Abstract
A multiplex PCR (mPCR) protocol was developed for simultaneous detection of the gyrB gene in Streptococcus pneumoniae, Streptococcus mitis, and Streptococcus oralis, and the specificity was evaluated using 141 coccus strains. Genomic DNAs purified from S. pneumoniae, S. mitis, and S. oralis strains were efficiently detected with size differences, whereas no PCR products were amplified from any of the reference strains tested. A pilot study of 47 human oral swab specimens was conducted in parallel, and the mPCR assay identified S. pneumoniae in 1 sample, S. mitis in 8 samples, and S. oralis in 2 samples, providing a powerful means for characterization at the level of species compared with traditional culture analysis. Our results suggest that the mPCR protocol presented here is a sensitive and promising tool for the rapid detection and discrimination of S. pneumoniae, S. mitis, and S. oralis from clinical specimens.
INTRODUCTION
Streptococcus pneumoniae is an important human pathogen associated with pneumonia, bacterial meningitis, otitis media, and nongonoccal urethritis (1–3). In contrast, two viridans group streptococci (VGS), Streptococcus mitis and Streptococcus oralis, are recognized as important etiological agents of dental caries and subacute bacterial endocarditis and septicemia (4–6), and recently, pancreatic diseases, including pancreatic cancer, have been associated with S. mitis. Thus, the pathogenesis and clinical importance of these cocci are increasing (7).
Precise discrimination among closely related bacterial species is crucial for accurate diagnosis and treatment. Because S. pneumoniae is often isolated with S. mitis, S. oralis, and other commensals in the oral cavity, accurate discrimination among these strains is important for diagnosis and treatment (8). However, identification of these species has traditionally been difficult using current clinical laboratory techniques, because they have a close, common genetic ancestry (5, 9, 10). Moreover, identification of these organisms at the species level by genotypic and phenotypic methods is challenging, since biological and biochemical profiles may be ambiguous due to natural competence and other genetic transfer events (8, 11). Thus, clinical laboratories can encounter “atypical pneumococci” that are optochin resistant, bile insoluble, or unencapsulated (9).
Molecular genetic analyses based on the 16S rRNA gene have provided new insights into the phylogenetic interrelationships of many bacteria (12) and a powerful means for species characterization (13, 14). Unfortunately, the 16S rRNA gene nucleotide sequences from S. mitis and S. oralis are almost identical to that of S. pneumoniae; as such, this gene sequence cannot be used to distinguish between strains at the species level (15).
More-sensitive routine diagnostic methods, such as PCR, could be useful for these bacteria. Several PCR-based methods have been developed for the detection of S. pneumoniae, targeting pneumococcal virulence factors. These factors include autolysin (lytA) (16), pneumolysin (ply) (17), pneumococcal surface antigen A (psaA) (18), manganese-dependent superoxide dismutase (sodA) (19), penicillin-binding protein (20), and a unknown putative gene (21). However, nonpneumococcal isolates containing these genes have also been described, making it difficult to confidently distinguish between S. mitis and S. pneumoniae isolates using these markers (22–24). Furthermore, S. mitis and S. oralis might become evolved from the pathogenic S. pneumoniae by genomic reduction, implying that both inter- and intraspecies recombination events have occurred among these species (25, 26).
Previously, we reported a specific single-step PCR assay to identify S. pneumoniae (27), S. oralis (28), and S. mitis (28). However, simultaneous detection methods specific to S. pneumoniae and the closely related S. mitis and S. oralis strains have not yet been reported. Housekeeping protein-coding genes that are thought to evolve faster than rRNA genes have been proposed as suitable specific markers for the identification and classification of bacteria (29, 30). Among these, DNA gyrase is an essential bacterial enzyme that catalyzes the ATP-dependent negative supercoiling of double-stranded closed-circular DNA. It is composed of two A and two B subunits encoded by the gyrA and gyrB genes, respectively (31). Using the gyrB genes for analysis could be more useful than 16S rRNA gene-based methods because there is only a single copy locus in most bacteria, lateral gene transfer of this gene is rare, and the evolution rate is higher than that of the 16S rRNA gene, making it more discriminatory at the species level. In this study, we developed a multiplex PCR (mPCR) protocol targeting the gyrB gene for the rapid, accurate, simple, and species-specific detection of S. pneumoniae, S. mitis, and S. oralis in oral environments and clinical specimens.
MATERIALS AND METHODS
Ethics statement.
Oral samples were collected by the Seyee dental clinic (Ulsan, Republic of Korea) and were analyzed in the Department of Microbiology in the College of Medicine at Chung-Ang University. All participants gave written informed consent. For all cases, the Chung-Ang University College of Medicine IRB (protocol 2009-12) approved the collection and analysis of all samples.
Bacterial strains and genomic DNA preparation.
