Abstract
Stenotrophomonas maltophilia is a multidrug-resistant organism increasingly isolated from the lungs of cystic fibrosis (CF) patients. One hundred twenty-five S. maltophilia isolates from 85 CF patients underwent planktonic and biofilm susceptibility testing against 9 different antibiotics, alone and in double antibiotic combinations. When S. maltophilia isolates were grown as a biofilm, 4 of the 10 most effective antibiotic combinations included high-dose levofloxacin and 7 of the 10 combinations included colistin at doses achievable by aerosolization.
TEXT
Stenotrophomonas maltophilia is one of the most common multidrug-resistant pathogens infecting the airways of cystic fibrosis (CF) patients (1–3). Antibiotics to treat CF pulmonary infections are chosen based on conventional antimicrobial susceptibility testing of organisms grown planktonically (“free-floating”) in liquid. However, it is known that organisms such as S. maltophilia actually grow as biofilms (communities of bacteria) on airway epithelial cells, suggesting that antibiotics chosen based on biofilm susceptibility testing may be more effective in CF (4, 5).
The objectives of this study were to compare biofilm antimicrobial susceptibility to conventional, planktonic antimicrobial susceptibility (as is currently done in clinical microbiology laboratories) for S. maltophilia, highlight the differences in antibiotic combinations derived using the two methods, and identify potentially more effective choices for inhibiting biofilm growth of S. maltophilia in the CF lung.
A total of 125 CF S. maltophilia isolates from sputum and bronchoalveolar lavage were prospectively collected from the microbiology laboratories at the Hospital for Sick Children (74 isolates from 51 CF patients; maximum of 2 isolates per patient) and St. Michael's Hospital (51 isolates from 34 CF patients; maximum of 2 isolates per patient) in Toronto, Canada, between January 2011 and July 2012. Planktonic susceptibility testing of S. maltophilia isolates was performed by broth microdilution according to CLSI guidelines (6). Isolates were also grown as biofilms using a modification of the Calgary biofilm technique (7). The following antibiotics were tested alone and in double combination: ceftazidime, ticarcillin-clavulanate, tobramycin, levofloxacin, moxifloxacin, trimethoprim-sulfamethoxazole, doxycycline, colistin, and azithromycin. Tobramycin (100 mg/liter and 200 mg/liter) (8) and colistin (100 mg/liter and 200 mg/liter) (9) were tested at concentrations achievable in CF sputum by aerosolization. Levofloxacin was tested at both high concentrations (50 mg/liter and 100 mg/liter, corresponding to achievable sputum levels by aerosolization) (10, 11) and low concentrations (2 mg/liter and 4 mg/liter, corresponding to achievable serum levels).
