II. Ultrastructure of the β-granule. J Cell Biol 41:162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michael J, Carroll R, Swift HH, Steiner DF. 1987. Studies on the molecular organization of rat insulin secretory granules. J Biol Chem 262:16531–16535 [PubMed] [Google Scholar]
- 8. Helle KB. 2000. The chromogranins. Historical perspectives. Adv Exp Med Biol 482:3–20 [DOI] [PubMed] [Google Scholar]
- 9. Winkler H, Fischer-Colbrie R. 1992. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49:497–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkler H, Westhead E. 1980. The molecular organization of adrenal chromaffin granules. Neuroscience 5:1803–1823 [DOI] [PubMed] [Google Scholar]
- 11. Borges R, Díaz-Vera J, Domínguez N, Arnau MR, Machado JD. 2010. Chromogranins as regulators of exocytosis. J Neurochem 114:335–343 [DOI] [PubMed] [Google Scholar]
- 12. Helle KB, Reed RK, Pihl KE, Serck-Hanssen G. 1985. Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand 123:21–33 [DOI] [PubMed] [Google Scholar]
- 13. Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. 1999. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci 2:440–446 [DOI] [PubMed] [Google Scholar]
- 14. Leung YM, Sheu L, Kwan E, Wang G, Tsushima R, Gaisano H. 2002. Visualization of sequential exocytosis in rat pancreatic islet β-cells. Biochem Biophys Res Commun 292:980–986 [DOI] [PubMed] [Google Scholar]
- 15. Michael DJ, Ritzel RA, Haataja L, Chow RH. 2006. Pancreatic β-cells secrete insulin in fast- and slow-release forms. Diabetes 55:600–607 [DOI] [PubMed] [Google Scholar]
- 16. Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. 1991. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA 88:10754–10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis EJ, Asnani LP. 1992. Soluble and membrane-bound forms of dopamine β-hydroxylase are encoded by the same mRNA. J Biol Chem 267:494–500 [PubMed] [Google Scholar]
- 18. Parmer RJ, Miles LA. 1998. Targeting of tissue plasminogen activator to the regulated pathway of secretion. Trends Cardiovasc Med 8:306–312 [DOI] [PubMed] [Google Scholar]
- 19. Steiner DF. 1998. The proprotein convertases. Curr Opin Chem Biol 2:31–39 [DOI] [PubMed] [Google Scholar]
- 20. Seidah NG, Chrétien M. 1999. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res 848:45–62 [DOI] [PubMed] [Google Scholar]
- 21. Loh YP, Snell CR, Cool DR. 1997. Receptor-mediated targeting of hormones to secretory granules: role of carboxypeptidase E. Trends Endocrinol Metab 8:130–137 [DOI] [PubMed] [Google Scholar]
- 22. Michael DJ, Cai H, Xiong W, Ouyang J, Chow RH. 2006. Mechanisms of peptide hormone secretion. Trends Endocrinol Metab 17:408–415 [DOI] [PubMed] [Google Scholar]
- 23. Heinemann C, Chow RH, Neher E, Zucker RS. 1994. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J 67:2546–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trifaró JM, Glavinovic M, Rosé SD. 1997. Secretory vesicle pools and rate and kinetics of single vesicle exocytosis in neurosecretory cells. Neurochem Res 22:831–841 [DOI] [PubMed] [Google Scholar]
- 25. Olofsson CS, Göpel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. 2002. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch 444:43–51 [DOI] [PubMed] [Google Scholar]
- 26. Straub SG, Shanmugam G, Sharp GW. 2004. Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the β-cells of rat pancreatic islets. Diabetes 53:3179–3183 [DOI] [PubMed] [Google Scholar]
- 27. Heinemann C, von Rüden L, Chow RH, Neher E. 1993. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch 424:105–112 [DOI] [PubMed] [Google Scholar]
- 28. Voets T. 2000. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron 28:537–545 [DOI] [PubMed] [Google Scholar]
- 29. Aravanis AM, Pyle JL, Tsien RW. 2003. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423:643–647 [DOI] [PubMed] [Google Scholar]
- 30. Kim T, Tao-Cheng JH, Eiden LE, Loh YP. 2001. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106:499–509 [DOI] [PubMed] [Google Scholar]
- 31. Huh YH, Jeon SH, Yoo SH. 2003. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem 278:40581–40589 [DOI] [PubMed] [Google Scholar]
- 32. Courel M, Soler-Jover A, Rodriguez-Flores JL, Mahata SK, Elias S, Montero-Hadjadje M, Anouar Y, Giuly RJ, O'Connor DT, Taupenot L. 2010. Pro-hormone secretogranin II regulates dense core secretory granule biogenesis in catecholaminergic cells. J Biol Chem 285:10030–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosaka M, Watanabe T. 2010. Secretogranin III: a bridge between core hormone aggregates and the secretory granule membrane. Endocr J 57:275–286 [DOI] [PubMed] [Google Scholar]
- 34. Banks P, Helle K. 1965. The release of protein from the stimulated adrenal medulla. Biochem J 97:40C–41C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirshner N, Sage HJ, Smith WJ, Kirshner AG. 1966. Release of catecholamines and specific protein from adrenal glands. Science 154:529–531 [DOI] [PubMed] [Google Scholar]
- 36. Helle KB. 1966. Some chemical and physical properties of the soluble protein fraction of bovine adrenal chromaffin granules. Mol Pharmacol 2:298–310 [PubMed] [Google Scholar]
- 37. Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. 1967. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature 215:58–59 [DOI] [PubMed] [Google Scholar]
- 38. Smith AD, Winkler H. 1967. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J 103:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider FH, Smith AD, Winkler H. 1967. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother 31:94–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohn DV, Zangerle R, Fischer-Colbrie R, Chu LL, Elting JJ, Hamilton JW, Winkler H. 1982. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci USA 79:6056–6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Connor DT, Frigon RP. 1984. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem 259:3237–3247 [PubMed] [Google Scholar]
- 42. Gerdes HH, Phillips E, Huttner WB. 1988. The primary structure of rat secretogranin II deduced from a cDNA sequence. Nucleic Acids Res 16:11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taupenot L, Harper KL, O'Connor DT. 2003. The chromogranin-secretogranin family. N Engl J Med 348:1134–1149 [DOI] [PubMed] [Google Scholar]
- 44. Helle KB. 2004. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol Rev Camb Philos Soc 79:769–794 [DOI] [PubMed] [Google Scholar]
- 45. Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata SK, Anouar Y. 2008. Chromogranins A and B and secretogranin II: evolutionary and functional aspects. Acta Physiol (Oxf) 192:309–324 [DOI] [PubMed] [Google Scholar]
- 46. Yoo SH. 2010. Secretory granules in inositol 1,4,5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells. FASEB J 24:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoo SH, Huh YH, Hur YS. 2010. Inositol 1,4,5-trisphosphate receptor in chromaffin secretory granules and its relation to chromogranins. Cell Mol Neurobiol 30:1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helle KB. 2010. Chromogranins A and B and secretogranin II as prohormones for regulatory peptides from the diffuse neuroendocrine system. Results Probl Cell Differ 50:21–44 [DOI] [PubMed] [Google Scholar]
- 49. Mahata SK, Mahata M, Fung MM, O'Connor DT. 2010. Catestatin: a multifunctional peptide from chromogranin A. Regul Pept 162:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sánchez-Margalet V, González-Yanes C, Najib S, Santos-Alvarez J. 2010. Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. Regul Pept 161:8–14 [DOI] [PubMed] [Google Scholar]
- 51. Kudo H, Liu J, Jansen EJ, Ozawa A, Panula P, Martens GJ, Lindberg I. 2009. Identification of proSAAS homologs in lower vertebrates: conservation of hydrophobic helices and convertase-inhibiting sequences. Endocrinology 150:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sahu BS, Sonawane PJ, Mahapatra NR. 2010. Chromogranin A: a novel susceptibility gene for essential hypertension. Cell Mol Life Sci 67:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanabe A, Yanagiya T, Iida A, Saito S, Sekine A, Takahashi A, Nakamura T, Tsunoda T, Kamohara S, Nakata Y, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Funahashi T, Miyazaki S, Tokunaga K, Hamaguchi K, Shimada T, Tanaka K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Sakata T, Matsuzawa Y, Kamatani N, Nakamura Y, Hotta K. 2007. Functional single-nucleotide polymorphisms in the secretogranin III (SCG3) gene that form secretory granules with appetite-related neuropeptides are associated with obesity. J Clin Endocrinol Metab 92:1145–1154 [DOI] [PubMed] [Google Scholar]
- 54. Rholam M, Nicolas P, Cohen P. 1986. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett 207:1–6 [DOI] [PubMed] [Google Scholar]
- 55. Rholam M, Brakch N, Germain D, Thomas DY, Fahy C, Boussetta H, Boileau G, Cohen P. 1995. Role of amino acid sequences flanking dibasic cleavage sites in precursor proteolytic processing. The importance of the first residue C-terminal of the cleavage site. Eur J Biochem 227:707–714 [DOI] [PubMed] [Google Scholar]
- 56. Rouillé Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva AA, Jr, Chan SJ, Steiner DF. 1995. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol 16:322–361 [DOI] [PubMed] [Google Scholar]
- 57. Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekström TJ, Hauser KF, Pickel VM, Bakalkin G. 2006. Prodynorphin storage and processing in axon terminals and dendrites. FASEB J 20:2124–2126 [DOI] [PubMed] [Google Scholar]
- 58. Glandieres JM, Hertzog M, Lazar N, Brakch N, Cohen P, Alpert B, Rholam M. 2002. Kinetics of precursor cleavage at the dibasic sites. Involvement of peptide dynamics. FEBS Lett 516:75–79 [DOI] [PubMed] [Google Scholar]
- 59. Ma GQ, Wang B, Wang HB, Wang Q, Bao L. 2008. Short elements with charged amino acids form clusters to sort protachykinin into large dense-core vesicles. Traffic 9 :2165–2179 [DOI] [PubMed] [Google Scholar]
- 60. Konecki DS, Benedum UM, Gerdes HH, Huttner WB. 1987. The primary structure of human chromogranin A and pancreastatin. J Biol Chem 262:17026–17030 [PubMed] [Google Scholar]
- 61. Iacangelo A, Affolter HU, Eiden LE, Herbert E, Grimes M. 1986. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature 323:82–86 [DOI] [PubMed] [Google Scholar]
- 62. Benedum UM, Baeuerle PA, Konecki DS, Frank R, Powell J, Mallet J, Huttner WB. 1986. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. Embo J 5:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iacangelo AL, Fischer-Colbrie R, Koller KJ, Brownstein MJ, Eiden LE. 1988. The sequence of porcine chromogranin A messenger RNA demonstrates chromogranin A can serve as the precursor for the biologically active hormone, pancreastatin. Endocrinology 122:2339–2341 [DOI] [PubMed] [Google Scholar]
- 64. Iacangelo A, Okayama H, Eiden LE. 1988. Primary structure of rat chromogranin A and distribution of its mRNA. FEBS Lett 227:115–121 [DOI] [PubMed] [Google Scholar]
- 65. Parmer RJ, Koop AH, Handa MT, O'Connor DT. 1989. Molecular cloning of chromogranin A from rat pheochromocytoma cells. Hypertension 14:435–444 [DOI] [PubMed] [Google Scholar]
- 66. Wu HJ, Rozansky DJ, Parmer RJ, Gill BM, O'Connor DT. 1991. Structure and function of the chromogranin A gene. Clues to evolution and tissue-specific expression. J Biol Chem 266:13130–13134 [PubMed] [Google Scholar]
- 67. Turquier V, Vaudry H, Jégou S, Anouar Y. 1999. Frog chromogranin A messenger ribonucleic acid encodes three highly conserved peptides. Coordinate regulation of proopiomelanocortin and chromogranin A gene expression in the pars intermedia of the pituitary during background color adaptation. Endocrinology 140:4104–4112 [DOI] [PubMed] [Google Scholar]
- 68. Sato F, Hasegawa T, Katayama Y, Iwanaga T, Yanaihara N, Kanno T, Ishida N. 2000. Molecular cloning of equine chromogranin A and its expression in endocrine and exocrine tissues. J Vet Med Sci 62:953–959 [DOI] [PubMed] [Google Scholar]