A total of 141 bacterial strains that were used in this study are listed in Table 1. The 24 S. pneumoniae strains, 20 S. mitis strains, and 10 S. oralis strains were obtained from the Korean Collection for Type Culture (KCTC; Daejeon, Republic of Korea), the Culture Collection of Antibiotic-Resistant Microbes (CCARM; Seoul, Republic of Korea), the Department of Oral Biochemistry, College of Dentistry, Chosun University (ChDC; Gwangju, Republic of Korea), the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig Germany), and the American Type Culture Collection (ATCC, Manassas, VA). These strains were used as positive controls for the mPCR assay. Sixty-one other Streptococcus and 26 other coccus strains were obtained from the Culture Collection of the University of Gothenburg (CCUG; Gothenburg, Sweden), the Belgian Coordinated Collections of Micro-organisms (BCCM/LMG Ghent, Belgium), KCTC, and DSMZ. These strains were used as negative controls. Oral streptococcus strains were grown on 5% sheep blood agar plates (Asan Pharm Co., Seoul, Republic of Korea) at 37°C for 20 h, while the other strains were cultured on brain heart infusion agar plates (Difco Laboratories, Detroit, MI) under the same conditions. Bacterial genomic DNA was prepared by the cetyltrimethylammonium bromide method (32). The purified DNA was quantified using a Nano Quant Infinite M200 spectrophotometer (Tecan, Männedorf, Switzerland) at a wavelength of 260 nm.
Table 1.
Streptococcus species and other bacteria used in this study
| Group | Species | Strain(s)a (n = 141) |
|---|---|---|
| Streptococcus (n = 115) | S. pneumoniae | KCTC 5080T, CCARM 4009, CCARM 4019, CCARM 4033, CCARM 4109, CCARM 4112, CCARM 4113, CCARM 4114, CCARM 4115, CCARM 4003, CCARM 4005, CCARM 4078, CCARM 4079, CCARM 4080, CCARM 4084, CCARM 4085, CCARM 4088, CCARM 4106, CCARM 4116, ChDC 4-0134, ChDC 4-0749, ChDC 6-4740, CCARM 4081, CCARM 4091 |
| S. mitis | KCTC 3556T, KCOM 1350, KCOM 1379, KCOM 1388, ChDC B183, ChDC B186, ChDC B193, ChDC B194, ChDC B227, ChDC B231, ChDC B239, ChDC B242, ChDC B253, ChDC B258, ChDC B260, ChDC B279, ChDC B286, ChDC B303-1, ChDC B315, ChDC B317 | |
| S. oralis | KCTC 13048T, ATCC 9811, DSMZ 20395, DSMZ 20066, ATCC 700233, KCOM 1401, KCOM 1407, KCOM 1408, KCOM 1414, KCOM 1416 | |
| S. pyogenes | KCTC 3984T, KCTC 3096, KCTC 3208 | |
| S. gordonii | KCTC 3286T, KCOM 1347, KCOM 1364, KCOM 1369, KCOM 1387, KCOM 1357 | |
| S. infantis | KCOM 1358, KCOM 1375 | |
| S. australis | KCOM 1386, KCOM 1439, KCOM 1441 | |
| S. sinensis | KCOM 1017, KCOM 1427, KCOM1018 | |
| S. sanguinis | KCTC 3284T, KCOM 1567, KCOM 1372, KCOM 1422, KCOM 1428, KCOM 1019, KCOM 1070, | |
| S. parasanguinis | KCTC 13046T, ChDC B185, ChDC B195, ChDC B215, KCOM 1365, KCOM 1366, KCOM 1370, KCOM 1585 | |
| S. constellatus | ATCC 27823T, ChDC B280, ChDC B284, ChDC B290 | |
| S. intermedius | KCTC 3268T, ChDC KB80, ChDC KB236, ChDC KB717 | |
| S. anginosus | ATCC 33397T, ChDC YA1, ChDC YA3, ChDC YA5, ChDC YA6, ChDC YA7, ChDC YA8, ChDC YA9, ChDC YA10, ChDC YA11, ChDC YA12, ChDC YA13, ChDC B181, ChDC YA201, ChDC B232, ChDC B248, ChDC B252, ChDC B263, ChDC B287, ChDC B311, ChDC B407-1 | |
| Other cocci (n = 26) | L. lactis subsp. cremoris | DSMZ 40699T |
| L. lactis subsp. hordniae | KCTC 3768T | |
| L. garvieae | LMG 8162T, LMG 8501, LMG 9472, LMG 14494 | |
| L. lactis subsp. lactis | KCTC 3769T, KCTC 3191, KCTC 2013, KCTC 3894, KCTC 3926 | |
| E. solitarius | KCTC 3553T | |
| E. hirae | KCTC 3616T | |
| E. mundtii | KCTC 3630T | |
| E. malodoratus | KCTC 3641T | |
| E. cecorum | KCTC 3642T | |
| E. saccharolyticus | KCTC 3643T | |
| E. villorum | KCTC 13904T | |
| E. moraviensis | KCTC 13911T | |
| E. phoeniculicola | KCTC 3818T | |
| E. raffinosus | KCTC 5189T | |
| E. avium | KCTC 5190T | |
| E. faecalis | KCTC 3206T | |
| V. fluvialis | LMG 9464T | |
| V. salmoninarum | LMG 1149T | |
| V. lutrae | LMG 19537T |
Abbreviations: KCTC, Korea Collection for Type Culture (Daejeon, Republic of Korea); CCARM, Culture Collection of Antibiotics Resistant Microbe (Seoul, Republic of Korea); ChDC, Chosun University Dental College (Gwangju, Republic of Korea); KCOM, Korean Collection for Oral Microbiology (Gwangju, Republic of Korea); DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany); ATCC, American Type Culture Collection (Manassas, VA); BCCM/LMG, Belgian Coordinated Collections of Micro-organisms (Ghent, Belgium).
