Abstract
In this report, we show that the overexpression of tissue transglutaminase (tTG) in the human neuroblastoma cell line SK-N-BE(2) renders these neural crest-derived cells highly susceptible to death by apoptosis. Cells transfected with a full-length tTG cDNA, under the control of a constitutive promoter, show a drastic reduction in proliferative capacity paralleled by a large increase in cell death rate. The dying tTG-transfected cells exhibit both cytoplasmic and nuclear changes characteristic of cells undergoing apoptosis. The tTG-transfected cells express high Bcl-2 protein levels as well as phenotypic neural cell adhesion molecule markers (NCAM and neurofilaments) of cells differentiating along the neuronal pathway. In keeping with these findings, transfection of neuroblastoma cells with an expression vector containing segments of the human tTG cDNA in antisense orientation resulted in a pronounced decrease of both spontaneous and retinoic acid (RA)-induced apoptosis. We also present evidence that (i) the apoptotic program of these neuroectodermal cells is strictly regulated by RA and (ii) cell death by apoptosis in the human neuroblastoma SK-N-BE(2) cells preferentially occurs in the substrate-adherent phenotype. For the first time, we report here a direct effect of tTG in the phenotypic maturation toward apoptosis. These results indicate that the tTG-dependent irreversible cross-linking of intracellular protein represents an important biochemical event in the induction of the structural changes featuring cells dying by apoptosis.
Full text
PDF







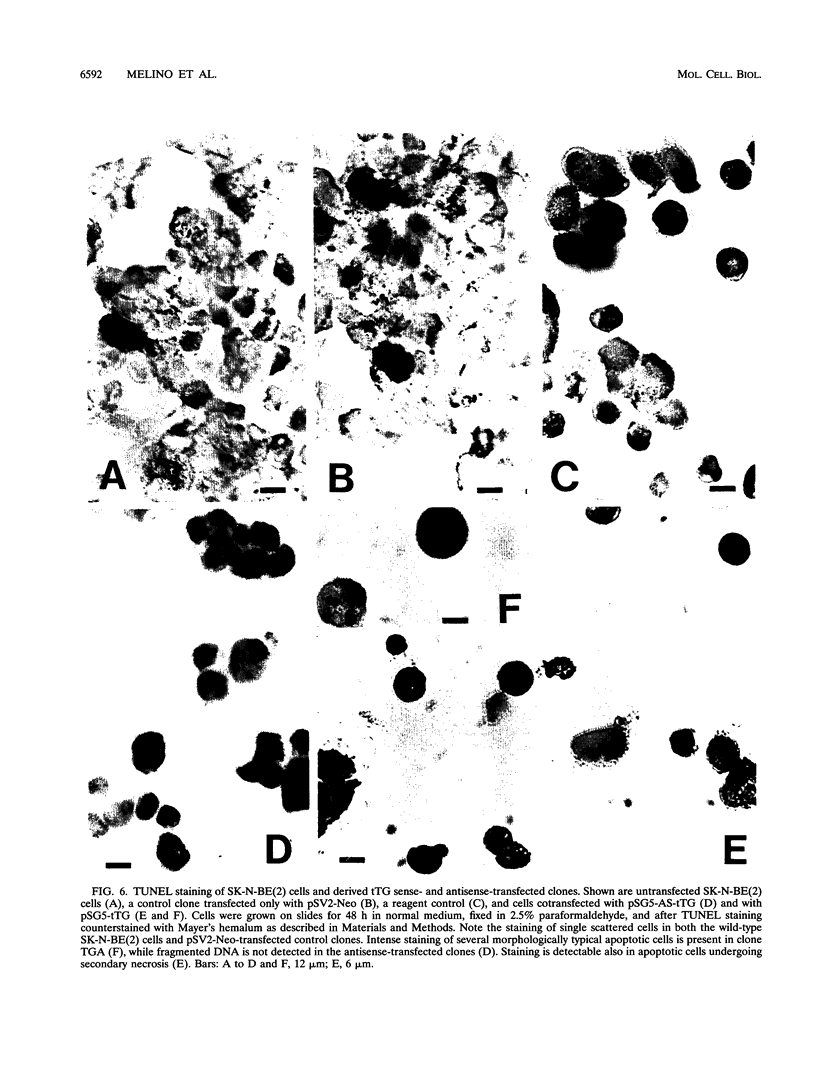
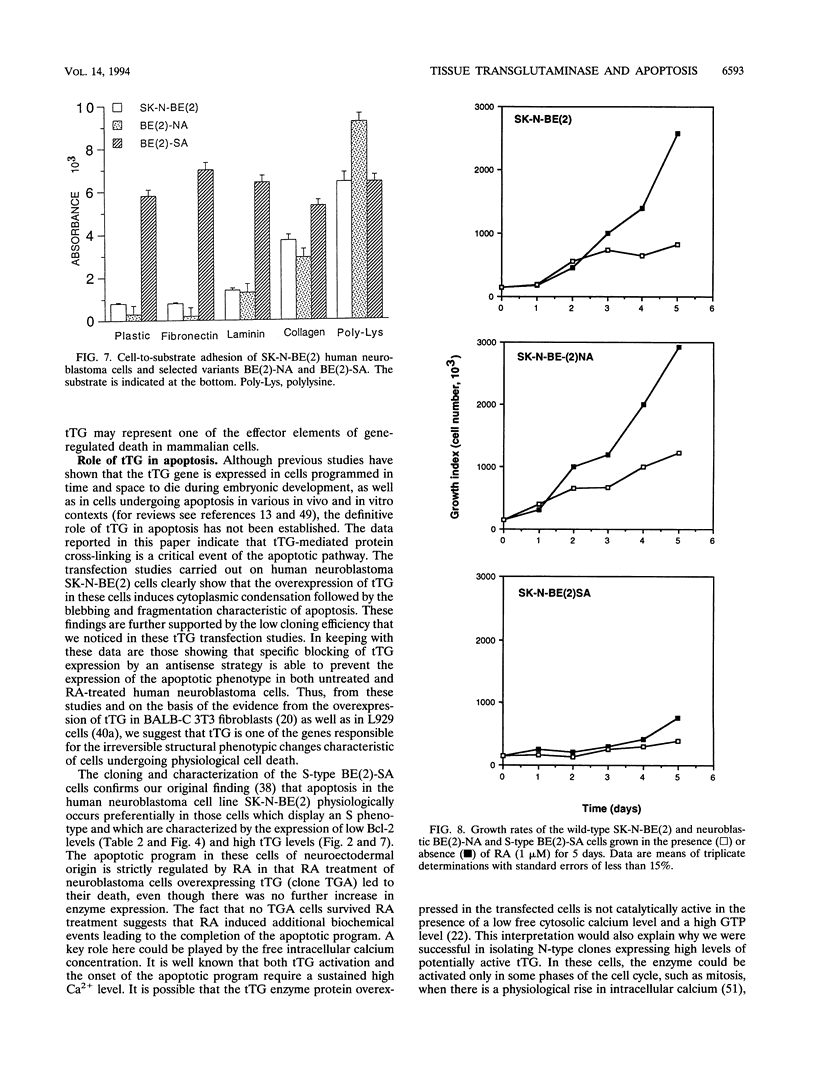



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A., Chang T. D., Ross R. A. Transdifferentiation of human neuroblastoma cells results in coordinate loss of neuronal and malignant properties. Prog Clin Biol Res. 1988;271:265–276. [PubMed] [Google Scholar]
- Bursch W., Kleine L., Tenniswood M. The biochemistry of cell death by apoptosis. Biochem Cell Biol. 1990 Sep;68(9):1071–1074. doi: 10.1139/o90-160. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciccarone V., Spengler B. A., Meyers M. B., Biedler J. L., Ross R. A. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989 Jan 1;49(1):219–225. [PubMed] [Google Scholar]
- Cooper M. J., Hutchins G. M., Cohen P. S., Helman L. J., Mennie R. J., Israel M. A. Human neuroblastoma tumor cell lines correspond to the arrested differentiation of chromaffin adrenal medullary neuroblasts. Cell Growth Differ. 1990 Apr;1(4):149–159. [PubMed] [Google Scholar]
- Dini L., Autuori F., Lentini A., Oliverio S., Piacentini M. The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 1992 Jan 20;296(2):174–178. doi: 10.1016/0014-5793(92)80373-o. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fesus L., Arato G. Quantitation of tissue transglutaminase by a sandwich ELISA system. J Immunol Methods. 1986 Nov 20;94(1-2):131–136. doi: 10.1016/0022-1759(86)90225-5. [DOI] [PubMed] [Google Scholar]
- Fesus L., Davies P. J., Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991 Dec;56(2):170–177. [PubMed] [Google Scholar]