- 69. Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. 2002. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev Dyn 225:384–391 [DOI] [PubMed] [Google Scholar]
- 70. Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, et al. 2002. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99:16899–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mosley CA, Taupenot L, Biswas N, Taulane JP, Olson NH, Vaingankar SM, Wen G, Schork NJ, Ziegler MG, Mahata SK, O'Connor DT. 2007. Biogenesis of the secretory granule: chromogranin A coiled-coil structure results in unusual physical properties and suggests a mechanism for granule core condensation. Biochemistry 46:10999–11012 [DOI] [PubMed] [Google Scholar]
- 72. Iacangelo AL, Grimes M, Eiden LE. 1991. The bovine chromogranin A gene: structural basis for hormone regulation and generation of biologically active peptides. Mol Endocrinol 5:1651–1660 [DOI] [PubMed] [Google Scholar]
- 73. Mouland AJ, Bevan S, White JH, Hendy GN. 1994. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J Biol Chem 269:6918–6926 [PubMed] [Google Scholar]
- 74. Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. 1993. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol 5:405–412 [DOI] [PubMed] [Google Scholar]
- 75. Tatemoto K, Efendiæ S, Mutt V, Makk G, Feistner GJ, Barchas JD. 1986. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324:476–478 [DOI] [PubMed] [Google Scholar]
- 76. Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. 1997. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100:1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. 2010. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol 11:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koshimizu H, Kim T, Cawley NX, Loh YP. 2010. Chromogranin A: a new proposal for trafficking, processing and induction of granule biogenesis. Regul Pept 160:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mahata SK, Kozak CA, Szpirer J, Szpirer C, Modi WS, Gerdes HH, Huttner WB, O'Connor DT. 1996. Dispersion of chromogranin/secretogranin secretory protein family loci in mammalian genomes. Genomics 33:135–139 [DOI] [PubMed] [Google Scholar]
- 80. Aït-Ali D, Turquier V, Alexandre D, Grumolato L, Jégou S, Vaudry H, Anouar Y. 2002. Molecular characterization of frog chromogranin B reveals conservation of selective sequences encoding potential novel regulatory peptides. FEBS Lett 511:127–132 [DOI] [PubMed] [Google Scholar]
- 81. Schober M, Fischer-Colbrie R, Schmid KW, Bussolati G, O'Connor DT, Winkler H. 1987. Comparison of chromogranins A, B, and secretogranin II in human adrenal medulla and pheochromocytoma. Lab Invest 57:385–391 [PubMed] [Google Scholar]
- 82. Lee JC, Hook V. 2009. Proteolytic fragments of chromogranins A and B represent major soluble components of chromaffin granules, illustrated by two-dimensional proteomics with NH2-terminal Edman peptide sequencing and MALDI-TOF MS. Biochemistry 48:5254–5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Flanagan T, Taylor L, Poulter L, Viveros OH, Diliberto EJ., Jr 1990. A novel 1745-dalton pyroglutamyl peptide derived from chromogranin B is in the bovine adrenomedullary chromaffin vesicle. Cell Mol Neurobiol 10:507–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kroesen S, Marksteiner J, Leitner B, Hogue-Angeletti R, Fischer-Colbrie R, Winkler H. 1996. Rat brain: distribution of immunoreactivity of PE-11, a peptide derived from chromogranin B. Eur J Neurosci 8:2679–2689 [DOI] [PubMed] [Google Scholar]
- 85. Strub JM, Garcia-Sablone P, Lonning K, Taupenot L, Hubert P, Van Dorsselaer A, Aunis D, Metz-Boutigue MH. 1995. Processing of chromogranin B in bovine adrenal medulla. Identification of secretolytin, the endogenous C-terminal fragment of residues 614–626 with antibacterial activity. Eur J Biochem 229:356–368 [DOI] [PubMed] [Google Scholar]
- 86. Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H. 1993. Secretoneurin–a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 53:359–365 [DOI] [PubMed] [Google Scholar]
- 87. Fischer-Colbrie R, Laslop A, Kirchmair R. 1995. Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog Neurobiol 46:49–70 [DOI] [PubMed] [Google Scholar]
- 88. Montero-Hadjadje M, Pelletier G, Yon L, Li S, Guillemot J, Magoul R, Tillet Y, Vaudry H, Anouar Y. 2003. Biochemical characterization and immunocytochemical localization of EM66, a novel peptide derived from secretogranin II, in the rat pituitary and adrenal glands. J Histochem Cytochem 51:1083–1095 [DOI] [PubMed] [Google Scholar]
- 89. Yajima A, Ikeda M, Miyazaki K, Maeshima T, Narita N, Narita M. 2004. Manserin, a novel peptide from secretogranin II in the neuroendocrine system. Neuroreport 15:1755–1759 [DOI] [PubMed] [Google Scholar]
- 90. Wolkersdorfer M, Laslop A, Lazure C, Fischer-Colbrie R, Winkler H. 1996. Processing of chromogranins in chromaffin cell culture: effects of reserpine and α-methyl-p-tyrosine. Biochem J 316:953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Leitner B, Schneitler C, Klocker H, Volknandt W, Zimmermann H, Winkler H, Fischer-Colbrie R. 1998. Formation and sequence analysis of secretoneurin, a neuropeptide derived from secretogranin II, in mammalian, bird, reptile, amphibian and fish brains. Neurosci Lett 248:105–108 [DOI] [PubMed] [Google Scholar]
- 92. Fischer-Colbrie R, Kirchmair R, Kähler CM, Wiedermann CJ, Saria A. 2005. Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci 6:373–385 [DOI] [PubMed] [Google Scholar]
- 93. Anouar Y, Desmoucelles C, Yon L, Leprince J, Breault L, Gallo-Payet N, Vaudry H. 1998. Identification of a novel secretogranin II-derived peptide (SgII187–252) in adult and fetal human adrenal glands using antibodies raised against the human recombinant peptide. J Clin Endocrinol Metab 83:2944–2951 [DOI] [PubMed] [Google Scholar]
- 94. Anouar Y, Jégou S, Alexandre D, Lihrmann I, Conlon JM, Vaudry H. 1996. Molecular cloning of frog secretogranin II reveals the occurrence of several highly conserved potential regulatory peptides. FEBS Lett 394:295–299 [DOI] [PubMed] [Google Scholar]
- 95. Holthuis JC, Martens GJ. 1996. The neuroendocrine proteins secretogranin II and III are regionally conserved and coordinately expressed with proopiomelanocortin in Xenopus intermediate pituitary. J Neurochem 66:2248–2256 [DOI] [PubMed] [Google Scholar]
- 96. Blázquez M, Bosma PT, Chang JP, Docherty K, Trudeau VL. 1998. Gamma-aminobutyric acid up-regulates the expression of a novel secretogranin-II messenger ribonucleic acid in the goldfish pituitary. Endocrinology 139:4870–4880 [DOI] [PubMed] [Google Scholar]
- 97. Holthuis JC, Jansen EJ, Martens GJ. 1996. Secretogranin III is a sulfated protein undergoing proteolytic processing in the regulated secretory pathway. J Biol Chem 271:17755–17760 [DOI] [PubMed] [Google Scholar]
- 98. Martens GJ, Braks JA, Eib DW, Zhou Y, Lindberg I. 1994. The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci USA 91:5784–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cameron A, Fortenberry Y, Lindberg I. 2000. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1. FEBS Lett 473:135–138 [DOI] [PubMed] [Google Scholar]
- 100. Mbikay M, Seidah NG, Chrétien M. 2001. Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J 357:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Westphal CH, Muller L, Zhou A, Zhu X, Bonner-Weir S, Schambelan M, Steiner DF, Lindberg I, Leder P. 1999. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing's disease. Cell 96:689–700 [DOI] [PubMed] [Google Scholar]
- 102. Laurent V, Kimble A, Peng B, Zhu P, Pintar JE, Steiner DF, Lindberg I. 2002. Mortality in 7B2 null mice can be rescued by adrenalectomy: involvement of dopamine in ACTH hypersecretion. Proc Natl Acad Sci USA 99:3087–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Williamson CM, Turner MD, Ball ST, Nottingham WT, Glenister P, Fray M, Tymowska-Lalanne Z, Plagge A, Powles-Glover N, Kelsey G, Maconochie M, Peters J. 2006. Identification of an imprinting control region affecting the expression of all transcripts in the Gnas cluster. Nat Genet 38:350–355 [DOI] [PubMed] [Google Scholar]
- 104. Weinstein LS, Xie T, Zhang QH, Chen M. 2007. Studies of the regulation and function of the Gsα gene Gnas using gene targeting technology. Pharmacol Ther 115:271–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bastepe M. 2008. The GNAS locus and pseudohypoparathyroidism. Adv Exp Med Biol 626:27–40 [DOI] [PubMed] [Google Scholar]
- 106. Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. 2010. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. J Clin Endocrinol Metab 95:3993–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, Wolkersdorfer M, Winkler H, Fischer-Colbrie R. 1997. Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J Biol Chem 272:11657–11662 [DOI] [PubMed] [Google Scholar]
- 108. Weiss U, Ischia R, Eder S, Lovisetti-Scamihorn P, Bauer R, Fischer-Colbrie R. 2000. Neuroendocrine secretory protein 55 (NESP55): alternative splicing onto transcripts of the GNAS gene and posttranslational processing of a maternally expressed protein. Neuroendocrinology 71:177–186 [DOI] [PubMed] [Google Scholar]
- 109. Levi A, Ferri GL, Watson E, Possenti R, Salton SR. 2004. Processing, distribution and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol 24:517–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. 1991. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem 266:14171–14174 [PubMed] [Google Scholar]
- 111. Kuehn MJ, Herrmann JM, Schekman R. 1998. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391:187–190 [DOI] [PubMed] [Google Scholar]
- 112. Kim T, Gondre-Lewis MC, Arnaoutova I, Loh YP. 2006. Dense-core secretory granule biogenesis. Physiology (Bethesda) 21:124–133 [DOI] [PubMed] [Google Scholar]
- 113. Chanat E, Huttner WB. 1991. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol 115:1505–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jain RK, Chang WT, Geetha C, Joyce PB, Gorr SU. 2002. In vitro aggregation of the regulated secretory protein chromogranin A. Biochem J 368:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Krömer A, Glombik MM, Huttner WB, Gerdes HH. 1998. Essential role of the disulfide-bonded loop of chromogranin B for sorting to secretory granules is revealed by expression of a deletion mutant in the absence of endogenous granin synthesis. J Cell Biol 140:1331–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gerdes HH, Glombik MM. 2000. Signal-mediated sorting of chromogranins to secretory granules. Adv Exp Med Biol 482:41–54 [DOI] [PubMed] [Google Scholar]
- 117. Gorr SU, Jain RK, Kuehn U, Joyce PB, Cowley DJ. 2001. Comparative sorting of neuroendocrine secretory proteins: a search for common ground in a mosaic of sorting models and mechanisms. Mol Cell Endocrinol 172:1–6 [DOI] [PubMed] [Google Scholar]
- 118. Rustom A, Bajohrs M, Kaether C, Keller P, Toomre D, Corbeil D, Gerdes HH. 2002. Selective delivery of secretory cargo in Golgi-derived carriers of nonepithelial cells. Traffic 3:279–288 [DOI] [PubMed] [Google Scholar]
- 119. Hosaka M, Watanabe T, Sakai Y, Uchiyama Y, Takeuchi T. 2002. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic β-cells. Mol Biol Cell 13:3388–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Garcia AL, Han SK, Janssen WG, Khaing ZZ, Ito T, Glucksman MJ, Benson DL, Salton SR. 2005. A prohormone convertase cleavage site within a predicted α-helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway. J Biol Chem 280:41595–41608 [DOI] [PubMed] [Google Scholar]
- 121. Courel M, Rodemer C, Nguyen ST, Pance A, Jackson AP, O'connor DT, Taupenot L. 2006. Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J Biol Chem 281:38038–38051 [DOI] [PubMed] [Google Scholar]