Design of multiplex PCR primers.
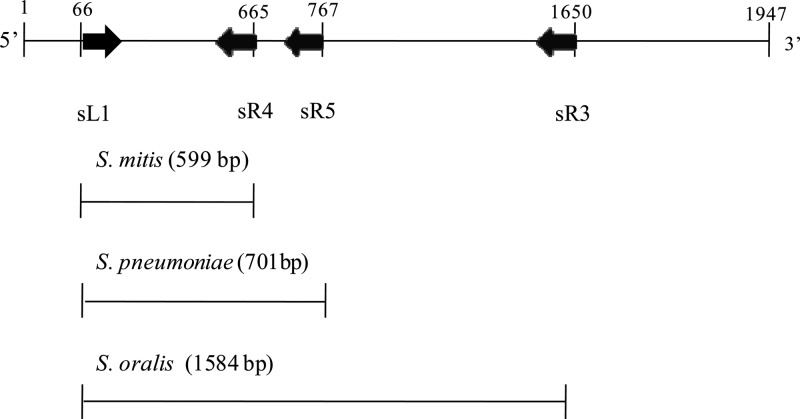
The oligonucleotide primer sets, sL1 (5′-GGCTTAGAGGCTGTTCGT-3′), sR1 (5′-TCACTTCCCACTTTAACCC-3′), sR2 (5′-AGTTTGTTCTAGCCCCTCA-3′), and sR3 (5′-ATCTCACCGTCTGTATAGA-3′), for mPCR amplification of the gyrB region were designed according to the gyrB nucleotide sequences of S. pneumoniae JJA (GenBank accession number NC_012466), S. pneumoniae P1031 (NC_012467), S. pneumoniae 70585 (NC_012468), S. pneumoniae ATCC 700669 (NC_011900), S. mitis B6 (NC_013853), S. oralis Uo5 (NC_015291), and S. oralis Uo5 (NC_015291) published in the NCBI GenBank database, using Oligo V6 software (Molecular Biology Insights, Cascade, CO). The gyrB gene of S. pneumoniae KCTC 5080T and the primers are illustrated in Fig. 1. During the mPCR process, amplicon sizes were selected to enable the identification of S. pneumoniae, S. mitis, and S. oralis species, and the amplification conditions were optimized in terms of primer concentrations, annealing temperatures, and other thermocycling conditions.
Fig 1.
The gyrB gene of S. pneumoniae KCTC 5080T and primers sL1, sR1, sR2, and sR3 for multiplex PCR amplification and nucleotide sequence analysis.
Establishment of the multiplex PCR assay.
Three reference strains, S. pneumoniae KCTC 5080T, S. mitis KCTC 3556T, and S. oralis 13048T, were used to develop and evaluate the mPCR assay in combination with each other; namely, S. pneumoniae, S. mitis, and S. oralis alone, S. pneumoniae plus S. mitis, S. pneumoniae plus S. oralis, S. mitis plus S. oralis, and S. pneumoniae plus S. mitis plus S. oralis. The mPCR reaction was conducted in a 20-μl-total reaction mixture containing 100 ng genomic DNA template, 1 μl each primer, 2 μl 10× reaction buffer, 0.2 mM deoxynucleoside triphosphates (dNTP)s, 1.5 mM MgCl2, and 2.5 U Taq polymerase. Primer concentrations were selected as 1, 2, 3, 5, 10, 15, 20, 25, and 30 μM each primer (sL1, sR1, sR2, and sR3). PCR amplification was performed using a TGradient thermal cycler (Biometra, Goettingen, Germany) with the following conditions: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, annealing temperature for 30 s, and 72°C for 40 s and a final extension at 72°C for 10 min. Ten gradient temperatures, including 49°C, 50°C, 51°C, 52°C, 53°C, 54°C, 55°C, 56°C, 57°C, 58°C, 59°C, and 60°C were used to optimize the annealing temperature. After PCR amplification, 5 μl of each PCR product was resolved using 1.2% SeaKem LE agarose gel (FMC Bioproducts, Rockland, ME), followed by ethidium bromide staining. A negative-control experiment was performed using sterile water instead of culture or DNA template. Electrophoresis was used to separate the amplicons, and DNA bands were viewed under a GelDoc XR image analysis system (Bio-Rad, Hercules, CA).
