Abstract
We report the discovery of an African American Y chromosome that carries the ancestral state of all SNPs that defined the basal portion of the Y chromosome phylogenetic tree. We sequenced ∼240 kb of this chromosome to identify private, derived mutations on this lineage, which we named A00. We then estimated the time to the most recent common ancestor (TMRCA) for the Y tree as 338 thousand years ago (kya) (95% confidence interval = 237–581 kya). Remarkably, this exceeds current estimates of the mtDNA TMRCA, as well as those of the age of the oldest anatomically modern human fossils. The extremely ancient age combined with the rarity of the A00 lineage, which we also find at very low frequency in central Africa, point to the importance of considering more complex models for the origin of Y chromosome diversity. These models include ancient population structure and the possibility of archaic introgression of Y chromosomes into anatomically modern humans. The A00 lineage was discovered in a large database of consumer samples of African Americans and has not been identified in traditional hunter-gatherer populations from sub-Saharan Africa. This underscores how the stochastic nature of the genealogical process can affect inference from a single locus and warrants caution during the interpretation of the geographic location of divergent branches of the Y chromosome phylogenetic tree for the elucidation of human origins.
Main Text
Characterizing the root of the phylogenetic tree of individual genetic loci has influenced many researchers in their attempts to infer the time and place of the origin of anatomically modern humans (AMHs).1–4 Analyses of the nonrecombining portion of the Y chromosome and mtDNA have suggested that recent common ancestors lived in sub-Saharan Africa within the last 200,000 years.5 However, estimates of the time to the most recent common ancestor (TMRCA) have been consistently lower for the Y chromosome (∼60–140 thousand years ago [kya])6–8 than for mtDNA (∼140–240 kya).9,10 A variety of evolutionary processes (e.g., natural selection, differential migration of males and females, and/or a skew in the breeding sex ratio) have been invoked to explain this difference.11–13
Genotyping of a DNA sample that was submitted to a commercial genetic-testing facility demonstrated that the Y chromosome of this African American individual carried the ancestral state of all known Y chromosome SNPs. To further characterize this lineage, which we dubbed A00 (see Figure S1, available online, for proposed nomenclature), we sequenced multiple regions (totaling ∼240 kb) of the X-degenerate portion of this chromosome, as well as a subset of these regions (∼180 kb) on a chromosome belonging to the previously known basal lineage A1b (which we rename here as A0). We note that all sampling procedures described herein were approved by and performed according to the ethical standards of the University of Arizona Human Subjects Committee. The ancestral state of each polymorphic site was inferred with pairwise alignments between the human (UCSC Genome Browser hg18) and chimpanzee (panTro3) reference sequences, and mutations were assigned to the branches (i.e., A00 and A0) shown as thick lines in Figure 1 on the basis of an infinite-sites model (Table S1). To estimate the TMRCA of the Y chromosome tree that incorporates the newly discovered root defined by A00, we developed a likelihood-based method that uses mutation rates recently estimated by Kong et al.,14 who performed high-coverage whole-genome sequencing on 78 human pedigrees.
Figure 1.