- 122. Courel M, Vasquez MS, Hook VY, Mahata SK, Taupenot L. 2008. Sorting of the neuroendocrine secretory protein secretogranin II into the regulated secretory pathway: role of N- and C-terminal α-helical domains. J Biol Chem 283:11807–11822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Han L, Suda M, Tsuzuki K, Wang R, Ohe Y, Hirai H, Watanabe T, Takeuchi T, Hosaka M. 2008. A large form of secretogranin III functions as a sorting receptor for chromogranin A aggregates in PC12 cells. Mol Endocrinol 22:1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, Gasman S, Anouar Y. 2009. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem 284:12420–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Taupenot L, Harper KL, Mahapatra NR, Parmer RJ, Mahata SK, O'Connor DT. 2002. Identification of a novel sorting determinant for the regulated pathway in the secretory protein chromogranin A. J Cell Sci 115:4827–4841 [DOI] [PubMed] [Google Scholar]
- 126. Cowley DJ, Moore YR, Darling DS, Joyce PB, Gorr SU. 2000. N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J Biol Chem 275:7743–7748 [DOI] [PubMed] [Google Scholar]
- 127. Kang YK, Yoo SH. 1997. Identification of the secretory vesicle membrane binding region of chromogranin A. FEBS Lett 404:87–90 [DOI] [PubMed] [Google Scholar]
- 128. Thiele C, Huttner WB. 1998. The disulfide-bonded loop of chromogranins, which is essential for sorting to secretory granules, mediates homodimerization. J Biol Chem 273:1223–1231 [DOI] [PubMed] [Google Scholar]
- 129. Stettler H, Beuret N, Prescianotto-Baschong C, Fayard B, Taupenot L, Spiess M. 2009. Determinants for chromogranin A sorting into the regulated secretory pathway are also sufficient to generate granule-like structures in non-endocrine cells. Biochem J 418:81–91 [DOI] [PubMed] [Google Scholar]
- 130. Glombik MM, Krömer A, Salm T, Huttner WB, Gerdes HH. 1999. The disulfide-bonded loop of chromogranin B mediates membrane binding and directs sorting from the trans-Golgi network to secretory granules. Embo J 18:1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hotta K, Hosaka M, Tanabe A, Takeuchi T. 2009. Secretogranin II binds to secretogranin III and forms secretory granules with orexin, neuropeptide Y, and POMC. J Endocrinol 202:111–121 [DOI] [PubMed] [Google Scholar]
- 132. Hosaka M, Suda M, Sakai Y, Izumi T, Watanabe T, Takeuchi T. 2004. Secretogranin III binds to cholesterol in the secretory granule membrane as an adapter for chromogranin A. J Biol Chem 279:3627–3634 [DOI] [PubMed] [Google Scholar]
- 133. Gondré-Lewis MC, Petrache HI, Wassif CA, Harries D, Parsegian A, Porter FD, Loh YP. 2006. Abnormal sterols in cholesterol-deficiency diseases cause secretory granule malformation and decreased membrane curvature. J Cell Sci 119:1876–1885 [DOI] [PubMed] [Google Scholar]
- 134. Gentile F, Calì G, Zurzolo C, Corteggio A, Rosa P, Calegari F, Levi A, Possenti R, Puri C, Tacchetti C, Nitsch L. 2004. The neuroendocrine protein VGF is sorted into dense-core granules and is secreted apically by polarized rat thyroid epithelial cells. Exp Cell Res 295:269–280 [DOI] [PubMed] [Google Scholar]
- 135. Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. 2004. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem 279:20242–20249 [DOI] [PubMed] [Google Scholar]
- 136. Kim T, Zhang CF, Sun Z, Wu H, Loh YP. 2005. Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci 25:6958–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. 2005. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115:1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hendy GN, Li T, Girard M, Feldstein RC, Mulay S, Desjardins R, Day R, Karaplis AC, Tremblay ML, Canaff L. 2006. Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol Endocrinol 20:1935–1947 [DOI] [PubMed] [Google Scholar]
- 139. Obermüller S, Calegari F, King A, Lindqvist A, Lundquist I, Salehi A, Francolini M, Rosa P, Rorsman P, Huttner WB, Barg S. 2010. Defective secretion of islet hormones in chromogranin-B deficient mice. PLoS One 5:e8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Elias S, Delestre C, Courel M, Anouar Y, Montero-Hadjadje M. 2010. Chromogranin A as a crucial factor in the sorting of peptide hormones to secretory granules. Cell Mol Neurobiol 30:1189–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Machado JD, Díaz-Vera J, Domínguez N, Alvarez CM, Pardo MR, Borges R. 2010. Chromogranins A and B as regulators of vesicle cargo and exocytosis. Cell Mol Neurobiol 30:1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kim T, Loh YP. 2006. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol Biol Cell 17:789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP. 2011. Serpinin: A novel chromogranin A-derived, secreted peptide up-regulates protease-nexin 1 expression and granule biogenesis in endocrine cells. Mol Endocrinol 25:732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Knoch KP, Bergert H, Borgonovo B, Saeger HD, Altkrüger A, Verkade P, Solimena M. 2004. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat Cell Biol 6:207–214 [DOI] [PubMed] [Google Scholar]
- 145. Eiden LE. 1987. Is chromogranin a prohormone? Nature 325:301. [DOI] [PubMed] [Google Scholar]
- 146. González-Yanes C, Sánchez-Margalet V. 2000. Pancreastatin modulates insulin signaling in rat adipocytes: mechanisms of cross-talk. Diabetes 49:1288–1294 [DOI] [PubMed] [Google Scholar]
- 147. Sánchez-Margalet V, Calvo JR, Goberna R. 1992. Glucogenolytic and hyperglycemic effect of 33–49 C-terminal fragment of pancreastatin in the rat in vivo. Horm Metab Res 24:455–457 [DOI] [PubMed] [Google Scholar]
- 148. Sánchez-Margalet V, Calvo JR, Lucas M, Goberna R. 1992. Pancreastatin and its 33–49 C-terminal fragment inhibit glucagon-stimulated insulin in vivo. Gen Pharmacol 23:637–638 [DOI] [PubMed] [Google Scholar]
- 149. Ahrén B, Lindskog S, Tatemoto K, Efendiæ S. 1988. Pancreastatin inhibits insulin secretion and stimulates glucagon secretion in mice. Diabetes 37:281–285 [DOI] [PubMed] [Google Scholar]
- 150. Funakoshi S, Tamamura H, Ohta M, Yoshizawa K, Funakoshi A, Miyasaka K, Tateishi K, Tatemoto K, Nakano I, Yajima H. 1989. Isolation and characterization of a tumor-derived human pancreastatin-related protein. Biochem Biophys Res Commun 164:141–148 [DOI] [PubMed] [Google Scholar]
- 151. Efendiæ S, Tatemoto K, Mutt V, Quan C, Chang D, Ostenson CG. 1987. Pancreastatin and islet hormone release. Proc Natl Acad Sci USA 84:7257–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gomez G, Udupi V, Greeley GH., Jr 1997. Interaction of nicotine and a H2-receptor antagonist, famotidine, on gastrin and chromogranin A expression. Regul Pept 69:77–82 [DOI] [PubMed] [Google Scholar]
- 153. Drees BM, Hamilton JW. 1992. Pancreastatin and bovine parathyroid cell secretion. Bone Miner 17:335–346 [DOI] [PubMed] [Google Scholar]
- 154. Miyasaka K, Funakoshi A, Matsumoto M, Jimi A, Shikado F, Kitani K. 1991. Absence of luminal bile increases duodenal content of cholecystokinin in rats. Proc Soc Exp Biol Med 197:175–180 [DOI] [PubMed] [Google Scholar]
- 155. O'Connor DT, Cadman PE, Smiley C, Salem RM, Rao F, Smith J, Funk SD, Mahata SK, Mahata M, Wen G, Taupenot L, Gonzalez-Yanes C, Harper KL, Henry RR, Sanchez-Margalet V. 2005. Pancreastatin: multiple actions on human intermediary metabolism in vivo, variation in disease, and naturally occurring functional genetic polymorphism. J Clin Endocrinol Metab 90:5414–5425 [DOI] [PubMed] [Google Scholar]
- 156. Gayen JR, Saberi M, Schenk S, Biswas N, Vaingankar SM, Cheung WW, Najjar SM, O'Connor DT, Bandyopadhyay G, Mahata SK. 2009. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. J Biol Chem 284:28498–28509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN, Lee C, Elmquist JK, Lechan RM, Mobbs CV, Salton SR. 2002. VGF is required for obesity induced by diet, gold thioglucose treatment and agouti, and is differentially regulated in POMC- and NPY-containing arcuate neurons in response to fasting. J Neurosci 22:6929–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, Kelley KA, Takahashi JS, Pintar JE, Roberts JL, Mobbs CV, Salton SR. 1999. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron 23:537–548 [DOI] [PubMed] [Google Scholar]
- 159. Watson E, Fargali S, Okamoto H, Sadahiro M, Gordon RE, Chakraborty T, Sleeman MW, Salton SR. 2009. Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Watson E, Hahm S, Mizuno TM, Windsor J, Montgomery C, Scherer PE, Mobbs CV, Salton SR. 2005. VGF ablation blocks the development of hyperinsulinemia and hyperglycemia in several mouse models of obesity. Endocrinology 146:5151–5163 [DOI] [PubMed] [Google Scholar]
- 161. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 162. Ross AW, Bell LM, Littlewood PA, Mercer JG, Barrett P, Morgan PJ. 2005. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinology 146:1940–1947 [DOI] [PubMed] [Google Scholar]
- 163. Bartolomucci A, La Corte G, Possenti R, Locatelli V, Rigamonti AE, Torsello A, Bresciani E, Bulgarelli I, Rizzi R, Pavone F, D'Amato FR, Severini C, Mignogna G, Giorgi A, Schininà ME, Elia G, Brancia C, Ferri GL, Conti R, Ciani B, Pascucci T, Dell'Omo G, Muller EE, Levi A, Moles A. 2006. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci USA 103:14584–14589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Brancia C, Cocco C, D'Amato F, Noli B, Sanna F, Possenti R, Argiolas A, Ferri GL. 2010. Selective expression of Tlqp-21 and other Vgf peptides in gastric neuroendocrine cells, and modulation by feeding. J Endocrinol 207:329–341 [DOI] [PubMed] [Google Scholar]
- 165. Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JW, Carter WG, Bolton N, Bruggraber M, Morgan PJ, Barrett P, Ebling FJ. 2007. VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology 148:4044–4055 [DOI] [PubMed] [Google Scholar]
- 166. Pinilla L, Pineda R, Gaytán F, Romero M, García-Galiano D, Sánchez-Garrido MA, Ruiz-Pino F, Tena-Sempere M, Aguilar E. 2011. Characterization of the reproductive effects of the anorexigenic VGF-derived peptide, TLQP-21: in vivo and in vitro studies in male rats. Am J Physiol Endocrinol Metab 300:E837–E847 [DOI] [PubMed] [Google Scholar]
- 167. Bartolomucci A, Bresciani E, Bulgarelli I, Rigamonti AE, Pascucci T, Levi A, Possenti R, Torsello A, Locatelli V, Muller EE, Moles A. 2009. Chronic intracerebroventricular injection of TLQP-21 prevents high fat diet induced weight gain in fast weight-gaining mice. Genes Nutr 4:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bartolomucci A, Possenti R, Levi A, Pavone F, Moles A. 2007. The role of the vgf gene and VGF-derived peptides in nutrition and metabolism. Genes Nutr 2:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yamaguchi H, Sasaki K, Satomi Y, Shimbara T, Kageyama H, Mondal MS, Toshinai K, Date Y, González LJ, Shioda S, Takao T, Nakazato M, Minamino N. 2007. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J Biol Chem 282:26354–26360 [DOI] [PubMed] [Google Scholar]
- 170. Toshinai K, Nakazato M. 2009. Neuroendocrine regulatory peptide-1 and -2: novel bioactive peptides processed from VGF. Cell Mol Life Sci 66:1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Toshinai K, Yamaguchi H, Kageyama H, Matsuo T, Koshinaka K, Sasaki K, Shioda S, Minamino N, Nakazato M. 2010. Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am J Physiol Endocrinol Metab 299:E394–E401 [DOI] [PubMed] [Google Scholar]
- 172. Bartolomucci A, Moles A, Levi A, Possenti R. 2008. Pathophysiological role of TLQP-21: gastrointestinal and metabolic functions. Eat Weight Disord 13:e49–54 [PubMed] [Google Scholar]
- 173. Snyder SE, Peng B, Pintar JE, Salton SR. 2003. Expression of VGF mRNA in developing neuroendocrine and endocrine tissues. J Endocrinol 179:227–235 [DOI] [PubMed] [Google Scholar]
- 174. Snyder SE, Pintar JE, Salton SR. 1998. Developmental expression of VGF mRNA in the prenatal and postnatal rat. J Comp Neurol 394:64–90 [PubMed] [Google Scholar]
- 175. Ferri GL, Levi A, Possenti R. 1992. A novel neuroendocrine gene product: selective VGF8a gene expression and immuno-localisation of the VGF protein in endocrine and neuronal populations. Brain Res Mol Brain Res 13:139–143 [DOI] [PubMed] [Google Scholar]