DNA sequence analysis.
All PCR products were evaluated by sequence analysis. Cycle sequencing was performed using the BigDye terminator version 3.1 cycle sequencing kit, and the sequencing reactions were analyzed using an automated DNA sequencer (model 3730; Applied Biosystems, Foster City, CA). Assembly and editing of nucleotide sequences were performed using BioEdit (33) software and the CLUSTAL_X 1.81 program (34). The nucleotide sequence homologies of each amplified PCR product were evaluated using BLAST searches of the National Center for Biotechnology Information (NCBI) databases.
Specificity and sensitivity of the multiplex PCR assay.
The specificity of the mPCR primers was examined using DNA templates from each of the 24 S. pneumoniae, 20 S. mitis, 10 S. oralis, 61 other Streptococcus, and 26 other coccus strains. mPCR was performed with 100 ng genomic DNA template in a 20-μl reaction mixture containing 1 μl of each primer (sL1, sR1, sR2, and sR3 at 20 μM, 20 μM, 1 μM, and 2 μM, respectively), 2 μl 10× reaction buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, and 2.5 U Taq polymerase. The sensitivity of the PCR assay was evaluated using a 10-fold series of purified DNA from a mixed culture of S. pneumoniae KCTC 5080T, S. mitis KCTC 3556T, and S. oralis 13048T (each at 102 CFU/ml). Amplification was conducted using the GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) with PCR cycling conditions consisting of an initial denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 40 s, with final extension at 72°C for 10 min. After PCR amplification, 5 μl of each PCR product was resolved using agarose gel electrophoresis as described above.
Pilot application study using clinical samples.
For a pilot application study, 47 samples collected from the oral cavity of outpatients in the Seyee Dental Clinic were used to test the mPCR protocol. The median age of patients was 32 years old (range, 4 to 78 years). All samples were taken using a 3M Quick swab, and genomic DNA was isolated as described previously. One microliter (10 μg/ml) of each DNA sample was subjected to species-specific detection for S. pneumoniae, S. mitis, and S. oralis. At the same time, 100 μl of each dilution was cultured on 5% sheep blood agar plates (Asan Pharmaceutical, Seoul, Republic of Korea) and the 3 oral pathogens were identified according to standard microbiological methods, including the API 20 Strep method (BioMérieux SA, Marcy l'Etoile, France).
RESULTS AND DISCUSSION
Optimization of the multiplex PCR assay.
Since mPCR protocols have been developed for the detection of S. pneumoniae and other respiratory pathogens, including S. pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, and Chlamydophila pneumoniae (35), or for serotyping of S. pneumoniae (36), no protocol has been developed for the simultaneous detection of S. pneumoniae and its closest phylogenetic relatives, S. mitis and S. oralis. In order to determine the optimal conditions for mPCR, genomic DNA extracted from 7 strain combinations, including S. pneumoniae, S. mitis, S. oralis, S. pneumoniae plus S. mitis, S. pneumoniae plus S. oralis, S. mitis plus S. oralis, and S. pneumoniae plus S. mitis plus S. oralis, were used as target templates. The discrimination of S. pneumoniae, S. mitis, and S. oralis is shown in Fig. 2. The amplification generated 3 PCR products as detected on agarose gels, comprising 701-bp, 599-bp, and 1,584-bp amplicons for S. pneumoniae, S. mitis, and S. oralis, respectively. No size variation was detected among the strains investigated. The PCR product showed the highest levels of resolution of specificity when primer concentrations of sL1, sR1, sR2, and sR3 were at 20 μM, 20 μM, 1 μM, and 2 μM and amplification was at an annealing temperature of 56°C compared to the specificity of products obtained under other conditions. The identity of PCR amplicons exactly matched the gyrB gene of each species as determined using nucleotide sequencing and BLAST homology searches.
Fig 2.