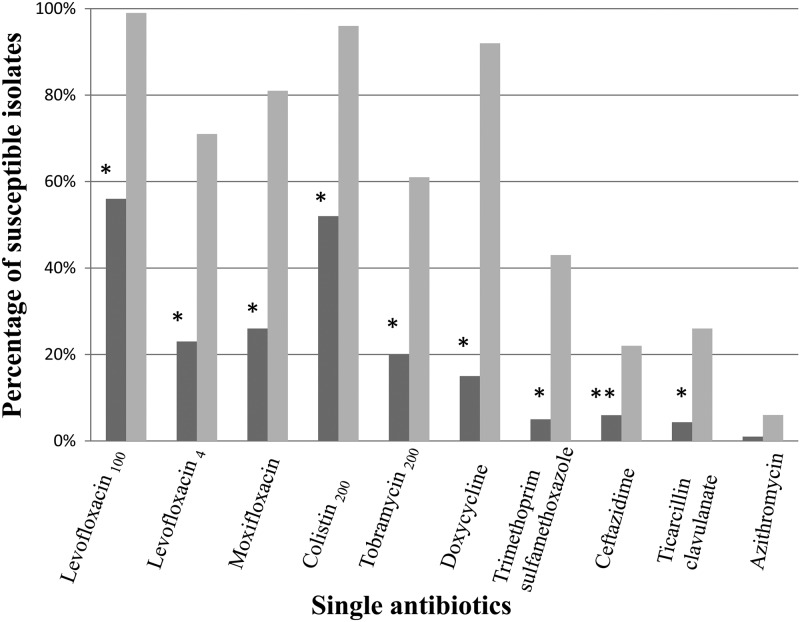
Biofilm inocula of the 125 S. maltophilia isolates tested fell between 2.5 × 104 and 4.6 × 106 CFU/ml (median, 5.5 × 105 CFU/ml), requiring a range of 4.5 h to over 24 h (median, 6.5 h) for biofilm generation. When tested against individual antibiotics, significantly fewer S. maltophilia isolates were susceptible to fluoroquinolones, colistin, tobramycin, doxycycline, trimethoprim-sulfamethoxazole, and β-lactams when grown as biofilms than when grown planktonically (Fig. 1). High-dose levofloxacin was the most effective antibiotic against S. maltophilia in both the planktonic and biofilm forms. S. maltophilia isolates were then tested against double combinations of antibiotics grown as a biofilm and planktonically. When S. maltophilia isolates were grown planktonically, 6 of the 10 most effective antibiotic combinations included high-dose (achievable by aerosolization) levofloxacin and 5 of the 10 most effective antibiotic combinations included colistin at doses achievable by aerosolization (Tables 1 and 2; see also the supplemental material for complete results). In contrast, only 4 of the 10 most effective antibiotic combinations included high-dose (achievable by aerosolization) levofloxacin and 7 of the 10 most effective antibiotic combinations included colistin at doses achievable by aerosolization when isolates were grown as a biofilm.
Fig 1.
Percentage of S. maltophilia isolates susceptible to single antibiotics when grown as a biofilm (dark gray) compared to planktonic (light gray) (*, P < 0.0001; **, P < 0.05, by Fisher's exact test). Levofloxacin100, levofloxacin tested at a maximum concentration of 100 mg/liter achievable in sputum after aerosolization; levofloxacin4, levofloxacin tested at a maximum concentration of 4 mg/liter achievable in serum; colistin200, colistin tested at a maximum concentration of 200 mg/liter achievable in sputum after aerosolization; and tobramycin200, tobramycin tested at a maximum concentration of 200 mg/liter achievable in sputum after aerosolization.
Table 1.
Most effective antibiotic combinations against planktonically grown S. maltophilia isolates
| Antibiotic combination | % susceptible isolates (no.) |
|---|---|
| Levofloxacin100-azithromycin | 99 (124) |
| Levofloxacin100–trimethoprim-sulfamethoxazole | 99 (124) |
| Levofloxacin100–ticarcillin-clavulanate | 99 (124) |
| Levofloxacin100-colistin200 | 99 (124) |
| Doxycycline-colistin200 | 98 (123) |
| Levofloxacin100-ceftazidime | 98 (123) |
| Colistin200–trimethoprim-sulfamethoxazole | 98 (123) |
| Tobramycin-levofloxacin100 | 98 (123) |
| Levofloxacin4-colistin200 | 98 (122) |
| Moxifloxacin-colistin200 | 98 (122) |
Table 2.