- 176. Severini C, La Corte G, Improta G, Broccardo M, Agostini S, Petrella C, Sibilia V, Pagani F, Guidobono F, Bulgarelli I, Ferri GL, Brancia C, Rinaldi AM, Levi A, Possenti R. 2009. In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. Br J Pharmacol 157:984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kanemasa K, Okamura H, Kodama T, Ibata Y. 1995. Induction of VGF mRNA in neurons of the rat nucleus tractus solitarius and the dorsal motor nucleus of vagus in duodenal ulceration by cysteamine. Brain Res Mol Brain Res 32:55–62 [DOI] [PubMed] [Google Scholar]
- 178. Kanemasa K, Okamura H, Kodama T, Kashima K, Ibata Y. 1995. Time course of the induction of VGF mRNA in the dorsal vagal complex in rats with cysteamine-induced peptic ulcers. Brain Res Mol Brain Res 34:309–314 [DOI] [PubMed] [Google Scholar]
- 179. Sibilia V, Pagani F, Bulgarelli I, Mrak E, Broccardo M, Improta G, Severini C, Possenti R, Guidobono F. 2010. TLQP-21, a VGF-derived peptide, prevents ethanol-induced gastric lesions: insights into its mode of action. Neuroendocrinology 92:189–197 [DOI] [PubMed] [Google Scholar]
- 180. Sibilia V, Pagani F, Bulgarelli I, Tulipano G, Possenti R, Guidobono F. 4. December 2010 Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a vgf-derived peptide, in rats. Amino Acids 10.1007/s00726-010-0818-6 [DOI] [PubMed] [Google Scholar]
- 181. Zhou A, Webb G, Zhu X, Steiner DF. 1999. Proteolytic processing in the secretory pathway. J Biol Chem 274:20745–20748 [DOI] [PubMed] [Google Scholar]
- 182. Hsi KL, Seidah NG, De Serres G, Chrétien M. 1982. Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett 147:261–266 [DOI] [PubMed] [Google Scholar]
- 183. Braks JA, Martens GJ. 1994. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell 78:263–273 [DOI] [PubMed] [Google Scholar]
- 184. Lee SN, Lindberg I. 2008. 7B2 prevents unfolding and aggregation of prohormone convertase 2. Endocrinology 149:4116–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lindberg I, van den Hurk WH, Bui C, Batie CJ. 1995. Enzymatic characterization of immunopurified prohormone convertase 2: potent inhibition by a 7B2 peptide fragment. Biochemistry 34:5486–5493 [DOI] [PubMed] [Google Scholar]
- 186. van Horssen AM, van den Hurk WH, Bailyes EM, Hutton JC, Martens GJ, Lindberg I. 1995. Identification of the region within the neuroendocrine polypeptide 7B2 responsible for the inhibition of prohormone convertase PC2. J Biol Chem 270:14292–14296 [DOI] [PubMed] [Google Scholar]
- 187. Zhu X, Rouille Y, Lamango NS, Steiner DF, Lindberg I. 1996. Internal cleavage of the inhibitory 7B2 carboxyl-terminal peptide by PC2: a potential mechanism for its inactivation. Proc Natl Acad Sci USA 93:4919–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Muller L, Zhu P, Juliano MA, Juliano L, Lindberg I. 1999. A 36-residue peptide contains all of the information required for 7B2-mediated activation of prohormone convertase 2. J Biol Chem 274:21471–21477 [DOI] [PubMed] [Google Scholar]
- 189. Hwang JR, Lindberg I. 2001. Inactivation of the 7B2 inhibitory CT peptide depends on a functional furin cleavage site. J Neurochem 79:437–444 [DOI] [PubMed] [Google Scholar]
- 190. Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. 2000. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci 20:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. 2001. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proc Natl Acad Sci USA 98:9971–9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Apletalina E, Appel J, Lamango NS, Houghten RA, Lindberg I. 1998. Identification of inhibitors of prohormone convertases 1 and 2 using a peptide combinatorial library. J Biol Chem 273:26589–26595 [DOI] [PubMed] [Google Scholar]
- 193. Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, Fricker LD. 2000. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem 275:23596–23601 [DOI] [PubMed] [Google Scholar]
- 194. Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chrétien M, Seidah NG. 2001. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J Biol Chem 276:32720–32728 [DOI] [PubMed] [Google Scholar]
- 195. Morgan DJ, Mzhavia N, Peng B, Pan H, Devi LA, Pintar JE. 2005. Embryonic gene expression and pro-protein processing of proSAAS during rodent development. J Neurochem 93:1454–1462 [DOI] [PubMed] [Google Scholar]
- 196. Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT. 2004. The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol 66:1180–1191 [DOI] [PubMed] [Google Scholar]
- 197. Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. 1998. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides 19:1241–1248 [DOI] [PubMed] [Google Scholar]
- 198. Krüger PG, Mahata SK, Helle KB. 2003. Catestatin (CgA344–364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul Pept 114:29–35 [DOI] [PubMed] [Google Scholar]
- 199. Mahata SK, Mahata M, Parmer RJ, O'Connor DT. 1999. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin a344–364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem 274:2920–2928 [DOI] [PubMed] [Google Scholar]
- 200. Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O'Connor DT. 2007. Catecholamine release-inhibitory peptide catestatin (chromogranin A352–372): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation 115:2271–2281 [DOI] [PubMed] [Google Scholar]
- 201. Saria A, Troger J, Kirchmair R, Fischer-Colbrie R, Hogue-Angeletti R, Winkler H. 1993. Secretoneurin releases dopamine from rat striatal slices: a biological effect of a peptide derived from secretogranin II (chromogranin C). Neuroscience 54:1–4 [DOI] [PubMed] [Google Scholar]
- 202. Agneter E, Sitte HH, Stöckl-Hiesleitner S, Fischer-Colbrie R, Winkler H, Singer EA. 1995. Sustained dopamine release induced by secretoneurin in the striatum of the rat: a microdialysis study. J Neurochem 65:622–625 [DOI] [PubMed] [Google Scholar]
- 203. You ZB, Saria A, Fischer-Colbrie R, Terenius L, Goiny M, Herrera-Marschitz M. 1996. Effects of secretogranin II-derived peptides on the release of neurotransmitters monitored in the basal ganglia of the rat with in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol 354:717–724 [DOI] [PubMed] [Google Scholar]
- 204. Zhao E, Basak A, Wong AO, Ko W, Chen A, López GC, Grey CL, Canosa LF, Somoza GM, Chang JP, Trudeau VL. 2009. The secretogranin II-derived peptide secretoneurin stimulates luteinizing hormone secretion from gonadotrophs. Endocrinology 150:2273–2282 [DOI] [PubMed] [Google Scholar]
- 205. Zhao E, McNeilly JR, McNeilly AS, Fischer-Colbrie R, Basak A, Seong JY, Trudeau VL. 2011. Secretoneurin stimulates the production and release of luteinizing hormone in mouse LβT2 gonadotropin cells. Am J Physiol Endocrinol Metab 301:E288–E297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB. 2003. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci 23:10800–10808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, Alberini CM, Huntley GW, Salton SR. 2008. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci 28:9857–9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Moss A, Ingram R, Koch S, Theodorou A, Low L, Baccei M, Hathway GJ, Costigan M, Salton SR, Fitzgerald M. 2008. Origins, actions and dynamic expression patterns of the neuropeptide VGF in rat peripheral and central sensory neurones following peripheral nerve injury. Mol Pain 4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Riedl MS, Braun PD, Kitto KF, Roiko SA, Anderson LB, Honda CN, Fairbanks CA, Vulchanova L. 2009. Proteomic analysis uncovers novel actions of the neurosecretory protein VGF in nociceptive processing. J Neurosci 29:13377–13388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. 2005. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021 [DOI] [PubMed] [Google Scholar]
- 211. Song G, Cechvala C, Resnick DK, Dempsey RJ, Rao VL. 2001. GeneChip analysis after acute spinal cord injury in rat. J Neurochem 79:804–815 [DOI] [PubMed] [Google Scholar]
- 212. Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. 2002. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Valder CR, Liu JJ, Song YH, Luo ZD. 2003. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem 87:560–573 [DOI] [PubMed] [Google Scholar]
- 214. Rizzi R, Bartolomucci A, Moles A, D'Amato F, Sacerdote P, Levi A, La Corte G, Ciotti MT, Possenti R, Pavone F. 2008. The VGF-derived peptide TLQP-21: a new modulatory peptide for inflammatory pain. Neurosci Lett 441:129–133 [DOI] [PubMed] [Google Scholar]
- 215. Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. 2004. The effect of a chromogranin A-derived peptide (CgA4–16) in the writhing nociceptive response induced by acetic acid in rats. Life Sci 75:1787–1799 [DOI] [PubMed] [Google Scholar]
- 216. Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. 2004. Effects of a chromogranin-derived peptide (CgA 47–66) in the writhing nociceptive response induced by acetic acid in rats. Regul Pept 119:199–207 [DOI] [PubMed] [Google Scholar]
- 217. van Kammen DP, Peters J, Yao J, Neylan T, Beuger M, Pontius E, O'Connor DT. 1991. CSF chromogranin A-like immunoreactivity in schizophrenia. Assessment of clinical and biochemical relationships. Schizophr Res 6:31–39 [DOI] [PubMed] [Google Scholar]
- 218. Landén M, Davidsson P, Gottfries CG, Grenfeldt B, Stridsberg M, Blennow K. 1999. Reduction of the small synaptic vesicle protein synaptophysin but not the large dense core chromogranins in the left thalamus of subjects with schizophrenia. Biol Psychiatry 46:1698–1702 [DOI] [PubMed] [Google Scholar]
- 219. Landén M, Grenfeldt B, Davidsson P, Stridsberg M, Regland B, Gottfries CG, Blennow K. 1999. Reduction of chromogranin A and B but not C in the cerebrospinal fluid in subjects with schizophrenia. Eur Neuropsychopharmacol 9:311–315 [DOI] [PubMed] [Google Scholar]
- 220. Miller C, Kirchmair R, Troger J, Saria A, Fleischhacker WW, Fischer-Colbrie R, Benzer A, Winkler H. 1996. CSF of neuroleptic-naive first-episode schizophrenic patients: levels of biogenic amines, substance P, and peptides derived from chromogranin A (GE-25) and secretogranin II (secretoneurin). Biol Psychiatry 39:911–918 [DOI] [PubMed] [Google Scholar]
- 221. Nowakowski C, Kaufmann WA, Adlassnig C, Maier H, Salimi K, Jellinger KA, Marksteiner J. 2002. Reduction of chromogranin B-like immunoreactivity in distinct subregions of the hippocampus from individuals with schizophrenia. Schizophr Res 58:43–53 [DOI] [PubMed] [Google Scholar]
- 222. Cattaneo A, Sesta A, Calabrese F, Nielsen G, Riva MA, Gennarelli M. 2010. The expression of VGF is reduced in leukocytes of depressed patients and it is restored by effective antidepressant treatment. Neuropsychopharmacology 35:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Thakker-Varia S, Jean YY, Parikh P, Sizer CF, Jernstedt Ayer J, Parikh A, Hyde TM, Buyske S, Alder J. 2010. The neuropeptide VGF is reduced in human bipolar postmortem brain and contributes to some of the behavioral and molecular effects of lithium. J Neurosci 30:9368–9380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Huang JT, Leweke FM, Oxley D, Wang L, Harris N, Koethe D, Gerth CW, Nolden BM, Gross S, Schreiber D, Reed B, Bahn S. 2006. Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis. PLoS Med 3:e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Huang JT, Leweke FM, Tsang TM, Koethe D, Kranaster L, Gerth CW, Gross S, Schreiber D, Ruhrmann S, Schultze-Lutter F, Klosterkötter J, Holmes E, Bahn S. 2007. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS One 2:e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. 2007. Antidepressant actions of the exercise-regulated gene VGF. Nat Med 13:1476–1482 [DOI] [PubMed] [Google Scholar]
- 227. Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, Black IB, Alder J. 2007. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci 27:12156–12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wakonigg G, Zernig G, Berger I, Fischer-Colbrie R, Laslop A, Saria A. 2002. Lack of a distinctive behavioural effect of chromogranin-derived peptides in rodents. Regul Pept 103:85–91 [DOI] [PubMed] [Google Scholar]
