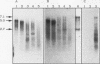
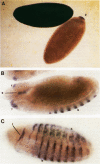
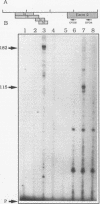
Abstract
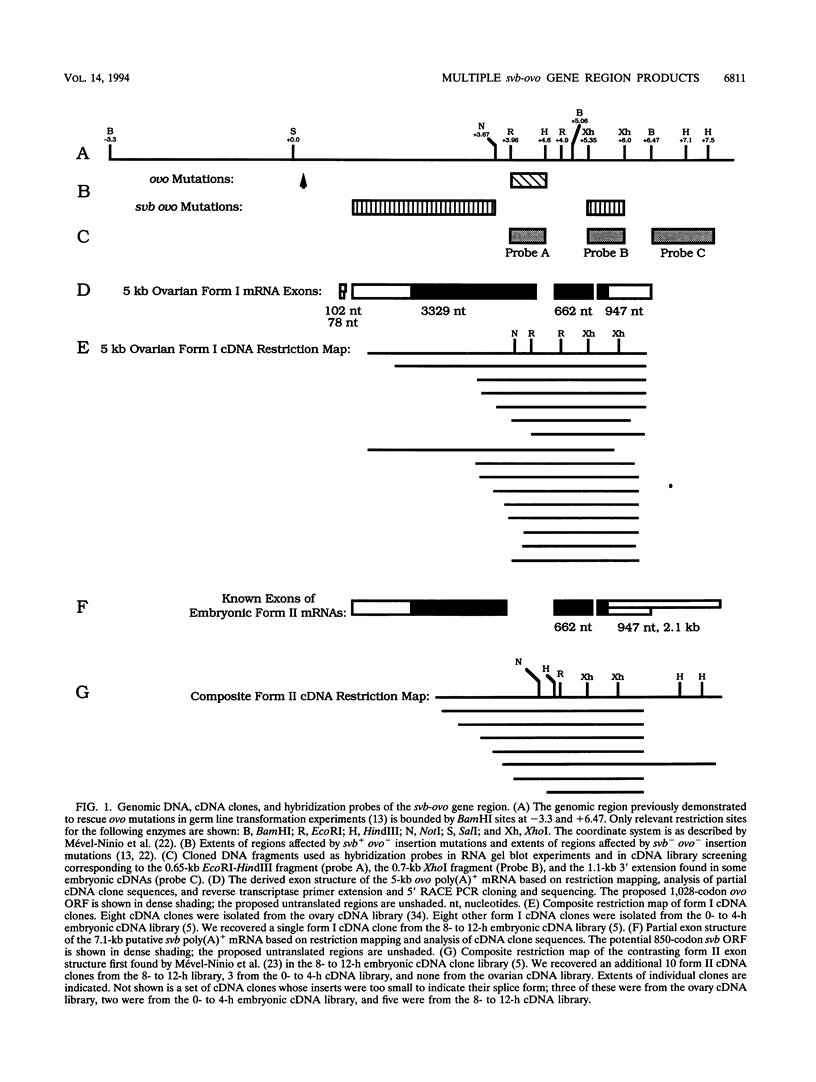
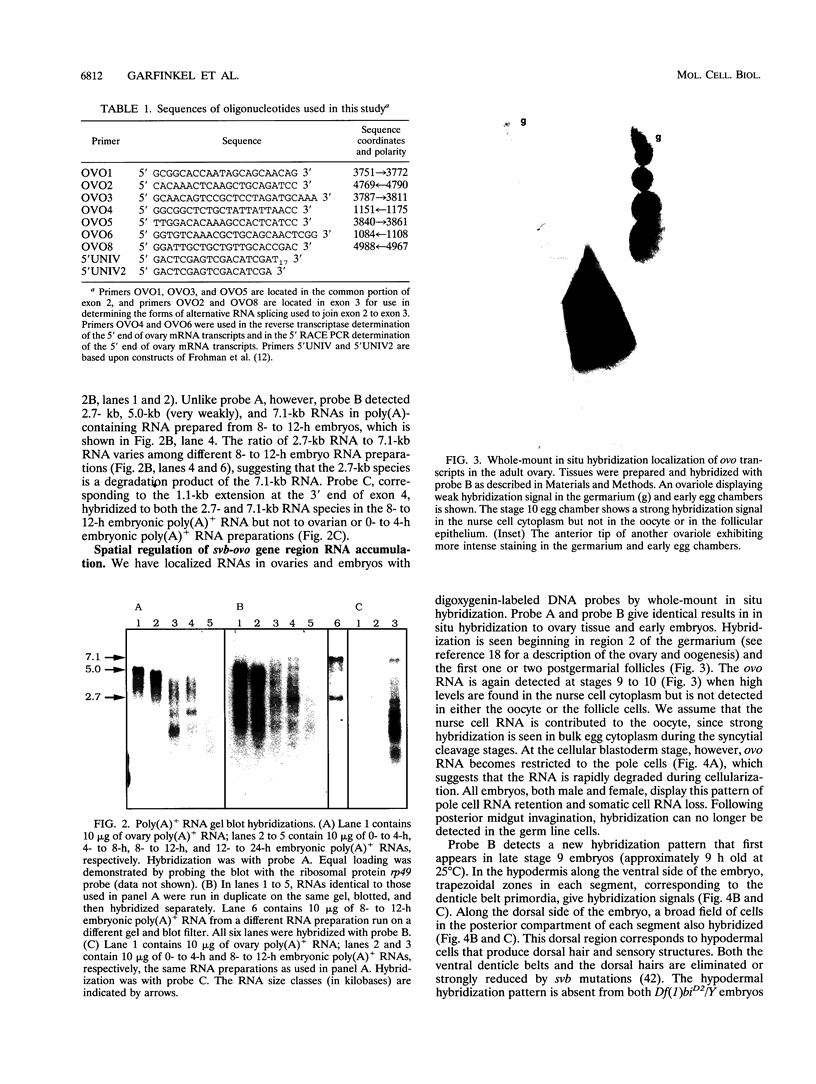
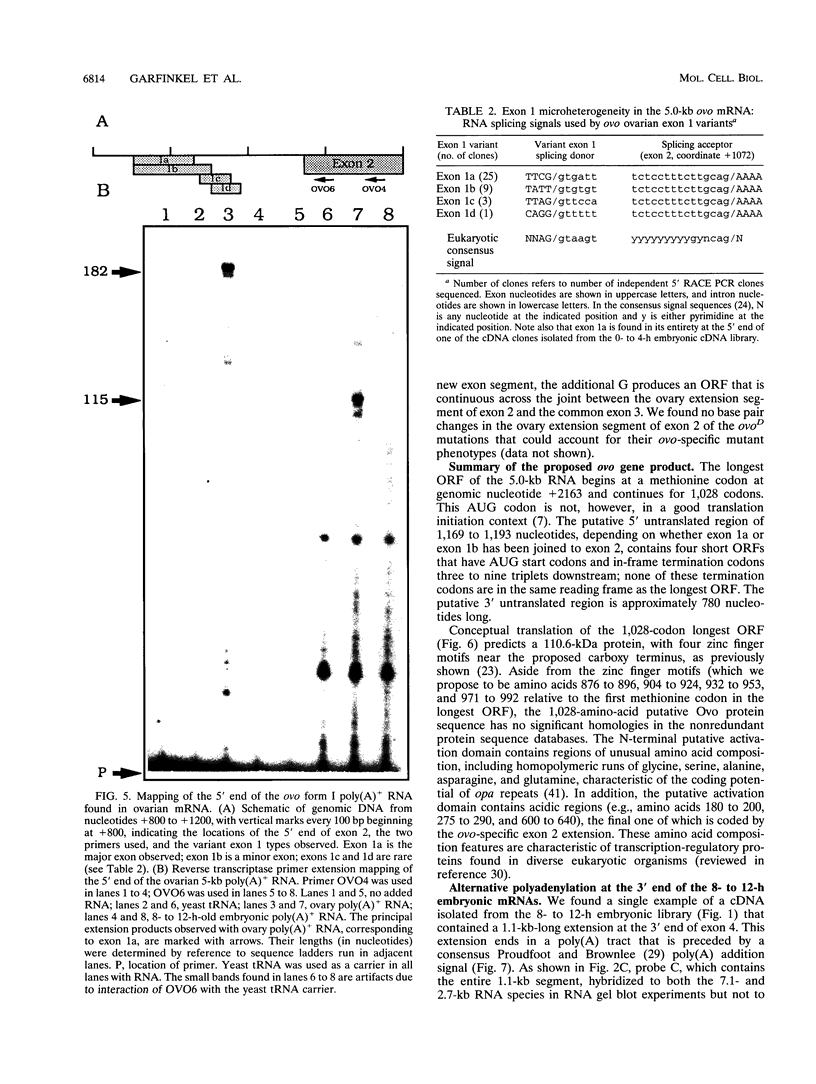
The Drosophila melanogaster shavenbaby (svb)-ovo gene region is a complex locus, containing two distinct but comutable genetic functions. ovo is required for survival and differentiation of female germ line cells and plays a role in germ line sex determination. In contrast, svb is required in both male and female embryos for the production of epidermal locomotor and sensory structures. Sequences required for the two genetic functions are partially overlapping. ovo corresponds to a previously described germ line-dependent 5.0-kb poly(A)+ mRNA that first appears in the germarium and accumulates in nurse cells during oogenesis. The 5.0-kb mRNA is stored in the egg, but it is rapidly lost in the embryos except for its continued presence in the germ line precursor pole cells. The ovo mRNA predicts a 1,028-amino-acid 110.6-kDa protein homologous with transcription factors. We have identified an embryonic mRNA, 7.1 kb in length, that contains exons partially overlapping those of the 5.0-kb poly(A)+ mRNA. The spatial distribution of this newly discovered transcript during midembryogenesis suggests that it corresponds to the svb function. The arrangement of exons common to the 5.0- and 7.1-kb mRNAs suggests that the Ovo and Svb proteins share DNA-binding specificity conferred by four Cys2-His2 zinc finger motifs but differ functionally in their capacity to interact with other components of the transcription machinery.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amrein H., Maniatis T., Nöthiger R. