Abstract
We recently reported the cloning of a mitogen-inducible prostaglandin synthase gene, TIS10/PGS2. In addition to growth factors and tumor promoters, the v-src oncogene induces TIS10/PGS2 expression in 3T3 cells. Deletion analysis, using luciferase reporters, identifies a region between -80 and -40 nucleotides 5' of the TIS10/PGS2 transcription start site that mediates pp60v-src induction in 3T3 cells. This region contains the sequence CGTCACGTG, which includes overlapping ATF/CRE (CGTCA) and E-box (CACGTG) sequences. Gel shift-oligonucleotide competition experiments with nuclear extracts from cells stably transfected with a temperature-sensitive v-src gene demonstrate that the CGTCACGTG sequence can bind proteins at both the ATF/CRE and E-box sequences. Dominant-negative CREB and Myc proteins that bind DNA, but do not transactivate, block v-src induction of a luciferase reporter driven by the first 80 nucleotides of the TIS10/PGS2 promoter. Mutational analysis distinguishes which TIS10/PGS2 cis-acting element mediates pp60v-src induction. E-box mutation has no effect on the fold induction in response to pp60v-src. In contrast, ATF/CRE mutation attenuates the pp60v-src response. Antibody supershift and methylation interference experiments demonstrate that CREB and at least one other ATF transcription factor in these extracts bind to the TIS10/PGS2 ATF/CRE element. Expression of a dominant-negative ras gene also blocks TIS10/PGS2 induction by v-src. Our data suggest that Ras mediates pp60v-src activation of an ATF transcription factor, leading to induced TIS10/PGS2 expression via the ATF/CRE element of the TIS10/PGS2 promoter. This is the first description of v-src activation of gene expression via an ATF/CRE element.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrisani O., Dixon J. E. Identification and purification of a novel 120-kDa protein that recognizes the cAMP-responsive element. J Biol Chem. 1990 Feb 25;265(6):3212–3218. [PubMed] [Google Scholar]
- Apel I., Yu C. L., Wang T., Dobry C., Van Antwerp M. E., Jove R., Prochownik E. V. Regulation of the junB gene by v-src. Mol Cell Biol. 1992 Aug;12(8):3356–3364. doi: 10.1128/mcb.12.8.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K., Aderem A., Hanafusa H. Modulation of arachidonic acid metabolism by Rous sarcoma virus. J Virol. 1989 Jul;63(7):2929–2935. doi: 10.1128/jvi.63.7.2929-2935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich C., Dürr I., Koenen M., Witzemann V. Two adjacent E box elements and a M-CAT box are involved in the muscle-specific regulation of the rat acetylcholine receptor beta subunit gene. Eur J Biochem. 1993 Sep 1;216(2):395–404. doi: 10.1111/j.1432-1033.1993.tb18157.x. [DOI] [PubMed] [Google Scholar]