Genealogy of A00, A0, and the Reference Sequence
Lineages on which mutations were identified and lineages that were used for placing those mutations on the genealogy are indicated with thick and thin lines, respectively. The numbers of identified mutations on a branch are indicated in italics (four mutations in A00 were not genotyped but are indicated as shared by Mbo in this tree). The time estimates (and confidence intervals) are indicated kya for three nodes: the most recent common ancestor, the common ancestor between A0 and the reference (ref), and the common ancestor of A00 chromosomes from an African American individual and the Mbo. Two sets of ages are shown: on the left are estimates (numbers in black) obtained with the mutation rate based on recent whole-genome-sequencing results as described in the main text, and on the right are estimates (numbers in gray) based on the higher mutation rate used by Cruciani et al.6
Assuming that mutations in the X-degenerate portion of the Y chromosome follow the linear model presented by Kong et al.,14 we adjusted for male-specific mutation processes. If μY is the mutation rate per year per base for the Y chromosome, L is the length of sequence analyzed for the autosomes, T is the average number of new mutations per genome in the autosomes, g is the generation time, b is the increment in mutation rate per base per year increment in the paternal age, F is the number of mutations originating in the mother, and g0 is the average age of fathers in the study,14 then . We obtained median values and generated 90% confidence intervals (CIs) for μY as a function of g (ranging from 20 to 40 years) by sampling 100,000 times from normal distributions for each of the parameters T ∼ N (63.2 [SD = 0.9]), b ∼ N (2.01 [SD = 0.17]), and F ∼ N (14.2 [SD = 3.12]), which result from the number of observed mutations (4,933 in 78 pedigrees), the linear coefficient in the linear fit of mutations as a function of paternal age (2.01 per year [SEM = 0.17 per year]), and the 71 mutations that can be confirmed as de novo and of maternal origin in five pedigrees,14 respectively. We then divided those values by 2.68 × 109 (the length of autosomal sequence covered in each of the 78 trios) to obtain estimates and CIs for μY (Figure S2). This resulted in a range for μY (across values of g) of 4.39 × 10−10 ≤ μY ≤ 7.07 × 10−10. To obtain point estimates of the ages of nodes in Figure 1, we used the median value of μY at age 30 years (6.17 × 10−10). To obtain upper and lower bounds of the CIs, we used the lower and upper bounds of μY, respectively. Estimates of mutation rate in Kong et al.14 are consistent with those from a variety of large-scale sequencing studies,15–19 making it unlikely that our TMRCA estimates are strongly biased by an excess of false negatives in genome sequence data from human pedigrees.
We estimated the TMRCA of human Y chromosomes as 338 kya (95% CI = 237–581 kya). Using a joint likelihood20 and the same mutation rate, we also estimated a divergence time between A0 chromosomes and the human reference as 202 kya (95% CI = 125–382 kya), a time that is older than that previously obtained by Cruciani et al. (142 kya).6 This discrepancy in the age of A0 is due to the fact that the earlier study did not utilize mutation rates based on recently obtained whole-genome sequence data.14–18 If we were to use the higher mutation rate (1.0 × 10−9 per base per year6) rather than a realistic range derived from whole-genome sequencing (4.39 × 10−10 − 7.07 × 10−10), the estimated TMRCA for the tree incorporating A00 as the basal lineage would be 209 kya, which is only slightly older than current estimates of the TMRCA of mtDNA and the age of the oldest AMH fossil remains. We note, however, that the higher mutation rate produces an estimate for the common ancestor of all non-African Y chromosome haplogroups (C through T) of ∼39 kya6 (i.e., versus ∼63 kya for the mutation rate used here). It is difficult to reconcile the younger estimate with the timing of the out-of-Africa dispersal on the basis of the analyses of autosomal DNA21 and the fossil record outside of Africa.22–25 Regardless of which mutation rate is applied, the analysis of relative ages of nodes26 shows that the TMRCA of the A00-rooted tree is 67% older (95% CI = 35%–126%) than that of the A0-rooted tree.
We then genotyped a set of six Y chromosome short tandem repeats (Y-STRs) (DYS19, DYS388, DYS390, DYS391, DYS392, and DYS39327) and found that the A00 chromosome carried the following alleles: 16-11-19-10-12-13. Upon searching a large pan-African database consisting of 5,648 samples from ten countries (Cameroon, Nigeria, Ghana, Senegal, Uganda, Tanzania, Malawi, Zimbabwe, Mozambique, and South Sudan), we identified 11 Y chromosomes that were invariant and identical to the A00 chromosome at five of the six Y-STRs (2 of the 11 chromosomes carried DYS19-16, whereas the others carried DYS19-15). These 11 chromosomes were all found in a sample of 174 (∼6.3%) Mbo individuals from western Cameroon (Figure 2). Seven of these Mbo chromosomes were available for further testing, and the genotypes were found to be identical at 37 of 39 SNPs known to be derived on the A00 chromosome (i.e., two of these genotyped SNPs were ancestral in the Mbo samples) (Table S1). Using the joint likelihood for the split of two lineages and a sublineage internal to one of them,20 we estimated that the most recent common ancestor of the African American and Mbo A00 chromosomes lived between 2.6 and 73 kya (95% CI, maximum likelihood estimate = 17 kya).