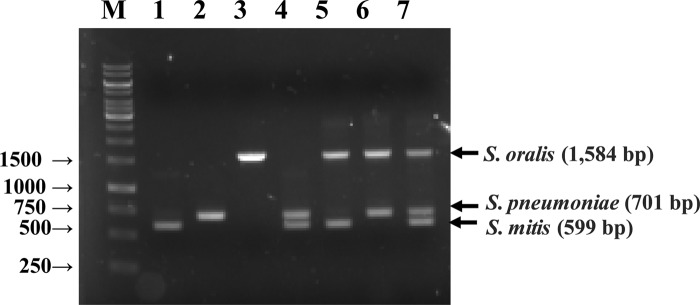
Electrophoretic examination of multiplex PCR products. Lane M, 1 kb plus DNA ladder (Life Technologies, Rockville, ME, U.S.A.); lane 1, S. mitis KCTC 3556T; lane 2, S. pneumoniae KCTC 5080T; lane 3, S. oralis KCTC 13048T; lane 4, S. mitis KCTC 3556T plus S. pneumoniae KCTC 5080T; lane 5, S. mitis KCTC 3556T plus S. oralis KCTC 13048T; lane 6, S. pneumoniae KCTC 5080T plus S. oralis KCTC 13048T; lane 7, S. mitis KCTC 3556T plus S. pneumoniae KCTC 5080T plus S. oralis KCTC 13048T.
Specificity of the multiplex PCR assay.
The specificity of the mPCR amplification was determined using DNA extracted from 24 S. pneumoniae, 20 S. mitis, and 10 S. oralis strains and 87 other strains. The amplified PCR products from the different bacterial strains resulted in the expected 701-bp PCR product common to the 24 S. pneumoniae strains, including type strains and clinical isolates. All S. mitis and S. oralis strains yielded specific amplifications for the 599-bp or 1,548-bp PCR products, respectively, showing positive results. This product was specifically detected because no amplifications of PCR products from the 87 strains of other closely related streptococci or Gram-positive cocci were detected (data not shown). In the present study, the following strains were mPCR negative based on gyrB amplification: 61 VGS strains, including 4 Streptococcus constellatus, 4 Streptococcus intermedius, 21 Streptococcus anginosus, 6 Streptococcus gordonii, 2 Streptococcus infantis, 3 Streptococcus australis, 3 Streptococcus sinensis, 7 Streptococcus sanguinis, and 8 Streptococcus parasanguinis; other streptococcus strains; 3 Streptococcus pyogenes strains; 11 lactococcus strains, including 1 Lactococcus lactis subsp. cremoris, 1 Lactococcus lactis subsp. hordniae, 4 Lactococcus garvieae, and 5 Lactococcus lactis subsp. lactis strains; 12 enterococcus strains, including 1 Enterococcus solitaries, 1 Enterococcus hirae, 1 Enterococcus mundtii, 1 Enterococcus malodoratus, 1 Enterococcus cecorum, 1 Enterococcus saccharolyticus, 1 Enterococcus villorum, 1 Enterococcus moraviensis, 1 Enterococcus phoeniculicola, 1 Enterococcus raffinosus, 1 Enterococcus avium, and 1 Enterococcus faecalis strain; and 3 vagococcus strains, including 1 Vagococcus fluvialis, 1 Vagococcus salmoninarum, and 1 Vagococcus lutrae strain.
Because S. pneumoniae shares over 99% 16S rRNA gene sequence homology with other VGS, the identification of novel genetic markers specific for S. pneumoniae is needed for accurate diagnosis of pneumococcal disease. However, this organism evolved closely with related VGS from a common genetic ancestor (5, 9, 10); therefore, serious specificity problems were noted with these assays, including a false-positive amplification with the ply (17) and a putative noncoding region Spn9802 and Spn9828 (21) primer sets from the genomic DNA of S. mitis, S. gordonii, and S. sanguinis, while a few S. pneumoniae strains did not amplify with the lytA primers (27). Even though the frequency was probably low in the mPCR protocol, 3.3% of S. mitis strains shared the lytA gene with S. pneumoniae (35).
More recently, the cpsA gene was identified as a novel genomic marker specific to S. pneumoniae and a quantitative PCR (qPCR) assay was developed to detect and enumerate this pathogen (37). In S. oralis, the rgg gene, known to be involved in the biosynthesis of glucosyltransferase, allowed for the easy and reliable discrimination of S. oralis from other streptococci (38). Thus, it should be noted that in our study, we were able to fully discriminate S. pneumoniae, S. mitis, and S. oralis species from one another and from related streptococci by the current mPCR protocol, promising the rapid diagnosis of streptococcal diseases.
Sensitivity of multiplex PCR assay.
The sensitivity of mPCR using the gyrB gene-based primers was determined by analyzing 10-fold serially diluted genomic DNA (6.3 ng to 6.3 fg) extracted from each S. pneumoniae KCTC 5080T, S. mitis KCTC 3556T, and S. oralis 13048T culture at 107 CFU ml−1. The detection limit of the conventional PCR assay was 630 fg with S. pneumoniae DNA, 6.3 pg with S. mitis DNA, and 63 fg with S. oralis DNA (Table 2).