- Fesus L., Thomazy V., Autuori F., Ceru M. P., Tarcsa E., Piacentini M. Apoptotic hepatocytes become insoluble in detergents and chaotropic agents as a result of transglutaminase action. FEBS Lett. 1989 Mar 13;245(1-2):150–154. doi: 10.1016/0014-5793(89)80210-8. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile V., Saydak M., Chiocca E. A., Akande O., Birckbichler P. J., Lee K. N., Stein J. P., Davies P. J. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991 Jan 5;266(1):478–483. [PubMed] [Google Scholar]
- Gentile V., Thomazy V., Piacentini M., Fesus L., Davies P. J. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J Cell Biol. 1992 Oct;119(2):463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Greenberg C. S., Birckbichler P. J., Rice R. H. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991 Dec;5(15):3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Jones T. L., Lafrenz D. Quantitative determination of the induction of apoptosis in a murine B cell line using flow cytometric bivariate cell cycle analysis. Cell Immunol. 1992 Jul;142(2):348–360. doi: 10.1016/0008-8749(92)90296-2. [DOI] [PubMed] [Google Scholar]
- Kim H. C., Lewis M. S., Gorman J. J., Park S. C., Girard J. E., Folk J. E., Chung S. I. Protransglutaminase E from guinea pig skin. Isolation and partial characterization. J Biol Chem. 1990 Dec 15;265(35):21971–21978. [PubMed] [Google Scholar]
- Klein J. D., Guzman E., Kuehn G. D. Purification and partial characterization of transglutaminase from Physarum polycephalum. J Bacteriol. 1992 Apr;174(8):2599–2605. doi: 10.1128/jb.174.8.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight C. R., Rees R. C., Griffin M. Apoptosis: a potential role for cytosolic transglutaminase and its importance in tumour progression. Biochim Biophys Acta. 1991 Jun 5;1096(4):312–318. doi: 10.1016/0925-4439(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Knight R. L., Hand D., Piacentini M., Griffin M. Characterization of the transglutaminase-mediated large molecular weight polymer from rat liver; its relationship to apoptosis. Eur J Cell Biol. 1993 Feb;60(1):210–216. [PubMed] [Google Scholar]
- Melino G., Farrace M. G., Ceru' M. P., Piacentini M. Correlation between transglutaminase activity and polyamine levels in human neuroblastoma cells. Effect of retinoic acid and alpha-difluoromethylornithine. Exp Cell Res. 1988 Dec;179(2):429–445. doi: 10.1016/0014-4827(88)90281-9. [DOI] [PubMed] [Google Scholar]
- Melino G., Knight R. A., Thiele C. J. New insight on the biology of neuroectodermal tumors. Workshop report from the University of Rome Tor Vergata and the IDI-IRCCS on the genetics and control of growth, differentiation, and programmed cell death. Cancer Res. 1993 Feb 15;53(4):926–928. [PubMed] [Google Scholar]
- Melino G., Piacentini M., Patel K., Annicchiarico-Petruzzelli M., Piredda L., Kemshead J. T. Retinoic acid and alpha-difluoromethylornithine induce different expression of neural-specific cell adhesion molecules in differentiating neuroblastoma cells. Prog Clin Biol Res. 1991;366:283–291. [PubMed] [Google Scholar]