- 229. Succu S, Cocco C, Mascia MS, Melis T, Melis MR, Possenti R, Levi A, Ferri GL, Argiolas A. 2004. Pro-VGF-derived peptides induce penile erection in male rats: possible involvement of oxytocin. Eur J Neurosci 20:3035–3040 [DOI] [PubMed] [Google Scholar]
- 230. Succu S, Mascia MS, Melis T, Sanna F, Melis MR, Possenti R, Argiolas A. 2005. Pro-VGF-derived peptides induce penile erection in male rats: Involvement of paraventricular nitric oxide. Neuropharmacology 49:1017–1025 [DOI] [PubMed] [Google Scholar]
- 231. Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR, Schratzberger P, Djanani AM, Mahata SK, Kirchmair R. 2008. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol 598:104–111 [DOI] [PubMed] [Google Scholar]
- 232. Zhang D, Shooshtarizadeh P, Laventie BJ, Colin DA, Chich JF, Vidic J, de Barry J, Chasserot-Golaz S, Delalande F, Van Dorsselaer A, Schneider F, Helle K, Aunis D, Prévost G, Metz-Boutigue MH. 2009. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One 4:e4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH, Patsch JR, Wolf D, Schratzberger P, Mahata SK, Kirchmair R. 2010. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res 107:1326–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Storch MK, Fischer-Colbrie R, Smith T, Rinner WA, Hickey WF, Cuzner ML, Winkler H, Lassmann H. 1996. Co-localization of secretoneurin immunoreactivity and macrophage infiltration in the lesions of experimental autoimmune encephalomyelitis. Neuroscience 71:885–893 [DOI] [PubMed] [Google Scholar]
- 235. Ferrero E, Scabini S, Magni E, Foglieni C, Belloni D, Colombo B, Curnis F, Villa A, Ferrero ME, Corti A. 2004. Chromogranin A protects vessels against tumor necrosis factor α-induced vascular leakage. FASEB J 18:554–556 [DOI] [PubMed] [Google Scholar]
- 236. Blois A, Srebro B, Mandalà M, Corti A, Helle KB, Serck-Hanssen G. 2006. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regul Pept 135:78–84 [DOI] [PubMed] [Google Scholar]
- 237. Belloni D, Scabini S, Foglieni C, Veschini L, Giazzon A, Colombo B, Fulgenzi A, Helle KB, Ferrero ME, Corti A, Ferrero E. 2007. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. FASEB J 21:3052–3062 [DOI] [PubMed] [Google Scholar]
- 238. Helle KB. 2010. Regulatory peptides from chromogranin A and secretogranin II: putative modulators of cells and tissues involved in inflammatory conditions. Regul Pept 165:45–51 [DOI] [PubMed] [Google Scholar]
- 239. Shooshtarizadeh P, Zhang D, Chich JF, Gasnier C, Schneider F, Haïkel Y, Aunis D, Metz-Boutigue MH. 2010. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept 165:102–110 [DOI] [PubMed] [Google Scholar]
- 240. Takiyyuddin MA, Baron AD, Cervenka JH, Barbosa JA, Neumann HP, Parmer RJ, Sullivan PA, O'Connor DT. 1991. Suppression of chromogranin-A release from neuroendocrine sources in man: pharmacological studies. J Clin Endocrinol Metab 72:616–622 [DOI] [PubMed] [Google Scholar]
- 241. Dimsdale JE, O'Connor DT, Ziegler M, Mills P. 1992. Chromogranin A correlates with norepinephrine release rate. Life Sci 51:519–525 [DOI] [PubMed] [Google Scholar]
- 242. Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA. 1995. Chromogranin A in human hypertension. Influence of heredity. Hypertension 26:213–220 [DOI] [PubMed] [Google Scholar]
- 243. O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. 2002. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345 [DOI] [PubMed] [Google Scholar]
- 244. O'Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. 2008. Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation 118:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, Ziegler MG, Mahata SK, O'Connor DT. 2010. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens 28:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, Torpey JW, O'Connor DT, Mahata SK. 2008. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology 149:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kirchmair R, Egger M, Walter DH, Eisterer W, Niederwanger A, Woell E, Nagl M, Pedrini M, Murayama T, Frauscher S, Hanley A, Silver M, Brodmann M, Sturm W, Fischer-Colbrie R, Losordo DW, Patsch JR, Schratzberger P. 2004. Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation 110:1121–1127 [DOI] [PubMed] [Google Scholar]
- 248. Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, Murayama T, Kaneider N, Sturm W, Kearny M, Fischer-Colbrie R, Kircher B, Gaenzer H, Wiedermann CJ, Ropper AH, Losordo DW, Patsch JR, Schratzberger P. 2004. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 109:777–783 [DOI] [PubMed] [Google Scholar]
- 249. Egger M, Schgoer W, Beer AG, Jeschke J, Leierer J, Theurl M, Frauscher S, Tepper OM, Niederwanger A, Ritsch A, Kearney M, Wanschitz J, Gurtner GC, Fischer-Colbrie R, Weiss G, Piza-Katzer H, Losordo DW, Patsch JR, Schratzberger P, Kirchmair R. 2007. Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1α- and basic FGF-dependent pathway in muscle cells. FASEB J 21:2906–2917 [DOI] [PubMed] [Google Scholar]
- 250. Schgoer W, Theurl M, Jeschke J, Beer AG, Albrecht K, Gander R, Rong S, Vasiljevic D, Egger M, Wolf AM, Frauscher S, Koller B, Tancevski I, Patsch JR, Schratzberger P, Piza-Katzer H, Ritsch A, Bahlmann FH, Fischer-Colbrie R, Wolf D, Kirchmair R. 2009. Gene therapy with the angiogenic cytokine secretoneurin induces therapeutic angiogenesis by a nitric oxide-dependent mechanism. Circ Res 105:994–1002 [DOI] [PubMed] [Google Scholar]
- 251. Shyu WC, Lin SZ, Chiang MF, Chen DC, Su CY, Wang HJ, Liu RS, Tsai CH, Li H. 2008. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest 118:133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Aardal S, Helle KB. 1992. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept 41:9–18 [DOI] [PubMed] [Google Scholar]
- 253. Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. 2007. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 28:1117–1127 [DOI] [PubMed] [Google Scholar]
- 254. Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. 2008. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Mazza R, Gattuso A, Mannarino C, Brar BK, Barbieri SF, Tota B, Mahata SK. 2008. Catestatin (chromogranin A344–364) is a novel cardiosuppressive agent: inhibition of isoproterenol and endothelin signaling in the frog heart. Am J Physiol Heart Circ Physiol 295:H113–H122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Imbrogno S, Garofalo F, Cerra MC, Mahata SK, Tota B. 2010. The catecholamine release-inhibitory peptide catestatin (chromogranin A344–364) modulates myocardial function in fish. J Exp Biol 213:3636–3643 [DOI] [PubMed] [Google Scholar]
- 257. Mazza R, Imbrogno S, Tota B. 2010. The interplay between chromogranin A-derived peptides and cardiac natriuretic peptides in cardioprotection against catecholamine-evoked stress. Regul Pept 165:86–94 [DOI] [PubMed] [Google Scholar]
- 258. Tota B, Cerra MC, Gattuso A. 2010. Catecholamines, cardiac natriuretic peptides and chromogranin A: evolution and physiopathology of a ‘whip-brake’ system of the endocrine heart. J Exp Biol 213:3081–3103 [DOI] [PubMed] [Google Scholar]
- 259. Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O'Connor DT, Hamilton BA. 2004. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet 74:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Zhang B, Tan Z, Zhang C, Shi Y, Lin Z, Gu N, Feng G, He L. 2002. Polymorphisms of chromogranin B gene associated with schizophrenia in Chinese Han population. Neurosci Lett 323:229–233 [DOI] [PubMed] [Google Scholar]
- 261. Iijima Y, Inada T, Ohtsuki T, Senoo H, Nakatani M, Arinami T. 2004. Association between chromogranin b gene polymorphisms and schizophrenia in the Japanese population. Biol Psychiatry 56:10–17 [DOI] [PubMed] [Google Scholar]
- 262. Halperin E, Eskin E. 2004. Haplotype reconstruction from genotype data using Imperfect Phylogeny. Bioinformatics 20:1842–1849 [DOI] [PubMed] [Google Scholar]
- 263. Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, Salem RM, Shih PA, Das M, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, O'Connor DT. 2008. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int 74:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Comings DE, MacMurray JP. 2000. Molecular heterosis: a review. Mol Genet Metab 71:19–31 [DOI] [PubMed] [Google Scholar]
- 265. Zhang K, Rao F, Rana BK, Gayen JR, Calegari F, King A, Rosa P, Huttner WB, Stridsberg M, Mahata M, Vaingankar S, Mahboubi V, Salem RM, Rodriguez-Flores JL, Fung MM, Smith DW, Schork NJ, Ziegler MG, Taupenot L, Mahata SK, OConnor DT. 2009. Autonomic function in hypertension; role of genetic variation at the catecholamine storage vesicle protein chromogranin B. Circ Cardiovasc Genet 2:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O'Connor DT. 2008. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol 52:1468–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, Carmona E, Castañeyra A, Viveros OH, O'Connor DT, Mahata SK, Borges R. 2008. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci 28:3350–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Díaz-Vera J, Morales YG, Hernández-Fernaud JR, Camacho M, Montesinos MS, Calegari F, Huttner WB, Borges R, Machado JD. 2010. Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles. J Neurosci 30:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gayen JR, Zhang K, RamachandraRao SP, Mahata M, Chen Y, Kim HS, Naviaux RK, Sharma K, Mahata SK, O'Connor DT. 2010. Role of reactive oxygen species in hyper-adrenergic hypertension: biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin A (Chga) gene. Circ Cardiovasc Genet 3:414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Vaingankar SM, Li Y, Corti A, Biswas N, Gayen J, O'Connor DT, Mahata SK. 2010. Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic “rescue” of disease phenotypes in the mouse Chga knockout. Physiol Genomics 41:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. 1989. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA 86:8000–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Sarac MS, Zieske AW, Lindberg I. 2002. The lethal form of Cushing's in 7B2 null mice is caused by multiple metabolic and hormonal abnormalities. Endocrinology 143:2324–2332 [DOI] [PubMed] [Google Scholar]
- 273. Levi A, Eldridge JD, Paterson BM. 1985. Molecular cloning of a gene sequence regulated by nerve growth factor. Science 229:393–395 [DOI] [PubMed] [Google Scholar]
- 274. Cho KO, Skarnes WC, Minsk B, Palmieri S, Jackson-Grusby L, Wagner JA. 1989. Nerve growth factor regulates gene expression by several distinct mechanisms. Mol Cell Biol 9:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Salton SR, Fischberg DJ, Dong KW. 1991. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol Cell Biol 11:2335–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Possenti R, Eldridge JD, Paterson BM, Grasso A, Levi A. 1989. A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. Embo J 8:2217–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Possenti R, Levi A. 2000. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol 21:199–219 [DOI] [PubMed] [Google Scholar]
- 278. Wei S, Feng Y, Che FY, Pan H, Mzhavia N, Devi LA, McKinzie AA, Levin N, Richards WG, Fricker LD. 2004. Obesity and diabetes in transgenic mice expressing proSAAS. J Endocrinol 180:357–368 [DOI] [PubMed] [Google Scholar]
- 279. Morgan DJ, Wei S, Gomes I, Czyzyk T, Mzhavia N, Pan H, Devi LA, Fricker LD, Pintar JE. 2010. The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation. J Neurochem 113:1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. 2002. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA 99:10293–10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Kass J, Jacob TC, Kim P, Kaplan JM. 2001. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci 21:9265–9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Lindberg I, Tu B, Muller L, Dickerson IM. 1998. Cloning and functional analysis of C. elegans 7B2. DNA Cell Biol 17:727–734 [DOI] [PubMed] [Google Scholar]