Most effective antibiotic combinations against biofilm-grown S. maltophilia isolates
| Antibiotic combination | % susceptible isolates (no.) |
|---|---|
| Ceftazidime-colistin200 | 65 (81) |
| Levofloxacin100–ticarcillin-clavulanate | 62 (78) |
| Colistin200–trimethoprim-sulfamethoxazole | 62 (78) |
| Moxifloxacin-colistin200 | 61 (76) |
| Doxycycline-colistin200 | 60 (75) |
| Levofloxacin100-ceftazidime | 59 (74) |
| Levofloxacin100-azithromycin | 58 (73) |
| Levofloxacin100-colistin200 | 58 (72) |
| Levofloxacin4-colistin200 | 58 (72) |
| Ticarcillin-clavulanate–colistin200 | 58 (72) |
This study is the first to examine the antimicrobial susceptibility of a large collection of predominantly CF S. maltophilia isolates grown both planktonically and in a biofilm. In a biofilm environment, traditional antibiotics used to treat CF patients, β-lactams and aminoglycosides, are not very effective, as β-lactams target rapidly dividing bacteria and aminoglycosides act on aerobically growing organisms (12, 13). Our study confirmed that S. maltophilia growing as a biofilm is very rarely susceptible to β-lactams and aminoglycosides (to which it is intrinsically resistant) (14), with fewer than 10% of isolates being susceptible to ceftazidime and ticarcillin-clavulanate and only 20% of isolates being susceptible to high-dose tobramycin which correlates with levels achievable by aerosolization. Trimethoprim-sulfamethoxazole is often considered the drug of choice in the treatment of S. maltophilia infections; however, S. maltophilia resistance to trimethoprim-sulfamethoxazole has been increasingly described (15). In our assays, only half of S. maltophilia isolates were susceptible to trimethoprim-sulfamethoxazole alone using planktonic susceptibility testing; fewer still (less than 10%) were susceptible when grown as a biofilm.
In our study, colistin was included in many of the most effective double antibiotic combinations, and the majority of S. maltophilia isolates were susceptible to colistin when grown planktonically or as a biofilm. It is important to note, however, that very high concentrations of colistin (to approximately the levels achievable by aerosolization) were used in this assay based on previous in vitro susceptibility reports (9) and high lung concentrations achieved in animal models (16–18). However, the pulmonary concentration of colistin that can be achieved through inhalation is limited by several factors, including significant bronchospasm and hypersensitivity pneumonitis (19–21). Colistin may thus be less effective in vivo with lower achievable pulmonary concentrations (22, 23) than has been demonstrated in vitro against S. maltophilia.
The most effective antibiotic tested alone against planktonic and biofilm-grown S. maltophilia isolates in our study was high-dose levofloxacin. Previous in vitro studies have demonstrated that fluoroquinolones, such as levofloxacin, can disrupt S. maltophilia biofilms and significantly reduce S. maltophilia biofilm mass (24, 25). In addition, high lung concentrations of aerosolized levofloxacin can be achieved in mouse models of lung infection (10) and in CF patients (11, 26). Inhaled levofloxacin may thus represent a potentially effective suppressive antimicrobial therapy for patients chronically infected with S. maltophilia, although antimicrobial resistance may develop with long-term use.
This study has several limitations. Based on current clinical practice, double, not triple, antibiotic combinations known to have in vitro activity against S. maltophilia were tested (9, 27, 28). However, current practices are losing efficacy, and different solutions may be required. Results may also be biased toward patients with repeated samples, although the majority of susceptibility results of isolates from the same patient were different in our study.
In conclusion, both colistin and levofloxacin, at levels achievable by inhalation, were effective at inhibiting the growth of CF S. maltophilia isolates under biofilm conditions. Further prospective studies are needed to determine whether aerosolized levofloxacin treatment can significantly decrease the pulmonary burden of S. maltophilia and improve clinical outcomes in CF patients.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Danuta Kovach in the development of the biofilm assay for S. maltophilia and Patricia Schneider for her help with data entry.
This work was supported by the Canadian Foundation for Infectious Diseases.
We have no conflicts of interest to declare.
This study was performed at The Hospital for Sick Children, Toronto, Canada.
Footnotes
Published ahead of print 7 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02215-12.