Table 2.
Limits of detection for genomic DNA in combination with S. pneumoniae KCTC 5080T, S. mitis KCTC 3556T, and S. oralis 13048T in multiplex PCR
| Lane | DNA concn (ng/μl) | Cell no. | PCR resultsa |
||
|---|---|---|---|---|---|
| S. pneumoniae | S. mitis | S. oralis | |||
| 1 | 6.3 × 100 | 1 × 107 | + | + | + |
| 2 | 6.3 × 10−1 | 1 × 106 | + | + | + |
| 3 | 6.3 × 10−2 | 1 × 105 | + | + | + |
| 4 | 6.3 × 10−3 | 1 × 104 | + | + | + |
| 5 | 6.3 × 10−4 | 1 × 103 | + | ND | + |
| 6 | 6.3 × 10−5 | 1 × 102 | ND | ND | + |
| 7 | 6.3 × 10−6 | 1 × 101 | ND | ND | ND |
| 8 | Negative control | 0 | ND | ND | ND |
ND, not detected.
Genome equivalents were calculated assuming one molecule of S. pneumoniae, S. mitis and S. oralis DNA. Considering a genome size of 2.1 Mb for S. pneumoniae and S. mitis, a corresponding 2.2 fg of DNA was calculated and 2.1 fg was determined for S. oralis DNA based on a 2.0-Mb genome. These numbers were determined according to the following equation: DNA amount in fg = bp × 660 Da/bp × 1.6 × 10−27 kg/Da × 1 × 10−18 fg/kg (39). Considering a genome size of 2.1 Mb as determined for S. pneumoniae (GenBank accession number FM_211187), S. mitis (GenBank accession number NC_013853), and S. oralis (GenBank accession number NC_015291) DNAs, the number of genomic copies in the nucleic acid extracts from each strain was determined using the following formula: genome copies = quantity of DNA in extract/2.2 fg or quantity of DNA in extract/2.1 fg. Therefore, we concluded that the minimum limits of detection of 630 fg, 6.3 pg, and 63 fg were equivalent to approximately 286, 2,863, and 30 S. pneumoniae, S. mitis, and S. oralis genomes, respectively.
Application of multiplex PCR in clinical samples.
The oral cavity is a reservoir of bacteria comprising more than 700 species or phylotypes, of which approximately 35% have not been cultured (40). The VGS, a major oral streptococcal group, is known for its low pathogenicity and virulence, but several prospective studies have shown positive associations between oral inflammation and increased risk of cardiovascular and cerebrovascular disease (41), preterm birth (42), and certain cancers (43). Recently, additional studies have shown that S. mitis is implicated in pancreatic disease, including autoimmune pancreatitis and pancreatic ductal adenocarcinoma (7).
Table 3 shows the results of the current mPCR protocol and traditional culture methods for the 47 oral samples used in our pilot application study. mPCR identified S. pneumoniae, S. mitis, and S. oralis and a combination of these bacteria in all 7 culture-positive and in an additional 40 culture-negative oral samples. Among the 47 patients, mPCR and culture techniques identified S. pneumoniae in 4 samples (8.5%) and in 1 (2.1%) sample, respectively. S. mitis presented in 6 (12.8%) based on mPCR and in 3 (6.4%) based on culture. S. oralis presented in 7 (14.9%) based upon mPCR and in 1 (2.1%) based on culture. In the combination with 3 pathogens, S. pneumoniae and S. oralis were detected in 2 (4.3%) based on mPCR and in 1 (2.1%) based on culture. S. pneumoniae and S. mitis were revealed in 2 (4.3%) based on mPCR and in 1 (2.1%) based on culture. S. oralis and S. mitis were revealed in 3 (6.4%) based on mPCR and were not identified in culture. Detection of the three species was not revealed by mPCR or culture in this study.
Table 3.
Results of the multiplex PCR protocol compared with culture in a pilot application study using human oral samples
| Species | No. of positive samples/no. of samples tested (n = 47) |
|
|---|---|---|
| Multiplex PCR protocol | Culture | |
| S. pneumoniae | 4/47 | 1/47 |
| S. mitis | 6/47 | 3/47 |
| S. oralis | 7/47 | 1/47 |
| S. pneumoniae + S. mitis | 2/47 | 1/47 |
| S. pneumoniae + S. oralis | 2/47 | 1/47 |
| S. mitis + S. oralis | 3/47 | 0/47 |
| S. pneumoniae + S. mitis + S. oralis | 0/47 | 0/47 |
| Negative | 23/47 | 40/47 |
Because the 16S rRNA gene evolves so slowly, phylogenetic information based on this molecule may not always be sufficient to distinguish closely related species or to resolve their evolutionary relationships. In addition, when several copies of the 16S rRNA gene are present, sequence heterogeneity results in ambiguities in the sequence chromatograms derived from direct sequencing of the PCR products (44). Nucleotide sequences of housekeeping protein-coding genes evolve more rapidly than 16S rRNA and may represent useful alternatives or complements to the 16S rRNA gene (45–47). Otherwise, housekeeping genes have been considered good monitoring tools for bacterial identification and strain typing. The gyrB gene-based mPCR presented in this study may be valuable in clinical microbiology laboratories because it provides a fast and cost-effective means of analyzing large numbers of samples and allows the detection and discrimination of S. pneumoniae, S. oralis, and S. mitis from oral streptococci. Our protocol proved to be more accurate and sensitive than traditional culture methods.