- 283. Hwang JR, Siekhaus DE, Fuller RS, Taghert PH, Lindberg I. 2000. Interaction of Drosophila melanogaster prohormone convertase 2 and 7B2. Insect cell-specific processing and secretion. J Biol Chem 275:17886–17893 [DOI] [PubMed] [Google Scholar]
- 284. Rieker S, Fischer-Colbrie R, Eiden L, Winkler H. 1988. Phylogenetic distribution of peptides related to chromogranins A and B. J Neurochem 50:1066–1073 [DOI] [PubMed] [Google Scholar]
- 285. Trandaburu T, Ali SS. 1998. Granin proteins (chromogranin A and secretogranin II C23-3 and C26-3) in the intestine of amphibians. Ann Anat 180:523–528 [DOI] [PubMed] [Google Scholar]
- 286. Trandaburu T, Ali SS, Trandaburu I. 1999. Granin proteins (chromogranin A and secretogranin II C23-3 and C26-3) in the endocrine pancreas of amphibians. Ann Anat 181:585–592 [DOI] [PubMed] [Google Scholar]
- 287. Trandaburu T, Ali SS, Trandaburu I. 1999. Granin proteins (chromogranin A and secretogranin II C23-3 and C26-3) in the intestine of reptiles. Ann Anat 181:261–268 [DOI] [PubMed] [Google Scholar]
- 288. Xie J, Wang WQ, Liu TX, Deng M, Ning G. 2008. Spatio-temporal expression of chromogranin A during zebrafish embryogenesis. J Endocrinol 198:451–458 [DOI] [PubMed] [Google Scholar]
- 289. Biomarkers DWG. 2001. Biomarkers and surrogate endpoints: preferred definitions and conceptural framework. Clin Pharmacol Ther 69:89–95 [DOI] [PubMed] [Google Scholar]
- 290. Conlon JM. 2010. Granin-derived peptides as diagnostic and prognostic markers for endocrine tumors. Regul Pept 165:5–11 [DOI] [PubMed] [Google Scholar]
- 291. Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, Goel R, Mathivanan S, Marimuthu A, Kashyap M, Vizza RF, Mayer RJ, Decaprio JA, Srivastava S, Hanash SM, Hruban RH, Pandey A. 2009. A compendium of potential biomarkers of pancreatic cancer. PLoS Med 6:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J, Pape UF, Plöckinger U. 2009. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology 90:194–202 [DOI] [PubMed] [Google Scholar]
- 293. Bartolomucci A, Pasinetti GM, Salton SR. 2010. Granins as disease-biomarkers: translational potential for psychiatric and neurological disorders. Neuroscience 170:289–297 [DOI] [PubMed] [Google Scholar]
- 294. Nilsson O, Jakobsen AM, Kölby L, Bernhardt P, Forssell-Aronsson E, Ahlman H. 2004. Importance of vesicle proteins in the diagnosis and treatment of neuroendocrine tumors. Ann NY Acad Sci 1014:280–283 [DOI] [PubMed] [Google Scholar]
- 295. Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. 2010. Granins and granin-related peptides in neuroendocrine tumours. Regul Pept 165:12–20 [DOI] [PubMed] [Google Scholar]
- 296. Yon L, Guillemot J, Montero-Hadjadje M, Grumolato L, Leprince J, Lefebvre H, Contesse V, Plouin PF, Vaudry H, Anouar Y. 2003. Identification of the secretogranin II- derived peptide EM66 in pheochromocytomas as a potential marker for discriminating benign versus malignant tumors. J Clin Endocrinol Metab 88:2579–2585 [DOI] [PubMed] [Google Scholar]
- 297. Guillemot J, Anouar Y, Montero-Hadjadje M, Grouzmann E, Grumolato L, Roshmaninho-Salgado J, Turquier V, Duparc C, Lefebvre H, Plouin PF, Klein M, Muresan M, Chow BK, Vaudry H, Yon L. 2006. Circulating EM66 is a highly sensitive marker for the diagnosis and follow-up of pheochromocytoma. Int J Cancer 118:2003–2012 [DOI] [PubMed] [Google Scholar]
- 298. Stridsberg M, Eriksson B, Janson ET. 2008. Measurements of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul Pept 148:95–98 [DOI] [PubMed] [Google Scholar]
- 299. Jakobsen AM, Ahlman H, Kölby L, Abrahamsson J, Fischer-Colbrie R, Nilsson O. 2003. NESP55, a novel chromogranin-like peptide, is expressed in endocrine tumours of the pancreas and adrenal medulla but not in ileal carcinoids. Br J Cancer 88:1746–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Srivastava A, Padilla O, Fischer-Colbrie R, Tischler AS, Dayal Y. 2004. Neuroendocrine secretory protein-55 (NESP-55) expression discriminates pancreatic endocrine tumors and pheochromocytomas from gastrointestinal and pulmonary carcinoids. Am J Surg Pathol 28:1371–1378 [DOI] [PubMed] [Google Scholar]
- 301. Srivastava A, Hornick JL. 2009. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol 33:626–632 [DOI] [PubMed] [Google Scholar]
- 302. Mitra A, Fillmore RA, Metge BJ, Rajesh M, Xi Y, King J, Ju J, Pannell L, Shevde LA, Samant RS. 2008. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res 10:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Moss AC, Jacobson GM, Walker LE, Blake NW, Marshall E, Coulson JM. 2009. SCG3 transcript in peripheral blood is a prognostic biomarker for REST-deficient small cell lung cancer. Clin Cancer Res 15:274–283 [DOI] [PubMed] [Google Scholar]
- 304. Waha A, Koch A, Hartmann W, Milde U, Felsberg J, Hübner A, Mikeska T, Goodyer CG, Sörensen N, Lindberg I, Wiestler OD, Pietsch T, Waha A. 2007. SGNE1/7B2 is epigenetically altered and transcriptionally downregulated in human medulloblastomas. Oncogene 26:5662–5668 [DOI] [PubMed] [Google Scholar]
- 305. Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, Naya Y, Ichikawa T, Fuse H. 2009. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 16:37–44 [DOI] [PubMed] [Google Scholar]
- 306. Massironi S, Conte D, Sciola V, Spampatti MP, Ciafardini C, Valenti L, Rossi RE, Peracchi M. 2010. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am J Gastroenterol 105:2072–2078 [DOI] [PubMed] [Google Scholar]
- 307. Kimura N, Yoshida R, Shiraishi S, Pilichowska M, Ohuchi N. 2002. Chromogranin A and chromogranin B in noninvasive and invasive breast carcinoma. Endocr Pathol 13:117–122 [DOI] [PubMed] [Google Scholar]
- 308. Righi L, Sapino A, Marchiò C, Papotti M, Bussolati G. 2010. Neuroendocrine differentiation in breast cancer: established facts and unresolved problems. Semin Diagn Pathol 27:69–76 [DOI] [PubMed] [Google Scholar]
- 309. Guillemot J, Barbier L, Thouennon E, Vallet-Erdtmann V, Montero-Hadjadje M, Lefebvre H, Klein M, Muresan M, Plouin PF, Seidah N, Vaudry H, Anouar Y, Yon L. 2006. Expression and processing of the neuroendocrine protein secretogranin II in benign and malignant pheochromocytomas. Ann NY Acad Sci 1073:527–532 [DOI] [PubMed] [Google Scholar]
- 310. Corti A. 2010. Chromogranin A and the tumor microenvironment. Cell Mol Neurobiol 30:1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Guérin M, Guillemot J, Thouënnon E, Pierre A, El-Yamani FZ, Montero-Hadjadje M, Dubessy C, Magoul R, Lihrmann I, Anouar Y, Yon L. 2010. Granins and their derived peptides in normal and tumoral chromaffin tissue: implications for the diagnosis and prognosis of pheochromocytoma. Regul Pept 165:21–29 [DOI] [PubMed] [Google Scholar]
- 312. Fries RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O'Connor DT. 2004. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension 43:1301–1311 [DOI] [PubMed] [Google Scholar]
- 313. O'Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, Mahata SK, Wu H, Kennedy BP, Ziegler MG, Wright FA, Schlager G, Parmer RJ. 1999. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press 8:285–295 [DOI] [PubMed] [Google Scholar]
- 314. Schober M, Howe PR, Sperk G, Fischer-Colbrie R, Winkler H. 1989. An increased pool of secretory hormones and peptides in adrenal medulla of stroke-prone spontaneously hypertensive rats. Hypertension 13:469–474 [DOI] [PubMed] [Google Scholar]
- 315. Sánchez-Margalet V, Valle M, Lobón JA, Escobar-Jiménez F, Pérez-Cano R, Goberna R. 1995. Plasma pancreastatin-like immunoreactivity correlates with plasma norepinephrine levels in essential hypertension. Neuropeptides 29:97–101 [DOI] [PubMed] [Google Scholar]
- 316. Sánchez-Margalet V, Valle M, Lobón JA, Maldonado A, Escobar-Jimenez F, Oliván J, Pérez-Cano R, Goberna R. 1995. Increased plasma pancreastatin-like immunoreactivity levels in non-obese patients with essential hypertension. J Hypertens 13:251–258 [PubMed] [Google Scholar]
- 317. Omland T, Dickstein K, Syversen U. 2003. Association between plasma chromogranin A concentration and long-term mortality after myocardial infarction. Am J Med 114:25–30 [DOI] [PubMed] [Google Scholar]
- 318. Estensen ME, Hognestad A, Syversen U, Squire I, Ng L, Kjekshus J, Dickstein K, Omland T. 2006. Prognostic value of plasma chromogranin A levels in patients with complicated myocardial infarction. Am Heart J 152:927.e1–927.e6 [DOI] [PubMed] [Google Scholar]
- 319. Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K. 2009. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J 30:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Tota B, Angelone T, Mazza R, Cerra MC. 2008. The chromogranin A-derived vasostatins: new players in the endocrine heart. Curr Med Chem 15:1444–1451 [DOI] [PubMed] [Google Scholar]
- 321. Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. 2002. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 23:967–974 [DOI] [PubMed] [Google Scholar]
- 322. Corti A, Ferrari R, Ceconi C. 2000. Chromogranin A and tumor necrosis factor-α (TNF) in chronic heart failure. Adv Exp Med Biol 482:351–359 [DOI] [PubMed] [Google Scholar]
- 323. Røsjø H, Masson S, Latini R, Flyvbjerg A, Milani V, La Rovere MT, Revera M, Mezzani A, Tognoni G, Tavazzi L, Omland T. 2010. Prognostic value of chromogranin A in chronic heart failure: data from the GISSI-Heart Failure trial. Eur J Heart Fail 12:549–556 [DOI] [PubMed] [Google Scholar]
- 324. Penna C, Alloatti G, Gallo MP, Cerra MC, Levi R, Tullio F, Bassino E, Dolgetta S, Mahata SK, Tota B, Pagliaro P. 2010. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cell Mol Neurobiol 30:1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Di Comite G, Morganti A. 2011. Chromogranin A: a novel factor acting at the cross road between the neuroendocrine and the cardiovascular systems. J Hypertens 29:409–414 [DOI] [PubMed] [Google Scholar]
- 326. Wohlschlaeger J, von Winterfeld M, Milting H, El Banayosy A, Schmitz KJ, Takeda A, Takeda N, Azhari P, Schmid C, August C, Schmid KW, Baba HA. 2008. Decreased myocardial chromogranin a expression and colocalization with brain natriuretic peptide during reverse cardiac remodeling after ventricular unloading. J Heart Lung Transplant 27:442–449 [DOI] [PubMed] [Google Scholar]
- 327. Greenwood TA, Cadman PE, Stridsberg M, Nguyen S, Taupenot L, Schork NJ, O'Connor DT. 2004. Genome-wide linkage analysis of chromogranin B expression in the CEPH pedigrees: implications for exocytotic sympathochromaffin secretion in humans. Physiol Genomics 18:119–127 [DOI] [PubMed] [Google Scholar]
- 328. Greenwood TA, Rao F, Stridsberg M, Mahapatra NR, Mahata M, Lillie EO, Mahata SK, Taupenot L, Schork NJ, O'Connor DT. 2006. Pleiotropic effects of novel trans-acting loci influencing human sympathochromaffin secretion. Physiol Genomics 25:470–479 [DOI] [PubMed] [Google Scholar]
- 329. Takiyyuddin MA, De Nicola L, Gabbai FB, Dinh TQ, Kennedy B, Ziegler MG, Sabban EL, Parmer RJ, O'Connor DT. 1993. Catecholamine secretory vesicles. Augmented chromogranins and amines in secondary hypertension. Hypertension 21:674–679 [DOI] [PubMed] [Google Scholar]
- 330. Røsjø H, Husberg C, Dahl MB, Stridsberg M, Sjaastad I, Finsen AV, Carlson CR, Oie E, Omland T, Christensen G. 2010. Chromogranin B in heart failure: a putative cardiac biomarker expressed in the failing myocardium. Circ Heart Fail 3:503–511 [DOI] [PubMed] [Google Scholar]
- 331. Wiedermann CJ. 2000. Secretoneurin: a functional neuropeptide in health and disease. Peptides 21:1289–1298 [DOI] [PubMed] [Google Scholar]
- 332. Wen G, Wessel J, Zhou W, Ehret GB, Rao F, Stridsberg M, Mahata SK, Gent PM, Das M, Cooper RS, Chakravarti A, Zhou H, Schork NJ, O'connor DT, Hamilton BA. 2007. An ancestral variant of secretogranin II confers regulation by PHOX2 transcription factors and association with hypertension. Hum Mol Genet 16:1752–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Sprott H, Pap T, Rethage J, Wintersberger W, Gay RE, Bradley LA, Uebelhart D, Gay S. 2000. Expression of the precursor of secretoneurin, secretogranin II, in the synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 27:2347–2350 [PubMed] [Google Scholar]