- Oberhammer F., Fritsch G., Schmied M., Pavelka M., Printz D., Purchio T., Lassmann H., Schulte-Hermann R. Condensation of the chromatin at the membrane of an apoptotic nucleus is not associated with activation of an endonuclease. J Cell Sci. 1993 Feb;104(Pt 2):317–326. doi: 10.1242/jcs.104.2.317. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Stewart B. E., Qin Q., Chakravarty R., Floyd E. E., Jetten A. M., Rice R. H. Primary structure of keratinocyte transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9333–9337. doi: 10.1073/pnas.87.23.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini M., Annicchiarico-Petruzzelli M., Oliverio S., Piredda L., Biedler J. L., Melino E. Phenotype-specific "tissue" transglutaminase regulation in human neuroblastoma cells in response to retinoic acid: correlation with cell death by apoptosis. Int J Cancer. 1992 Sep 9;52(2):271–278. doi: 10.1002/ijc.2910520220. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Autuori F., Dini L., Farrace M. G., Ghibelli L., Piredda L., Fesus L. "Tissue" transglutaminase is specifically expressed in neonatal rat liver cells undergoing apoptosis upon epidermal growth factor-stimulation. Cell Tissue Res. 1991 Feb;263(2):227–235. doi: 10.1007/BF00318764. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Ceru'-Argento M. P., Farrace M. G., Autuori F. Post-translational modifications of cellular proteins by polyamines and polyamine-derivatives. Adv Exp Med Biol. 1988;231:185–198. doi: 10.1007/978-1-4684-9042-8_15. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Fesus L., Farrace M. G., Ghibelli L., Piredda L., Melino G. The expression of "tissue" transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol. 1991 Apr;54(2):246–254. [PubMed] [Google Scholar]
- Piacentini M., Fesus L., Melino G. Multiple cell cycle access to the apoptotic death programme in human neuroblastoma cells. FEBS Lett. 1993 Apr 5;320(2):150–154. doi: 10.1016/0014-5793(93)80081-5. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Martinet N., Beninati S., Folk J. E. Free and protein-conjugated polyamines in mouse epidermal cells. Effect of high calcium and retinoic acid. J Biol Chem. 1988 Mar 15;263(8):3790–3794. [PubMed] [Google Scholar]
- Piacentini M., Sartori C., Beninati S., Bargagli A. M., Cerù-Argento M. P. Ornithine decarboxylase, transglutaminase, diamine oxidase and total diamines and polyamines in maternal liver and kidney throughout rat pregnancy. Biochem J. 1986 Mar 1;234(2):435–440. doi: 10.1042/bj2340435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Stemple D. L., Anderson D. J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992 Dec 11;71(6):973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R. R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992 May;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Thomázy V., Fésüs L. Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res. 1989 Jan;255(1):215–224. doi: 10.1007/BF00229084. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Borisy G. G. Intracellular free calcium and mitosis in mammalian cells: anaphase onset is calcium modulated, but is not triggered by a brief transient. J Cell Biol. 1989 Aug;109(2):627–636. doi: 10.1083/jcb.109.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G. Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem. 1984;58(1-2):51–61. doi: 10.1007/BF00240604. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]