- Birchenall-Roberts M. C., Ruscetti F. W., Kasper J., Lee H. D., Friedman R., Geiser A., Sporn M. B., Roberts A. B., Kim S. J. Transcriptional regulation of the transforming growth factor beta 1 promoter by v-src gene products is mediated through the AP-1 complex. Mol Cell Biol. 1990 Sep;10(9):4978–4983. doi: 10.1128/mcb.10.9.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle P. K., Montminy M. R. The CREB family of transcription activators. Curr Opin Genet Dev. 1992 Apr;2(2):199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Cai H., Szeberényi J., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990 Oct;10(10):5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Dhand R., Pilat D., Twamley G. M., Waterfield M. D., Roussel M. F. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J. 1993 Mar;12(3):943–950. doi: 10.1002/j.1460-2075.1993.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D. L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991 May 8;1083(2):121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- Dehbi M., Mbiguino A., Beauchemin M., Chatelain G., Bédard P. A. Transcriptional activation of the CEF-4/9E3 cytokine gene by pp60v-src. Mol Cell Biol. 1992 Apr;12(4):1490–1499. doi: 10.1128/mcb.12.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Fletcher B. S., Kujubu D. A., Perrin D. M., Herschman H. R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992 Mar 5;267(7):4338–4344. [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Fujii M., Shalloway D., Verma I. M. Gene regulation by tyrosine kinases: src protein activates various promoters, including c-fos. Mol Cell Biol. 1989 Jun;9(6):2493–2499. doi: 10.1128/mcb.9.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. P., Yoon S. O., Chikaraishi D. M. Sequences that direct rat tyrosine hydroxylase gene expression. J Neurochem. 1992 Jun;58(6):2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Habib A., Créminon C., Frobert Y., Grassi J., Pradelles P., Maclouf J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem. 1993 Nov 5;268(31):23448–23454. [PubMed] [Google Scholar]
- Hamasaki Y., Kitzler J., Hardman R., Nettesheim P., Eling T. E. Phorbol ester and epidermal growth factor enhance the expression of two inducible prostaglandin H synthase genes in rat tracheal epithelial cells. Arch Biochem Biophys. 1993 Jul;304(1):226–234. doi: 10.1006/abbi.1993.1343. [DOI] [PubMed] [Google Scholar]