Figure 2.
Map Showing Cameroon and the Approximate Location where Mbo Speakers Live
We also estimated the level of variation among nine A00 lineages (i.e., including one additional Mbo individual) by using a battery of 95 Y-STRs for which all individuals had no missing data; (Table S2). A median-joining network28 shows that the African American A00 lineage is 11 mutational steps from the nearest Mbo and that the maximum difference between any pair of Mbo is nine steps (Figure 3 and Table S2). On the basis of these levels of within- and between-group variation, we calculated a second divergence time estimate of 564–2,697 years (Table 1) by assuming a mean Y-STR mutation rate of 1.32 × 10−4 and 2.76 × 10−5 per year, respectively.29,30
Figure 3.
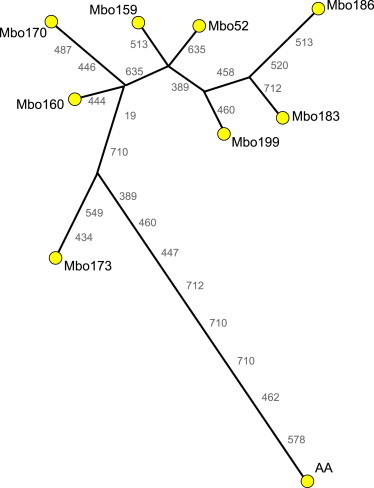
Median-Joining Network of A00 Haplotypes
The network is based on haplotypes (constructed with 95 Y-STRs) of eight Mbo and an African American (AA) individual. All mutations are assumed to be single step and were given equal weight during the construction of the network. Marker names are indicated without “DYS” at the beginning.
Table 1.
Pairwise and Average STR-Based Estimates of TMRCA for A00 Chromosomes
|
TMRCA (Years) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mbo 52 | Mbo 159 | Mbo 160 | Mbo 170 | Mbo 173 | Mbo 183 | Mbo 186 | Mbo 199 | African American | Average with Mboa | |
| Mbo 52 | - | 80 | 120 | 159 | 239 | 159 | 199 | 120 | 478 | 154 |
| Mbo 159 | 381 | - | 120 | 159 | 239 | 159 | 199 | 120 | 478 | 154 |
| Mbo 160 | 572 | 572 | - | 120 | 199 | 199 | 239 | 159 | 439 | 165 |
| Mbo 170 | 763 | 763 | 572 | - | 239 | 239 | 279 | 199 | 478 | 199 |
| Mbo 173 | 1,144 | 1,144 | 953 | 1,144 | - | 319 | 359 | 279 | 399 | 268 |
| Mbo 183 | 763 | 763 | 953 | 1,144 | 1,526 | - | 120 | 120 | 558 | 188 |
| Mbo 186 | 953 | 953 | 1,144 | 1,335 | 1,716 | 572 | - | 159 | 598 | 222 |
| Mbo 199 | 572 | 572 | 763 | 953 | 1,335 | 572 | 763 | - | 518 | 165 |
| African American | 2,288 | 2,288 | 2,098 | 2,288 | 1,907 | 2,670 | 2,860 | 2,479 | - | 564 |
| Average with Mbob | 736 | 736 | 790 | 953 | 1,280 | 899 | 1,062 | 790 | 2,697 | - |
We obtained point estimates of the TMRCA of two haplotypes by dividing the estimate of the number of mutational steps separating the haplotypes (as inferred from the network) by twice the mutational rate per STR and by the number of STRs scored in both haplotypes. Values above and below the diagonal separation correspond to estimates obtained with the high and low mutation rates, respectively (see text). The following abbreviation is used: TMRCA, time to the most recent common ancestor.