ACKNOWLEDGMENT
This paper was sponsored by Wonkwang University in 2012.
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. 1996. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 275:134–141 [PubMed] [Google Scholar]
- 2. Marrie TJ, Durant H, Yates L. 1989. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev. Infect. Dis. 11:586–599 [DOI] [PubMed] [Google Scholar]
- 3. Schmidt A, Bisle B, Kislinger T. 2009. Quantitative peptide and protein profiling by mass spectrometry. Methods Mol. Biol. 492:21–38 [DOI] [PubMed] [Google Scholar]
- 4. Paddick JS, Brailsford SR, Kidd EA, Gilbert SC, Clark DT, Alam S, Killick ZJ, Beighton D. 2003. Effect of the environment on genotypic diversity of Actinomyces naeslundii and Streptococcus oralis in the oral biofilm. Appl. Environ. Microbiol. 69:6475–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whiley RA, Beighton D. 1998. Current classification of the oral streptococci. Oral Microbiol. Immunol. 13:195–216 [DOI] [PubMed] [Google Scholar]
- 6. Wisplinghoff H, Reinert RR, Cornely O, Seifert H. 1999. Molecular relationships and antimicrobial susceptibilities of viridans group streptococci isolated from blood of neutropenic cancer patients. J. Clin. Microbiol. 37:1876–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DT. 2012. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61:582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. 2008. Accuracy of phenotypic methods for identification of Streptococcus pneumoniae isolates included in surveillance programs. J. Clin. Microbiol. 46:2184–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whatmore AM, Efstratiou A, Pickerill AP, Broughton K, Woodard G, Sturgeon D, George R, Dowson CG. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. 2003. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 30:644–654 [DOI] [PubMed] [Google Scholar]
- 11. Havarstein LS, Hakenbeck R, Gaustad P. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentley RW, Leigh JA, Collins MD. 1991. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 41:487–494 [DOI] [PubMed] [Google Scholar]
- 13. Stackebrandt E, Witt D, Kemmerling C, Kroppenstedt R, Liesack W. 1991. Designation of Streptomycete 16S and 23S rRNA-based target regions for oligonucleotide probes. Appl. Environ. Microbiol. 57:1468–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stackebrandt E, Goebel BM. 1994. Taxonomic note: a place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846–849 [Google Scholar]
- 15. Fox GE, Wisotzkey JD, Jurtshuk P., Jr 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:1 66–170 [DOI] [PubMed] [Google Scholar]
- 16. McAvin JC, Reilly PA, Roudabush RM, Barnes WJ, Salmen A, Jackson GW, Beninga KK, Astorga A, McCleskey FK, Huff WB, Niemeyer D, Lohman KL. 2001. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. J. Clin. Microbiol. 39:3446–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, Kaczmarski EB. 2001. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39:1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrison KE, Lake D, Crook J, Carlone GM, Ades E, Facklam R, Sampson JS. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawamura Y, Whiley RA, Shu SE, Ezaki T, Hardie JM. 1999. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 145(Pt 9):2605–2613 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill AM, Gillespie SH, Whiting GC. 1999. Detection of penicillin susceptibility in Streptococcus pneumoniae by pbp2b PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 37:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki N, Seki M, Nakano Y, Kiyoura Y, Maeno M, Yamashita Y. 2005. Discrimination of Streptococcus pneumoniae from viridans group streptococci by genomic subtractive hybridization. J. Clin. Microbiol. 43:4528–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhelst R, Kaijalainen T, De Baere T, Verschraegen G, Claeys G, Van Simaey L, De Ganck C, Vaneechoutte M. 2003. Comparison of five genotypic techniques for identification of optochin-resistant pneumococcus-like isolates. J. Clin. Microbiol. 41:3521–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvalho Mda G, Tondella ML, McCaustland K, Weidlich L, McGee L, Mayer LW, Steigerwalt A, Whaley M, Facklam RR, Fields B, Carlone G, Ades EW, Dagan R, Sampson JS. 2007. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 45:2460–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang S, Lin S, Khalil A, Gaydos C, Nuemberger E, Juan G, Hardick J, Bartlett JG, Auwaerter PG, Rothman RE. 2005. Quantitative PCR assay using sputum samples for rapid diagnosis of pneumococcal pneumonia in adult emergency department patients. J. Clin. Microbiol. 