REFERENCES
- 1. Gibson RL, Burns JL, Ramsey BW. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918–951 [DOI] [PubMed] [Google Scholar]
- 2. Ballestero S, Virseda I, Escobar H, Suarez L, Baquero F. 1995. Stenotrophomonas maltophilia in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:728–729 [DOI] [PubMed] [Google Scholar]
- 3. Steinkamp G, Wiedemann B, Rietschel E, Krahl A, Gielen J, Barmeier H, Ratjen F. 2005. Prospective evaluation of emerging bacteria in cystic fibrosis. J. Cyst. Fibros. 4:41–48 [DOI] [PubMed] [Google Scholar]
- 4. Pompilio A, Crocetta V, Confalone P, Nicoletti M, Petrucca A, Guarnieri S, Fiscarelli E, Savini V, Piccolomini R, Di Bonaventura G. 2010. Adhesion to and biofilm formation on IB3-1 bronchial cells by Stenotrophomonas maltophilia isolates from cystic fibrosis patients. BMC Microbiol. 10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keays T, Ferris W, Vandemheen KL, Chan F, Yeung SW, Mah TF, Ramotar K, Saginur R, Aaron SD. 2009. A retrospective analysis of biofilm antibiotic susceptibility testing: a better predictor of clinical response in cystic fibrosis exacerbations. J. Cyst. Fibros. 8:122–127 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dales L, Ferris W, Vandemheen K, Aaron SD. 2009. Combination antibiotic susceptibility of biofilm-grown Burkholderia cepacia and Pseudomonas aeruginosa isolated from patients with pulmonary exacerbations of cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 28:1275–1279 [DOI] [PubMed] [Google Scholar]
- 9. San Gabriel P, Zhou J, Tabibi S, Chen Y, Trauzzi M, Saiman L. 2004. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:3923–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit JS, Dudley MN. 2011. Pharmacokinetics and safety of MP-376 (levofloxacin inhalation solution) in cystic fibrosis subjects. Antimicrob. Agents Chemother. 55:2636–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ, Hwang SH, McDermott TR, Ochsner UA. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425–1443 [DOI] [PubMed] [Google Scholar]
- 13. Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Doring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leclercq R, Canton R, Brown DF, Giske CG, Heisig P, Macgowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM, Soussy CJ, Steinbakk M, Winstanley TG, Kahlmeter G. 2011. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469–0691.2011.03703.x [DOI] [PubMed] [Google Scholar]
- 15. Toleman MA, Bennett PM, Bennett DM, Jones RN, Walsh TR. 2007. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg. Infect. Dis. 13:559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, Rouby JJ. 2010. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 36:1147–1155 [DOI] [PubMed] [Google Scholar]
- 17. Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, Yamaguchi K. 2009. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 63:534–542 [DOI] [PubMed] [Google Scholar]
- 18. Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 54:3702–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beringer P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434–440 [DOI] [PubMed] [Google Scholar]
- 20. Cunningham S, Prasad A, Collyer L, Carr S, Lynn IB, Wallis C. 2001. Bronchoconstriction following nebulised colistin in cystic fibrosis. Arch. Dis. Child. 84:432–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leong KW, Ong S, Chee HL, Lee W, Kwa AL. 2010. Hypersensitivity pneumonitis due to high-dose colistin aerosol therapy. Int. J. Infect. Dis. 14:e1018–e1019 [DOI] [PubMed] [Google Scholar]
- 22. Reed MD, Stern RC, O'Riordan MA, Blumer JL. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645–654 [DOI] [PubMed] [Google Scholar]
- 23. Athanassa ZE, Markantonis SL, Fousteri MZ, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ. 2012. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med. [Epub ahead of print.] doi:10.1007/s00134-012-2628-7 [DOI] [PubMed] [Google Scholar]
- 24. Passerini de Rossi B, Garcia C, Calenda M, Vay C, Franco M. 2009. Activity of levofloxacin and ciprofloxacin on biofilms and planktonic cells of Stenotrophomonas maltophilia isolates from patients with device-associated infections. Int. J. Antimicrob. Agents. 34:260–264 [DOI] [PubMed] [Google Scholar]
- 25. Di Bonaventura G, Spedicato I, D'Antonio D, Robuffo I, Piccolomini R. 2004. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob. Agents Chemother. 48:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. 2011. Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 183:1510–1516 [DOI] [PubMed] [Google Scholar]
- 27. Waters V, Ratjen F. 2006. Multidrug-resistant organisms in cystic fibrosis: management and infection-control issues. Expert Rev. Anti-Infect. Ther. 4:807–819 [DOI] [PubMed] [Google Scholar]
- 28. Weiss K, Restieri C, De Carolis E, Laverdiere M, Guay H. 2000. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 45:363–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.