High mutation rate.
Low mutation rate.
In contrast to the estimates of all previous studies, our estimate of the Y chromosome TMRCA predates both that of known mtDNA lineages9,10 and of the likely time of origin of AMHs on the basis of current fossil evidence.31 Although we identified the A00 lineage in an African American, the unusual Y-STR profile associated with this individual’s Y chromosome allowed us to identify the same divergent lineage in a single ethnic group living in a small region of western Cameroon (Figure 2). Interestingly, contrary to previous Y chromosome and mtDNA studies, we did not identify the most basal lineage in a traditional hunter-gatherer population, such as the Khoisan or Pygmies.32–35 Rather, like the majority of groups that now occupy sub-Saharan Africa, the Mbo are a Bantu-speaking population. Given the large stochasticity expected in the genealogical process of a single locus,36,37 we should not be surprised that the Y chromosome genealogy does not match the currently accepted phylogeny of human populations on the basis of genome-wide data,38 which show that the earliest divergence events involved the ancestors of extant traditional hunter-gatherers.39–41 Given what we know about the coalescent process, the lack of dense sampling in sub-Saharan Africa (especially compared with Europe) has most likely contributed to the failure to identify more cases of incongruence between genealogical and population divergence.
The large sample size of African Americans was critical for the discovery of the A00 lineage given its very low frequency estimate in sub-Saharan Africa (0.19% [95% CI = 0.11%–0.35%]). However, even the large consumer-based African American data set examined here is a highly biased representation of sub-Saharan Africans because it captures only the genetic diversity inherited from ancestors living at a particular time and place (i.e., essentially the West African coastal source area of the Atlantic Slave Trade, which took place between the 15th and 19th centuries).42 It is likely that a much richer understanding of the Y chromosome phylogeny, as well as of genetic variation in general, would be achieved if more dense and even sampling were to be conducted across sub-Saharan Africa, especially given its high level of genetic diversity.
Although the stochastic nature of the evolutionary process can explain the aforementioned incongruences, the extreme age and rarity of the A00 lineage point to the possibility of a highly structured ancestral population, consistent with recent work on the autosomes.40,41,43,44 This could take the form of long-standing population structure among AMH populations45 or archaic introgression from an archaic form into the ancestors of AMHs.46 Interestingly, the Mbo live less than 800 km away from a Nigerian site known as Iwo Eleru, where human skeletal remains with both archaic and modern features were found and dated to ∼13 kya.47 Further surveys in sub-Saharan Africa and in the African Diaspora might uncover more diverged basal lineages, which will help to disentangle some of the complex evolutionary processes that shape patterns of Y chromosome diversity. Finally, the discovery of the A00 lineage demonstrates the power of public participation in the scientific process—a venture that is likely to continue in the current era of personal genomics.