43:3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Do T, Jolley KA, Maiden MC, Gilbert SC, Clark D, Wade WG, Beighton D. 2009. Population structure of Streptococcus oralis. Microbiology 155:2593–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kilian M, Poulsen K, Blomqvist T, Havarstein LS, Bek-Thomsen M, Tettelin H, Sorensen UB. 2008. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3:e2683 doi:10.1371/journal.pone.0002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park HK, Lee SJ, Yoon JW, Shin JW, Shin HS, Kook JK, Myung SC, Kim W. 2010. Identification of the cpsA gene as a specific marker for the discrimination of Streptococcus pneumoniae from viridans group streptococci. J. Med. Microbiol. 59:1146–1152 [DOI] [PubMed] [Google Scholar]
- 28. Park HK, Lee HJ, Jeong EG, Shin HS, Kim W. 2010. The rgg gene is a specific marker for Streptococcus oralis. J. Dent. Res. 89:1299–1303 [DOI] [PubMed] [Google Scholar]
- 29. Palys T, Berger E, Mitrica I, Nakamura LK, Cohan FM. 2000. Protein-coding genes as molecular markers for ecologically distinct populations: the case of two Bacillus species. Int. J. Syst. Evol. Microbiol. 50:1021–1028 [DOI] [PubMed] [Google Scholar]
- 30. Palys T, Nakamura LK, Cohan FM. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145–1156 [DOI] [PubMed] [Google Scholar]
- 31. Wang JC. 1996. DNA topoisomerases. Annu. Rev. Biochem. 65:635–692 [DOI] [PubMed] [Google Scholar]
- 32. Wilson K. 1993. Preparation of genomic DNA from bacteria, p 2.4.1–2.4.5 In Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Struhl K. (ed), Current protocols in molecular biology, vol 1 John Wiley & Sons, New York, NY: [DOI] [PubMed] [Google Scholar]
- 33. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 34. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stralin K, Backman A, Holmberg H, Fredlund H, Olcen P. 2005. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples. APMIS 113:99–111 [DOI] [PubMed] [Google Scholar]
- 36. Brito DA, Ramirez M, de Lencastre H. 2003. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 41:2378–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park HK, Lee HJ, Kim W. 2010. Real-time PCR assays for the detection and quantification of Streptococcus pneumoniae. FEMS Microbiol. Lett. 310:48–53 [DOI] [PubMed] [Google Scholar]
- 38. Vickerman MM, Sulavik MC, Clewell DB. 1995. Oral streptococci with genetic determinants similar to the glucosyltransferase regulatory gene, rgg. Infect. Immun. 63:4524–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez-Lazaro D, Lewis DA, Ocampo-Sosa AA, Fogarty U, Makrai L, Navas J, Scortti M, Hernandez M, Vazquez-Boland JA. 2006. Internally controlled real-time PCR method for quantitative species-specific detection and vapA genotyping of Rhodococcus equi. Appl. Environ. Microbiol. 72:4256–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meurman JH, Sanz M, Janket SJ. 2004. Oral health, atherosclerosis, and cardiovascular disease. Crit. Rev. Oral Biol. Med. 15:403–413 [DOI] [PubMed] [Google Scholar]
- 42. Goldenberg RL, Culhane JF. 2006. Preterm birth and periodontal disease. N. Engl. J. Med. 355:1925–1927 [DOI] [PubMed] [Google Scholar]
- 43. Meyer MS, Joshipura K, Giovannucci E, Michaud DS. 2008. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control 19:895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dahllof I, Baillie H, Kjelleberg S. 2000. rpoB-based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl. Environ. Microbiol. 66:3376–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maeda Y, Shinohara H, Kiba A, Ohnishi K, Furuya N, Kawamura Y, Ezaki T, Vandamme P, Tsushima S, Hikichi Y. 2006. Phylogenetic study and multiplex PCR-based detection of Burkholderia plantarii, Burkholderia glumae and Burkholderia gladioli using gyrB and rpoD sequences. Int. J. Syst. Evol. Microbiol. 56:1031–1038 [DOI] [PubMed] [Google Scholar]
- 46. Mahenthiralingam E, Vandamme P, Campbell ME, Henry DA, Gravelle AM, Wong LT, Davidson AG, Wilcox PG, Nakielna B, Speert DP. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 33:1469–1475 [DOI] [PubMed] [Google Scholar]
- 47. Payne GW, Vandamme P, Morgan SH, Lipuma JJ, Coenye T, Weightman AJ, Jones TH, Mahenthiralingam E. 2005. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl. Environ. Microbiol. 71:3917–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]