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. EMBO J. 1990 Nov;9(11):3619–3629. doi: 10.1002/j.1460-2075.1990.tb07573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bopp D., Bell L. R., Cline T. W., Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991 Mar;5(3):403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Busson D., Gans M., Komitopoulou K., Masson M. Genetic Analysis of Three Dominant Female-Sterile Mutations Located on the X Chromosome of DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):309–325. doi: 10.1093/genetics/105.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Cherbas P. The arthropod initiator: the capsite consensus plays an important role in transcription. Insect Biochem Mol Biol. 1993 Jan;23(1):81–90. doi: 10.1016/0965-1748(93)90085-7. [DOI] [PubMed] [Google Scholar]
- Cline T. W. The Drosophila sex determination signal: how do flies count to two? Trends Genet. 1993 Nov;9(11):385–390. doi: 10.1016/0168-9525(93)90138-8. [DOI] [PubMed] [Google Scholar]
- Freund R., Meselson M. Long terminal repeat nucleotide sequence and specific insertion of the gypsy transposon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4462–4464. doi: 10.1073/pnas.81.14.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel M. D., Lohe A. R., Mahowald A. P. Molecular genetics of the Drosophila melanogaster ovo locus, a gene required for sex determination of germline cells. Genetics. 1992 Apr;130(4):791–803. doi: 10.1093/genetics/130.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granadino B., Santamaria P., Sánchez L. Sex determination in the germ line of Drosophila melanogaster: activation of the gene Sex-lethal. Development. 1993 Jul;118(3):813–816. doi: 10.1242/dev.118.3.813. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Darlison M. G. Random-primed cDNA synthesis facilitates the isolation of multiple 5'-cDNA ends by RACE. Nucleic Acids Res. 1991 Jul 25;19(14):4002–4002. doi: 10.1093/nar/19.14.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Gomer R. H., Murtagh J. J., Jr Increasing specificity from the PCR-RACE technique. Biotechniques. 1992 Jan;12(1):58–59. [PubMed] [Google Scholar]
- Mattox W., Palmer M. J., Baker B. S. Alternative splicing of the sex determination gene transformer-2 is sex-specific in the germ line but not in the soma. Genes Dev. 1990 May;4(5):789–805. doi: 10.1101/gad.4.5.789. [DOI] [PubMed] [Google Scholar]
- McKeown M., Belote J. M., Boggs R. T. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell. 1988 Jun 17;53(6):887–895. doi: 10.1016/s0092-8674(88)90369-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Burks C., Hertz G., Stormo G. D., White O., Fields C. Splicing signals in Drosophila: intron size, information content, and consensus sequences. Nucleic Acids Res. 1992 Aug 25;20(16):4255–4262. doi: 10.1093/nar/20.16.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mével-Ninio M., Mariol M. C., Gans M. Mobilization of the gypsy and copia retrotransposons in Drosophila melanogaster induces reversion of the ovo dominant female-sterile mutations: molecular analysis of revertant alleles. EMBO J. 1989 May;8(5):1549–1558. doi: 10.1002/j.1460-2075.1989.tb03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mével-Ninio M., Terracol R., Kafatos F. C. The ovo gene of Drosophila encodes a zinc finger protein required for female germ line development. EMBO J. 1991 Aug;10(8):2259–2266. doi: 10.1002/j.1460-2075.1991.tb07762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B., Kim Y. J., Baker B. S. Sex-lethal, master and slave: a hierarchy of germ-line sex determination in Drosophila. Development. 1993 Nov;119(3):897–908. doi: 10.1242/dev.119.3.897. [DOI] [PubMed] [Google Scholar]