Acknowledgments
We thank Jacqueline Johnson and her male cousins, the descendants of Albert Perry (South Carolina), as well as participating Family Tree DNA customers. We also thank Brent Manning, Arjan Bormans, Ana Bestic, and Bennett Greenspan for technical support and Fulvio Cruciani and Andrea Massaia for providing the genomic coordinates for Y chromosome regions sequenced in Cruciani et al.6
Supplemental Data
Web Resources
The URL for data presented herein is as follows:
UCSC Genome Browser, http://genome.ucsc.edu
References
- 1.Batini C., Ferri G., Destro-Bisol G., Brisighelli F., Luiselli D., Sánchez-Diz P., Rocha J., Simonson T., Brehm A., Montano V. Signatures of the preagricultural peopling processes in sub-Saharan Africa as revealed by the phylogeography of early Y chromosome lineages. Mol. Biol. Evol. 2011;28:2603–2613. doi: 10.1093/molbev/msr089. [DOI] [PubMed] [Google Scholar]
- 2.Cann R.L., Stoneking M., Wilson A.C. Mitochondrial DNA and human evolution. Nature. 1987;325:31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- 3.Hammer M.F. A recent common ancestry for human Y chromosomes. Nature. 1995;378:376–378. doi: 10.1038/378376a0. [DOI] [PubMed] [Google Scholar]
- 4.Semino O., Santachiara-Benerecetti A.S., Falaschi F., Cavalli-Sforza L.L., Underhill P.A. Ethiopians and Khoisan share the deepest clades of the human Y-chromosome phylogeny. Am. J. Hum. Genet. 2002;70:265–268. doi: 10.1086/338306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Underhill P.A., Kivisild T. Use of y chromosome and mitochondrial DNA population structure in tracing human migrations. Annu. Rev. Genet. 2007;41:539–564. doi: 10.1146/annurev.genet.41.110306.130407. [DOI] [PubMed] [Google Scholar]
- 6.Cruciani F., Trombetta B., Massaia A., Destro-Bisol G., Sellitto D., Scozzari R. A revised root for the human Y chromosomal phylogenetic tree: The origin of patrilineal diversity in Africa. Am. J. Hum. Genet. 2011;88:814–818. doi: 10.1016/j.ajhg.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson R., Pritchard J.K., Shen P., Oefner P.J., Feldman M.W. Recent common ancestry of human Y chromosomes: Evidence from DNA sequence data. Proc. Natl. Acad. Sci. USA. 2000;97:7360–7365. doi: 10.1073/pnas.97.13.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei W., Ayub Q., Chen Y., McCarthy S., Hou Y., Carbone I., Xue Y., Tyler-Smith C. A calibrated human Y-chromosomal phylogeny based on resequencing. Genome Res. 2013;23:388–395. doi: 10.1101/gr.143198.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behar D.M., van Oven M., Rosset S., Metspalu M., Loogväli E.L., Silva N.M., Kivisild T., Torroni A., Villems R. A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 2012;90:675–684. doi: 10.1016/j.ajhg.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster S.C., Miller W., Ratan A., Tomsho L.P., Giardine B., Kasson L.R., Harris R.S., Petersen D.C., Zhao F., Qi J. Complete Khoisan and Bantu genomes from southern Africa. Nature. 2010;463:943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seielstad M.T., Minch E., Cavalli-Sforza L.L. Genetic evidence for a higher female migration rate in humans. Nat. Genet. 1998;20:278–280. doi: 10.1038/3088. [DOI] [PubMed] [Google Scholar]
- 12.Wilder J.A., Mobasher Z., Hammer M.F. Genetic evidence for unequal effective population sizes of human females and males. Mol. Biol. Evol. 2004;21:2047–2057. doi: 10.1093/molbev/msh214. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins J.F. Unraveling male and female histories from human genetic data. Curr. Opin. Genet. Dev. 2006;16:611–617. doi: 10.1016/j.gde.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S.A., Sigurdsson A., Jonasdottir A., Jonasdottir A. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad D.F., Keebler J.E., DePristo M.A., Lindsay S.J., Zhang Y., Casals F., Idaghdour Y., Hartl C.L., Torroja C., Garimella K.V., 1000 Genomes Project Variation in genome-wide mutation rates within and between human families. Nat. Genet. 