- 334. Fournier I, Gaucher D, Chich JF, Bach C, Shooshtarizadeh P, Picaud S, Bourcier T, Speeg-Schatz C, Strub JM, Van Dorsselaer A, Corti A, Aunis D, Metz-Boutigue MH. 2011. Processing of chromogranins/secretogranin in patients with diabetic retinopathy. Regul Pept 167:118–124 [DOI] [PubMed] [Google Scholar]
- 335. Zhang D, Lavaux T, Sapin R, Lavigne T, Castelain V, Aunis D, Metz-Boutigue MH, Schneider F. 2009. Serum concentration of chromogranin A at admission: an early biomarker of severity in critically ill patients. Ann Med 41:38–44 [DOI] [PubMed] [Google Scholar]
- 336. Yasuhara O, Kawamata T, Aimi Y, McGeer EG, McGeer PL. 1994. Expression of chromogranin A in lesions in the central nervous system from patients with neurological diseases. Neurosci Lett 170:13–16 [DOI] [PubMed] [Google Scholar]
- 337. Schiffer D, Cordera S, Giordana MT, Attanasio A, Pezzulo T. 1995. Synaptic vesicle proteins, synaptophysin and chromogranin A in amyotrophic lateral sclerosis. J Neurol Sci 129(Suppl):68–74 [DOI] [PubMed] [Google Scholar]
- 338. Schrott-Fischer A, Bitsche M, Humpel C, Walcher C, Maier H, Jellinger K, Rabl W, Glueckert R, Marksteiner J. 2009. Chromogranin peptides in amyotrophic lateral sclerosis. Regul Pept 152:13–21 [DOI] [PubMed] [Google Scholar]
- 339. Obayashi K, Sato K, Shimazaki R, Ishikawa T, Goto K, Ueyama H, Mori T, Ando Y, Kumamoto T. 2008. Salivary chromogranin A: useful and quantitative biochemical marker of affective state in patients with amyotrophic lateral sclerosis. Intern Med 47:1875–1879 [DOI] [PubMed] [Google Scholar]
- 340. Gros-Louis F, Andersen PM, Dupre N, Urushitani M, Dion P, Souchon F, D'Amour M, Camu W, Meininger V, Bouchard JP, Rouleau GA, Julien JP. 2009. Chromogranin B P413L variant as risk factor and modifier of disease onset for amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 106:21777–21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. 2006. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci 9:108–118 [DOI] [PubMed] [Google Scholar]
- 342. Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, Brown RH, Cudkowicz ME, Newhall K, Peskind E, Marcus S, Ho L. 2006. Identification of potential CSF biomarkers in ALS. Neurology 66:1218–1222 [DOI] [PubMed] [Google Scholar]
- 343. Zhao Z, Lange DJ, Ho L, Bonini S, Shao B, Salton SR, Thomas S, Pasinetti GM. 2008. Vgf is a novel biomarker associated with muscle weakness in amyotrophic lateral sclerosis (ALS), with a potential role in disease pathogenesis. Int J Med Sci 5:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Shimazawa M, Tanaka H, Ito Y, Morimoto N, Tsuruma K, Kadokura M, Tamura S, Inoue T, Yamada M, Takahashi H, Warita H, Aoki M, Hara H. 2010. An inducer of VGF protects cells against ER stress-induced cell death and prolongs survival in the mutant SOD1 animal models of familial ALS. PLoS One 5:e15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Weiler R, Lassmann H, Fischer P, Jellinger K, Winkler H. 1990. A high ratio of chromogranin A to synaptin/synaptophysin is a common feature of brains in Alzheimer and Pick disease. FEBS Lett 263:337–339 [DOI] [PubMed] [Google Scholar]
- 346. Munoz DG. 1991. Chromogranin A-like immunoreactive neurites are major constituents of senile plaques. Lab Invest 64:826–832 [PubMed] [Google Scholar]
- 347. Kikuchi K, Arawaka S, Koyama S, Kimura H, Ren CH, Wada M, Kawanami T, Kurita K, Daimon M, Kawakatsu S, Kadoya T, Goto K, Kato T. 2003. An N-terminal fragment of ProSAAS (a granin-like neuroendocrine peptide precursor) is associated with tau inclusions in Pick's disease. Biochem Biophys Res Commun 308:646–654 [DOI] [PubMed] [Google Scholar]
- 348. Lechner T, Adlassnig C, Humpel C, Kaufmann WA, Maier H, Reinstadler-Kramer K, Hinterhölzl J, Mahata SK, Jellinger KA, Marksteiner J. 2004. Chromogranin peptides in Alzheimer's disease. Exp Gerontol 39:101–113 [DOI] [PubMed] [Google Scholar]
- 349. Wada M, Ren CH, Koyama S, Arawaka S, Kawakatsu S, Kimura H, Nagasawa H, Kawanami T, Kurita K, Daimon M, Hirano A, Kato T. 2004. A human granin-like neuroendocrine peptide precursor (proSAAS) immunoreactivity in tau inclusions of Alzheimer's disease and parkinsonism-dementia complex on Guam. Neurosci Lett 356:49–52 [DOI] [PubMed] [Google Scholar]
- 350. Blennow K, Davidsson P, Wallin A, Ekman R. 1995. Chromogranin A in cerebrospinal fluid: a biochemical marker for synaptic degeneration in Alzheimer's disease? Dementia 6:306–311 [DOI] [PubMed] [Google Scholar]
- 351. O'Connor DT, Kailasam MT, Thal LJ. 1993. Cerebrospinal fluid chromogranin A is unchanged in Alzheimer dementia. Neurobiol Aging 14:267–269 [DOI] [PubMed] [Google Scholar]
- 352. Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, Hochstrasser DF, Sanchez JC. 2003. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer's disease. Proteomics 3:1486–1494 [DOI] [PubMed] [Google Scholar]
- 353. Simonsen AH, McGuire J, Podust VN, Hagnelius NO, Nilsson TK, Kapaki E, Vassilopoulos D, Waldemar G. 2007. A novel panel of cerebrospinal fluid biomarkers for the differential diagnosis of Alzheimer's disease versus normal aging and frontotemporal dementia. Dement Geriatr Cogn Disord 24:434–440 [DOI] [PubMed] [Google Scholar]
- 354. Davidsson P, Sjögren M, Andreasen N, Lindbjer M, Nilsson CL, Westman-Brinkmalm A, Blennow K. 2002. Studies of the pathophysiological mechanisms in frontotemporal dementia by proteome analysis of CSF proteins. Brain Res Mol Brain Res 109:128–133 [DOI] [PubMed] [Google Scholar]
- 355. Rüetschi U, Zetterberg H, Podust VN, Gottfries J, Li S, Hviid Simonsen A, McGuire J, Karlsson M, Rymo L, Davies H, Minthon L, Blennow K. 2005. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol 196:273–281 [DOI] [PubMed] [Google Scholar]
- 356. Mattsson N, Rüetschi U, Podust VN, Stridsberg M, Li S, Andersen O, Haghighi S, Blennow K, Zetterberg H. 2007. Cerebrospinal fluid concentrations of peptides derived from chromogranin B and secretogranin II are decreased in multiple sclerosis. J Neurochem 103:1932–1939 [DOI] [PubMed] [Google Scholar]
- 357. Stoop MP, Dekker LJ, Titulaer MK, Burgers PC, Sillevis Smitt PA, Luider TM, Hintzen RQ. 2008. Multiple sclerosis-related proteins identified in cerebrospinal fluid by advanced mass spectrometry. Proteomics 8:1576–1585 [DOI] [PubMed] [Google Scholar]
- 358. Stoop MP, Dekker LJ, Titulaer MK, Lamers RJ, Burgers PC, Sillevis Smitt PA, van Gool AJ, Luider TM, Hintzen RQ. 2009. Quantitative matrix-assisted laser desorption ionization-fourier transform ion cyclotron resonance (MALDI-FT-ICR) peptide profiling and identification of multiple-sclerosis-related proteins. J Proteome Res 8 :1404–1414 [DOI] [PubMed] [Google Scholar]
- 359. Kitao Y, Inada T, Arinami T, Hirotsu C, Aoki S, Iijima Y, Yamauchi T, Yagi G. 2000. A contribution to genome-wide association studies: search for susceptibility loci for schizophrenia using DNA microsatellite markers on chromosomes 19, 20, 21 and 22. Psychiatr Genet 10:139–143 [DOI] [PubMed] [Google Scholar]
- 360. Takahashi N, Ishihara R, Saito S, Maemo N, Aoyama N, Ji X, Miura H, Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Ozaki N, Inada T. 2006. Association between chromogranin A gene polymorphism and schizophrenia in the Japanese population. Schizophr Res 83:179–183 [DOI] [PubMed] [Google Scholar]
- 361. Anouar Y, Yon L, Guillemot J, Thouennon E, Barbier L, Gimenez-Roqueplo AP, Bertherat J, Lefebvre H, Klein M, Muresan M, Grouzmann E, Plouin PF, Vaudry H, Elkahloun AG. 2006. Development of novel tools for the diagnosis and prognosis of pheochromocytoma using peptide marker immunoassay and gene expression profiling approaches. Ann NY Acad Sci 1073:533–540 [DOI] [PubMed] [Google Scholar]
- 362. Portela-Gomes GM, Grimelius L, Stridsberg M. 2010. Immunohistochemical and biochemical studies with region-specific antibodies to chromogranins A and B and secretogranins II and III in neuroendocrine tumors. Cell Mol Neurobiol 30:1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Stridsberg M, Eriksson B, Fellström B, Kristiansson G, Tiensuu Janson E. 2007. Measurements of chromogranin B can serve as a complement to chromogranin A. Regul Pept 139:80–83 [DOI] [PubMed] [Google Scholar]
- 364. Stridsberg M, Eriksson B, Oberg K, Janson ET. 2003. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol 177:337–341 [DOI] [PubMed] [Google Scholar]
- 365. Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT, Piodi L. 2009. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 15:867–871 [DOI] [PubMed] [Google Scholar]
- 366. Herrero CJ, Alés E, Pintado AJ, López MG, García-Palomero E, Mahata SK, O'Connor DT, García AG, Montiel C. 2002. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J Neurosci 22:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Severini C, Ciotti MT, Biondini L, Quaresima S, Rinaldi AM, Levi A, Frank C, Possenti R. 2008. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem 104:534–544 [DOI] [PubMed] [Google Scholar]
- 368. Helle KB. 2010. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res 85:9–16 [DOI] [PubMed] [Google Scholar]
- 369. Huttner WB, Gerdes HH, Rosa P. 1991. The granin (chromogranin/secretogranin) family. Trends Biochem Sci 16:27–30 [DOI] [PubMed] [Google Scholar]
- 370. Simon JP, Aunis D. 1989. Biochemistry of the chromogranin A protein family. Biochem J 262:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Somogyi P, Hodgson AJ, DePotter RW, Fischer-Colbrie R, Schober M, Winkler H, Chubb IW. 1984. Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res 320:193–230 [DOI] [PubMed] [Google Scholar]
- 372. Zhao E, Zhang D, Basak A, Trudeau VL. 2009. New insights into granin-derived peptides: evolution and endocrine roles. Gen Comp Endocrinol 164:161–174 [DOI] [PubMed] [Google Scholar]
- 373. Helle KB, Aunis D, Chromogranins: functional and clinical aspects. Proc 10th International Symposium of Chromaffin Cell Biology, Session VII, New York, 2000 [Google Scholar]
- 374. Helle KB. 2010. Regulatory peptides from chromogranin A and secretogranin II. Cell Mol Neurobiol 30:1145–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Aunis D. 2010. GRANINS: thirty-five happy years in the granulosome world. Preface. Regul Pept 165:3–4 [DOI] [PubMed] [Google Scholar]
- 376. Portela-Gomes GM, Grimelius L, Stridsberg M. 2010. Secretogranin III in human neuroendocrine tumours A comparative immunohistochemical study with chromogranins A and B and secretogranin II. Regul Pept 165:30–35 [DOI] [PubMed] [Google Scholar]
- 377. Schafer MK, Mahata SK, Stroth N, Eiden LE, Weihe E. 2010. Cellular distribution of chromogranin A in excitatory, inhibitory, aminergic and peptidergic neurons of the rodent central nervous system. Regul Pept 165:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Mahata SK, Mahata M, Fung MM, O'Connor DT. 2010. Catestatin: a multifunctional peptide from chromogranin A. Regul Pept 165:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Brar BK, Helgeland E, Mahata SK, Zhang K, O'Connor DT, Helle KB, Jonassen AK. 2010. Human catestatin peptides differentially regulate infarct size in the ischemic-reperfused rat heart. Regul Pept 165:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Sánchez-Margalet V, González-Yanes C, Najib S, Santos-Álvarez J. 2010. Reprint of: Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. Regul Pept 165:71–77 [DOI] [PubMed] [Google Scholar]
- 381. Angelone T, Quintieri AM, Goumon Y, Di Felice V, Filice E, Gattuso A, Mazza R, Corti A, Tota B, Metz-Boutigue MH, Cerra MC. 2010. Cytoskeleton mediates negative inotropism and lusitropism of chromogranin A-derived peptides (human vasostatin1–78 and rat CgA) in the rat heart. Regul Pept 165:78–85 [DOI] [PubMed] [Google Scholar]
- 382. Koshimizu H, Kim T, Cawley NX, Loh YP. 2010. Reprint of: Chromogranin A: a new proposal for trafficking, processing and induction of granule biogenesis. Regul Pept 165:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Vitale N, Thiersé D, Bader MF. 2010. Melittin promotes exocytosis in neuroendocrine cells through the activation of phospholipase A. Regul Pept 165:111–116 [DOI] [PubMed] [Google Scholar]