- Han J. W., Sadowski H., Young D. A., Macara I. G. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1990 May;87(9):3373–3377. doi: 10.1073/pnas.87.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lee K. A. Dimeric transcription factor families: it takes two to tango but who decides on partners and the venue? J Cell Sci. 1992 Sep;103(Pt 1):9–14. doi: 10.1242/jcs.103.1.9. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Leshkowitz D., Aronheim A., Walker M. D. Insulin-producing cells contain a cell-specific repressor activity that functions through multiple E-box sequences. DNA Cell Biol. 1992 Sep;11(7):549–558. doi: 10.1089/dna.1992.11.549. [DOI] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., Herschman H. R. Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1(3):263–270. [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Maroney A. C., Qureshi S. A., Foster D. A., Brugge J. S. Cloning and characterization of a thermolabile v-src gene for use in reversible transformation of mammalian cells. Oncogene. 1992 Jun;7(6):1207–1214. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- O'Banion M. K., Sadowski H. B., Winn V., Young D. A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [PubMed] [Google Scholar]
- Peleg S. Modified binding of proteins from calcitonin-negative tumor cells to the neuroendocrine-specific CANNTG motif of the calcitonin gene. Nucleic Acids Res. 1993 Nov 25;21(23):5360–5365. doi: 10.1093/nar/21.23.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., Orth K., Calame K. L. Binding in vitro of multiple cellular proteins to immunoglobulin heavy-chain enhancer DNA. Mol Cell Biol. 1986 Dec;6(12):4168–4178. doi: 10.1128/mcb.6.12.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilbeam C. C., Kawaguchi H., Hakeda Y., Voznesensky O., Alander C. B., Raisz L. G. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 Dec 5;268(34):25643–25649. [PubMed] [Google Scholar]
- Qureshi S. A., Cao X. M., Sukhatme V. P., Foster D. A. v-Src activates mitogen-responsive transcription factor Egr-1 via serum response elements. J Biol Chem. 1991 Jun 15;266(17):10802–10806. [PubMed] [Google Scholar]
- Qureshi S. A., Joseph C. K., Rim M., Maroney A., Foster D. A. v-Src activates both protein kinase C-dependent and independent signaling pathways in murine fibroblasts. Oncogene. 1991 Jun;6(6):995–999. [PubMed] [Google Scholar]
- Rehfuss R. P., Walton K. M., Loriaux M. M., Goodman R. H. The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991 Oct 5;266(28):18431–18434. [PubMed] [Google Scholar]