2011;43:712–714. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach J.C., Glusman G., Smit A.F., Huff C.D., Hubley R., Shannon P.T., Rowen L., Pant K.P., Goodman N., Bamshad M. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010;328:636–639. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abecasis G.R., Altshuler D., Auton A., Brooks L.D., Durbin R.M., Gibbs R.A., Hurles M.E., McVean G.A., 1000 Genomes Project Consortium A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awadalla P., Gauthier J., Myers R.A., Casals F., Hamdan F.F., Griffing A.R., Côté M., Henrion E., Spiegelman D., Tarabeux J. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am. J. Hum. Genet. 2010;87:316–324. doi: 10.1016/j.ajhg.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson M.R., Wegmann D., Ehm M.G., Kessner D., St Jean P., Verzilli C., Shen J., Tang Z., Bacanu S.A., Fraser D. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science. 2012;337:100–104. doi: 10.1126/science.1217876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez F.L., Watkins J.C., Hammer M.F. A haplotype at STAT2 Introgressed from neanderthals and serves as a candidate of positive selection in Papua New Guinea. Am. J. Hum. Genet. 2012;91:265–274. doi: 10.1016/j.ajhg.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen M., Guo X., Wang Y., Lohmueller K.E., Rasmussen S., Albrechtsen A., Skotte L., Lindgreen S., Metspalu M., Jombart T. An Aboriginal Australian genome reveals separate human dispersals into Asia. Science. 2011;334:94–98. doi: 10.1126/science.1211177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowler J.M., Johnston H., Olley J.M., Prescott J.R., Roberts R.G., Shawcross W., Spooner N.A. New ages for human occupation and climatic change at Lake Mungo, Australia. Nature. 2003;421:837–840. doi: 10.1038/nature01383. [DOI] [PubMed] [Google Scholar]
- 23.Shang H., Tong H., Zhang S., Chen F., Trinkaus E. An early modern human from Tianyuan Cave, Zhoukoudian, China. Proc. Natl. Acad. Sci. USA. 2007;104:6573–6578. doi: 10.1073/pnas.0702169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higham T., Compton T., Stringer C., Jacobi R., Shapiro B., Trinkaus E., Chandler B., Gröning F., Collins C., Hillson S. The earliest evidence for anatomically modern humans in northwestern Europe. Nature. 2011;479:521–524. doi: 10.1038/nature10484. [DOI] [PubMed] [Google Scholar]
- 25.Demeter F., Shackelford L.L., Bacon A.M., Duringer P., Westaway K., Sayavongkhamdy T., Braga J., Sichanthongtip P., Khamdalavong P., Ponche J.L. Anatomically modern human in Southeast Asia (Laos) by 46 ka. Proc. Natl. Acad. Sci. USA. 2012;109:14375–14380. doi: 10.1073/pnas.1208104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez F.L., Watkins J.C., Hammer M.F. Global genetic variation at OAS1 provides evidence of archaic admixture in Melanesian populations. Mol. Biol. Evol. 2012;29:1513–1520. doi: 10.1093/molbev/msr301. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M.G., Bradman N., Flinn H.M. High throughput analysis of 10 microsatellite and 11 diallelic polymorphisms on the human Y-chromosome. Hum. Genet. 1999;105:577–581. doi: 10.1007/s004399900181. [DOI] [PubMed] [Google Scholar]
- 28.Bandelt H.J., Forster P., Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 29.Ballantyne K.N., Goedbloed M., Fang R., Schaap O., Lao O., Wollstein A., Choi Y., van Duijn K., Vermeulen M., Brauer S. Mutability of Y-chromosomal microsatellites: rates, characteristics, molecular bases, and forensic implications. Am. J. Hum. Genet. 2010;87:341–353. doi: 10.1016/j.ajhg.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhivotovsky L.A., Underhill P.A., Cinnioğlu C., Kayser M., Morar B., Kivisild T., Scozzari R., Cruciani F., Destro-Bisol G., Spedini G. The effective mutation rate at Y chromosome short tandem repeats, with application to human population-divergence time. Am. J. Hum. Genet. 2004;74:50–61. doi: 10.1086/380911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougall I., Brown F.H., Fleagle J.G. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 32.Behar D.M., Villems R., Soodyall H., Blue-Smith J., Pereira L., Metspalu E., Scozzari R., Makkan H., Tzur S., Comas D., Genographic Consortium The dawn of human matrilineal diversity. Am. J. Hum. Genet. 2008;82:1130–1140. doi: 10.1016/j.ajhg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer M.F., Karafet T., Rasanayagam A., Wood E.T., Altheide T.K., Jenkins T., Griffiths R.C., Templeton A.R., Zegura S.L. Out of Africa and back again: Nested cladistic analysis of human Y chromosome variation. Mol. Biol. Evol. 1998;15:427–441. doi: 10.1093/oxfordjournals.molbev.a025939. [DOI] [PubMed] [Google Scholar]
- 34.Ingman M., Kaessmann H., Pääbo S., Gyllensten U. Mitochondrial genome variation and the origin of modern humans. Nature. 2000;408:708–713. doi: 10.1038/35047064. [DOI] [PubMed] [Google Scholar]
- 35.Underhill P.A., Shen P., Lin A.A., Jin L., Passarino G., Yang W.H., Kauffman E., Bonné-Tamir B., Bertranpetit J., Francalacci P. Y chromosome sequence variation and the history of human populations. Nat. Genet. 2000;26:358–361. doi: 10.1038/81685. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen R., Beaumont M.A. Statistical inferences in phylogeography. Mol. Ecol. 2009;18:1034–1047. doi: 10.1111/j.1365-294X.2008.04059.x. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg N.A., Nordborg M. Genealogical trees, coalescent theory and the analysis of genetic polymorphisms. Nat. Rev. Genet. 2002;3:380–390. doi: 10.1038/nrg795. [DOI] [PubMed] [Google Scholar]
- 38.Batini C., Jobling M.A. The jigsaw puzzle of our African ancestry: Unsolved, or unsolvable? Genome Biol. 2011;12:118. doi: 10.1186/gb-2011-12-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickrell J.K., Patterson N., Barbieri C., Berthold F., Gerlach L., Güldemann T., Kure B., Mpoloka S.W., Nakagawa H., Naumann C. The genetic prehistory of southern Africa. Nat Commun. 2012;3:1143. doi: 10.1038/ncomms2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlebusch C.M., Skoglund P., Sjödin P., Gattepaille L.M., Hernandez D., Jay F., Li S., De Jongh M., Singleton A., Blum M.G. Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science. 2012;338:374–379. doi: 10.1126/science.1227721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veeramah K.R., Wegmann D., Woerner A., Mendez F.L., Watkins J.C., Destro-Bisol G., Soodyall H., Louie L., Hammer M.F. An early divergence of KhoeSan ancestors from those of other modern humans is supported by an ABC-based analysis of autosomal resequencing data. Mol. Biol. Evol. 2012;29:617–630. doi: 10.1093/molbev/msr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltis D. The volume and structure of the transatlantic slave trade: A reassessment. William Mary Q. 2001;58:17–46. [PubMed] [Google Scholar]
- 43.Gronau I., Hubisz M.J., Gulko B., Danko C.G., Siepel A. Bayesian inference of ancient human demography from individual genome sequences. Nat. Genet. 2011;43:1031–1034. doi: 10.1038/ng.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scally A., Durbin R. Revising the human mutation rate: Implications for understanding human evolution. Nat. Rev. Genet. 2012;13:745–753. doi: 10.1038/nrg3295. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson A., Manica A. Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proc. Natl. Acad. Sci. USA. 2012;109:13956–13960. doi: 10.1073/pnas.1200567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer M.F., Woerner A.E., Mendez F.L., Watkins J.C., Wall J.D. Genetic evidence for archaic admixture in Africa. Proc. Natl. Acad. Sci. USA. 2011;108:15123–15128. doi: 10.1073/pnas.1109300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvati K., Stringer C., Grün R., Aubert M., Allsworth-Jones P., Folorunso C.A. The Later Stone Age calvaria from Iwo Eleru, Nigeria: Morphology and chronology. PLoS ONE. 2011;6:e24024. doi: 10.1371/journal.pone.0024024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