- 384. Zhao E, Hu H, Trudeau VL. 2010. Secretoneurin as a hormone regulator in the pituitary. Regul Pept 165:117–122 [DOI] [PubMed] [Google Scholar]
- 385. Teuchner B, Dimmer A, Troger J, Fischer-Colbrie R, Schmid E, Kieselbach G, Dietrich H, Bechrakis N. 2010. Secretoneurin and the tachykinins substance P and neurokinin-A/B in NMDA-induced excitotoxicity in the rat retina. Regul Pept 165:123–127 [DOI] [PubMed] [Google Scholar]
- 386. Jones DT. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292:195–202 [DOI] [PubMed] [Google Scholar]
- 387. Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT. 2005. Protein structure prediction servers at University College London. Nucleic Acids Res 33:W36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Makretsov N, Gilks CB, Coldman AJ, Hayes M, Huntsman D. 2003. Tissue microarray analysis of neuroendocrine differentiation and its prognostic significance in breast cancer. Hum Pathol 34:1001–1008 [DOI] [PubMed] [Google Scholar]
- 389. Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, Scherag A, Hahmann M, Müller HH, Barth P. 2008. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol 6:820–827 [DOI] [PubMed] [Google Scholar]
- 390. Syversen U, Ramstad H, Gamme K, Qvigstad G, Falkmer S, Waldum HL. 2004. Clinical significance of elevated serum chromogranin A levels. Scand J Gastroenterol 39:969–973 [DOI] [PubMed] [Google Scholar]
- 391. Yasuda D, Iguchi H, Funakoshi A, Wakasugi H, Sekiya K, Misawa T, Tateishi K, Bloom SR, Nawata H. 1993. Comparison of plasma pancreastatin and GAWK concentrations, presumed processing products of chromogranin A and B, in plasma of patients with pancreatic islet cell tumors. Horm Metab Res 25:593–595 [DOI] [PubMed] [Google Scholar]
- 392. Kimura N, Pilichowska M, Okamoto H, Kimura I, Aunis D. 2000. Immunohistochemical expression of chromogranins A and B, prohormone convertases 2 and 3, and amidating enzyme in carcinoid tumors and pancreatic endocrine tumors. Mod Pathol 13:140–146 [DOI] [PubMed] [Google Scholar]
- 393. Massironi S, Fraquelli M, Paggi S, Sangiovanni A, Conte D, Sciola V, Ciafardini C, Colombo M, Peracchi M. 2009. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig Liver Dis 41:31–35 [DOI] [PubMed] [Google Scholar]
- 394. Børglum T, Rehfeld JF, Drivsholm LB, Hilsted L. 2007. Processing-independent quantitation of chromogranin a in plasma from patients with neuroendocrine tumors and small-cell lung carcinomas. Clin Chem 53:438–446 [DOI] [PubMed] [Google Scholar]
- 395. Hanna FW, Ardill JE, Johnston CF, Cunningham RT, Curry WJ, Russell CF, Buchanan KD. 1997. Regulatory peptides and other neuroendocrine markers in medullary carcinoma of the thyroid. J Endocrinol 152:275–281 [DOI] [PubMed] [Google Scholar]
- 396. Peracchi M, Conte D, Gebbia C, Penati C, Pizzinelli S, Arosio M, Corbetta S, Spada A. 2003. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. Eur J Endocrinol 148:39–43 [DOI] [PubMed] [Google Scholar]
- 397. Cleary S, Phillips JK, Huynh TT, Pacak K, Fliedner S, Elkahloun AG, Munson P, Worrell RA, Eisenhofer G. 2007. Chromogranin a expression in phaeochromocytomas associated with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Horm Metab Res 39:876–883 [DOI] [PubMed] [Google Scholar]
- 398. Eder U, Fischer-Colbrie R, Kogner P, Leitner B, Bjellerup P, Winkler H. 1998. Levels and molecular forms of chromogranins in human childhood neuroblastomas and ganglioneuromas. Neurosci Lett 253:17–20 [DOI] [PubMed] [Google Scholar]
- 399. Kogner P, Bjellerup P, Svensson T, Theodorsson E. 1995. Pancreastatin immunoreactivity in favourable childhood neuroblastoma and ganglioneuroma. Eur J Cancer 31A:557–560 [DOI] [PubMed] [Google Scholar]
- 400. Heaney AP, Curry WJ, Pogue KM, Armstrong VL, Mirakhur M, Sheridan B, Johnston CF, Buchanan KD, Atkinson AB. 2000. Immunohistochemical evaluation of the post-translational processing of chromogranin A in human pituitary adenomas. Pituitary 3:67–75 [DOI] [PubMed] [Google Scholar]
- 401. Gussi IL, Young J, Baudin E, Bidart JM, Chanson P. 2003. Chromogranin A as serum marker of pituitary adenomas. Clin Endocrinol (Oxf) 59:644–648 [DOI] [PubMed] [Google Scholar]
- 402. Pruneri G, Galli S, Rossi RS, Roncalli M, Coggi G, Ferrari A, Simonato A, Siccardi AG, Carboni N, Buffa R. 1998. Chromogranin A and B and secretogranin II in prostatic adenocarcinomas: neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate 34:113–120 [DOI] [PubMed] [Google Scholar]
- 403. Fahrenkamp AG, Wibbeke C, Winde G, Ofner D, Böcker W, Fischer-Colbrie R, Schmid KW. 1995. Immunohistochemical distribution of chromogranins A and B and secretogranin II in neuroendocrine tumours of the gastrointestinal tract. Virchows Arch 426:361–367 [DOI] [PubMed] [Google Scholar]
- 404. Woussen-Colle MC, Gourlet P, Vandermeers A, Vandermeers-Piret MC, D'Haens J, Velkeniers B, Robberecht P. 1995. Identification of a new chromogranin B fragment (314–365) in endocrine tumors. Peptides 16:231–236 [DOI] [PubMed] [Google Scholar]
- 405. Brouwers FM, Gläsker S, Nave AF, Vortmeyer AO, Lubensky I, Huang S, Abu-Asab MS, Eisenhofer G, Weil RJ, Park DM, Linehan WM, Pacak K, Zhuang Z. 2007. Proteomic profiling of von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 pheochromocytomas reveals different expression of chromogranin B. Endocr Relat Cancer 14:463–471 [DOI] [PubMed] [Google Scholar]
- 406. Dahma H, Gourlet P, Vandermeers A, Vandermeers-Piret MC, Robberecht P. 2001. Evidence that the chromogranin B fragment 368–417 extracted from a pheochromocytoma is phosphorylated. Peptides 22:1491–1499 [DOI] [PubMed] [Google Scholar]
- 407. Prommegger R, Obrist P, Ensinger C, Schwelberger HG, Wolf C, Fischer-Colbrie R, Mikuz G, Bodner E. 1998. Secretoneurin in carcinoids of the appendix-immunohistochemical comparison with chromogranins A,B and secretogranin II. Anticancer Res 18:3999–4002 [PubMed] [Google Scholar]
- 408. Ferrero S, Buffa R, Pruneri G, Siccardi AG, Pelagi M, Lee AK, Coggi G, Bosari S. 1995. The prevalence and clinical significance of chromogranin A and secretogranin II immunoreactivity in colorectal adenocarcinomas. Virchows Arch 426:587–592 [DOI] [PubMed] [Google Scholar]
- 409. Le Gall F, Vallet VS, Thomas D, De Monti M, Duval J, Ramee MP. 1997. Immunohistochemical study of secretogranin II in 62 neuroendocrine tumours of the digestive tract and of the pancreas in comparison with other granins. Pathol Res Pract 193:179–185 [PubMed] [Google Scholar]
- 410. Tötsch M, Padberg BC, Schröder S, Ofner D, Böcker W, Fischer-Colbrie R, Schmid KW. 1995. Secretoneurin in bronchopulmonary carcinoids–immunohistochemical comparison with chromogranins A and B and secretogranin II. Histopathology 26:357–361 [DOI] [PubMed] [Google Scholar]
- 411. Ischia R, Hobisch A, Bauer R, Weiss U, Gasser RW, Horninger W, Bartsch G, Jr, Fuchs D, Bartsch G, Winkler H, Klocker H, Fischer-Colbrie R, Culig Z. 2000. Elevated levels of serum secretoneurin in patients with therapy resistant carcinoma of the prostate. J Urol 163:1161–1164; discussion 1164–1165 [PubMed] [Google Scholar]
- 412. Suzuki H, Ghatei MA, Williams SJ, Uttenthal LO, Facer P, Bishop AE, Polak JM, Bloom SR. 1986. Production of pituitary protein 7B2 immunoreactivity by endocrine tumors and its possible diagnostic value. J Clin Endocrinol Metab 63:758–765 [DOI] [PubMed] [Google Scholar]
- 413. Vieau D, Rojas-Miranda A, Verley JM, Lenne F, Bertagna X. 1991. The secretory granule peptides 7B2 and CCB are sensitive biochemical markers of neuro-endocrine bronchial tumours in man. Clin Endocrinol (Oxf) 35:319–325 [DOI] [PubMed] [Google Scholar]
- 414. Graham DA, Abbott GD, Suzuki Y, Suzuki H, Shimoda S. 1992. Neuroendocrine protein 7B2 in Prader-Willi syndrome. Aust N Z J Med 22:455–457 [PubMed] [Google Scholar]
- 415. Vieau D, Linard CG, Mbikay M, Lenne F, Chretien M, Luton JP, Bertagna X. 1992. Expression of the neuroendocrine cell marker 7B2 in human ACTH secreting tumours. Clin Endocrinol (Oxf) 36:597–603 [DOI] [PubMed] [Google Scholar]
- 416. Ostrow KL, Park HL, Hoque MO, Kim MS, Liu J, Argani P, Westra W, Van Criekinge W, Sidransky D. 2009. Pharmacologic unmasking of epigenetically silenced genes in breast cancer. Clin Cancer Res 15:1184–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Rindi G, Licini L, Necchi V, Bottarelli L, Campanini N, Azzoni C, Favret M, Giordano G, D'Amato F, Brancia C, Solcia E, Ferri GL. 2007. Peptide products of the neurotrophin-inducible gene vgf are produced in human neuroendocrine cells from early development and increase in hyperplasia and neoplasia. J Clin Endocrinol Metab 92:2811–2815 [DOI] [PubMed] [Google Scholar]
- 418. Possenti R, Rinaldi AM, Ferri GL, Borboni P, Trani E, Levi A. 1999. Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology 140:3727–3735 [DOI] [PubMed] [Google Scholar]
- 419. Matsumoto T, Kawashima Y, Nagashio R, Kageyama T, Kodera Y, Jiang SX, Okayasu I, Kameya T, Sato Y. 2009. A new possible lung cancer marker: VGF detection from the conditioned medium of pulmonary large cell neuroendocrine carcinoma-derived cells using secretome analysis. Int J Biol Markers 24:282–285 [DOI] [PubMed] [Google Scholar]
- 420. Rossi A, Granata F, Augusti-Tocco G, Canu N, Levi A, Possenti R. 1992. Expression in murine and human neuroblastoma cell lines of VGF, a tissue specific protein. Int J Dev Neurosci 10:527–534 [DOI] [PubMed] [Google Scholar]
- 421. Gupta N, Bark SJ, Lu WD, Taupenot L, O'Connor DT, Pevzner P, Hook V. 2010. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J Proteome Res 9:5065–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 423. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. 2009. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Grönberg M, Amini RM, Stridsberg M, Janson ET, Saras J. 2010. Neuroendocrine markers are expressed in human mammary glands. Regul Pept 160:68–74 [DOI] [PubMed] [Google Scholar]
- 425. Ranganathan S, Williams E, Ganchev P, Gopalakrishnan V, Lacomis D, Urbinelli L, Newhall K, Cudkowicz ME, Brown RH, Jr, Bowser R. 2005. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem 95:1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Perrin RJ, Craig-Schapiro R, Malone JP, Shah AR, Gilmore P, Davis AE, Roe CM, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, Kaye JA, Morris JC, Holtzman DM, Townsend RR, Fagan AM. 2011. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer's disease. PLoS One 6:e16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Di Comite G, Rossi CM, Marinosci A, Lolmede K, Baldissera E, Aiello P, Mueller RB, Herrmann M, Voll RE, Rovere-Querini P, Sabbadini MG, Corti A, Manfredi AA. 2009. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-α-elicited endothelial activation. J Leukoc Biol 85:81–87 [DOI] [PubMed] [Google Scholar]
- 428. Eder U, Leitner B, Kirchmair R, Pohl P, Jobst KA, Smith AD, Málly J, Benzer A, Riederer P, Reichmann H, Saria A, Winkler H. 1998. Levels and proteolytic processing of chromogranin A and B and secretogranin II in cerebrospinal fluid in neurological diseases. J Neural Transm 105:39–51 [DOI] [PubMed] [Google Scholar]
- 429. Korsgren M, Erjefält JS, Hinterholzl J, Fischer-Colbrie R, Emanuelsson CA, Andersson M, Persson CG, Mackay-Sim A, Sundler F, Greiff L. 2003. Neural expression and increased lavage fluid levels of secretoneurin in seasonal allergic rhinitis. Am J Respir Crit Care Med 167:1504–1508 [DOI] [PubMed] [Google Scholar]
- 430. Hernández-Cruz A, Eiden LE. 2010. Proceedings of the 15th International Symposium on Chromaffin Cell Biology: the chromaffin cell as a stress transducer. Nov 12–16th, 2009, Yucatan, Mexico. Cell Mol Neurobiol 30:1143–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Vaudry H, Metz-Boutigue M-H. 2010. GRANINS: Thirty-five happy years in the granulosome world. Regul Pept 165:1–128 [DOI] [PubMed] [Google Scholar]