- Rossi M. J., Clark M. A., Steiner S. M. Possible role of prostaglandins in the regulation of mouse myoblasts. J Cell Physiol. 1989 Oct;141(1):142–147. doi: 10.1002/jcp.1041410121. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Raynoschek C., Macdonald-Bravo H., Dorfman K., Mattéi M. G., Bravo R. Identification of an immediate early gene, pghs-B, whose protein product has prostaglandin synthase/cyclooxygenase activity. Cell Growth Differ. 1992 Jul;3(7):443–450. [PubMed] [Google Scholar]
- Sato H., Kita M., Seiki M. v-Src activates the expression of 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem. 1993 Nov 5;268(31):23460–23468. [PubMed] [Google Scholar]
- Sawyers C. L., Callahan W., Witte O. N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992 Sep 18;70(6):901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois J., Levy L. O., Simmons D. L., Richards J. S. Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem. 1993 Jun 5;268(16):12199–12206. [PubMed] [Google Scholar]
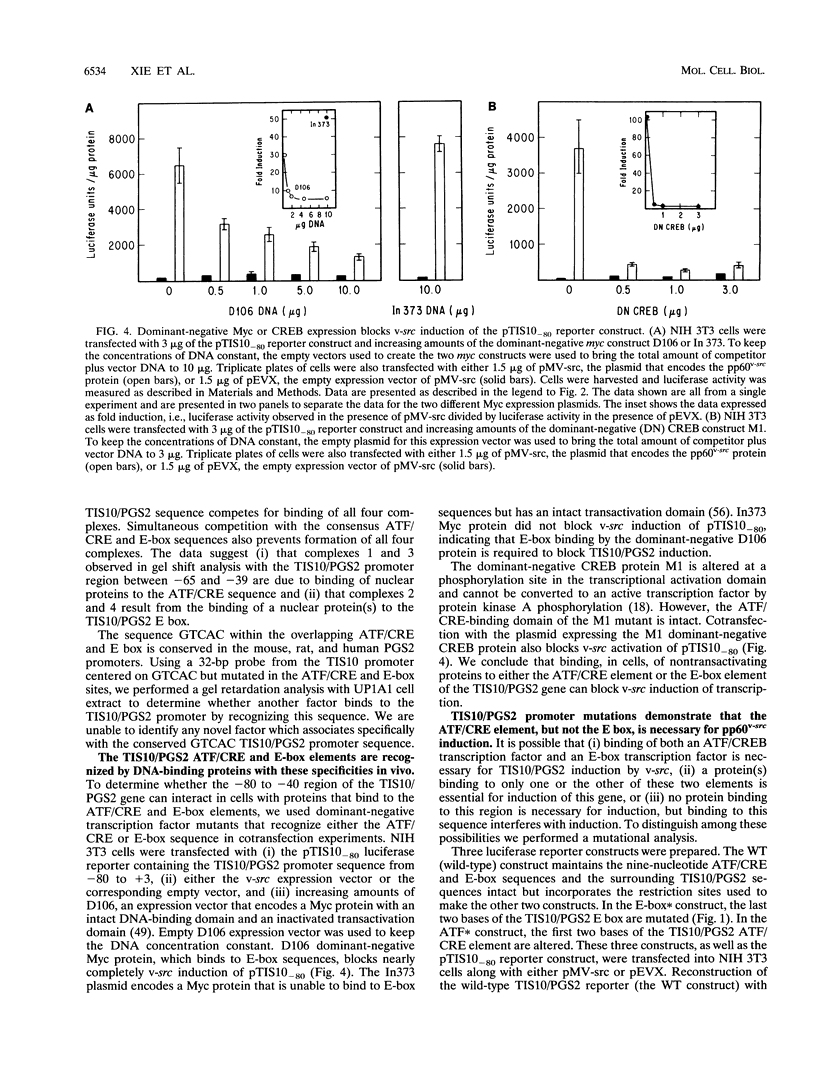
- Sirois J., Richards J. S. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP beta promoter element. J Biol Chem. 1993 Oct 15;268(29):21931–21938. [PubMed] [Google Scholar]
- Sirois J., Simmons D. L., Richards J. S. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem. 1992 Jun 5;267(16):11586–11592. [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
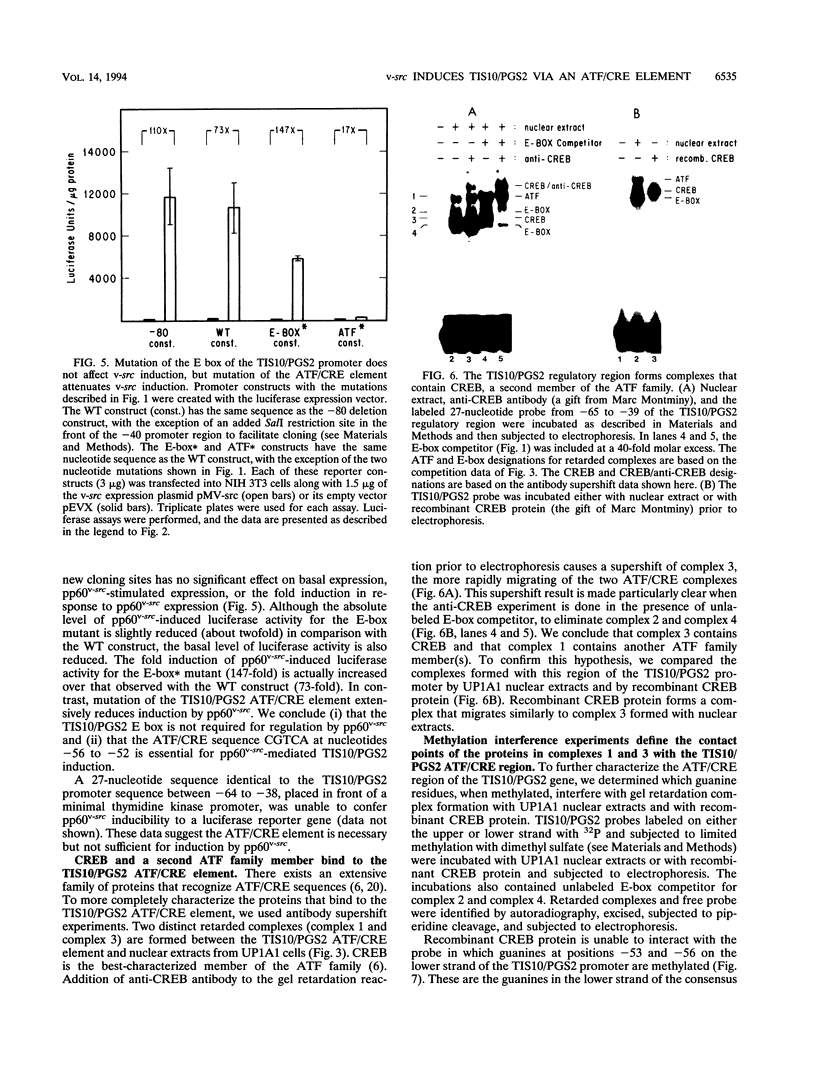
- Smith W. L., Marnett L. J. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta. 1991 Apr 24;1083(1):1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley-Stein G. M., Pepperkok R., Ansorge W., Courtneidge S. A. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B. C., Lim R. W., Kujubu D. A., Luner S. J., Kaufman S. E., Greenberger J. S., Gasson J. C., Herschman H. R. Granulocyte-macrophage colony-stimulating factor and tetradecanoyl phorbol acetate induce a distinct, restricted subset of primary-response TIS genes in both proliferating and terminally differentiated myeloid cells. Mol Cell Biol. 1989 Aug;9(8):3580–3583. doi: 10.1128/mcb.9.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. R., Hu Y., Hirschhorn R. R., Steiner S. M. MyoD and regulation of prostaglandin H synthase. Exp Cell Res. 1993 Aug;207(2):439–441. doi: 10.1006/excr.1993.1212. [DOI] [PubMed] [Google Scholar]
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984 Sep 6;311(5981):81–84. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993 Aug;11(2):371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990 Mar;6(3):69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]