- Oliver B., Pauli D., Mahowald A. P. Genetic evidence that the ovo locus is involved in Drosophila germ line sex determination. Genetics. 1990 Jul;125(3):535–550. doi: 10.1093/genetics/125.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B., Perrimon N., Mahowald A. P. The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev. 1987 Nov;1(9):913–923. doi: 10.1101/gad.1.9.913. [DOI] [PubMed] [Google Scholar]
- Pauli D., Mahowald A. P. Germ-line sex determination in Drosophila melanogaster. Trends Genet. 1990 Aug;6(8):259–264. doi: 10.1016/0168-9525(90)90208-n. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Steinhauer W. R., Walsh R. C., Kalfayan L. J. Sequence and structure of the Drosophila melanogaster ovarian tumor gene and generation of an antibody specific for the ovarian tumor protein. Mol Cell Biol. 1989 Dec;9(12):5726–5732. doi: 10.1128/mcb.9.12.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann-Zwicky M. How do germ cells choose their sex? Drosophila as a paradigm. Bioessays. 1992 Aug;14(8):513–518. doi: 10.1002/bies.950140803. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M., Schmid H., Nöthiger R. Cell-autonomous and inductive signals can determine the sex of the germ line of drosophila by regulating the gene Sxl. Cell. 1989 Apr 7;57(1):157–166. doi: 10.1016/0092-8674(89)90181-5. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M. Sex determination in Drosophila: sis-b, a major numerator element of the X:A ratio in the soma, does not contribute to the X:A ratio in the germ line. Development. 1993 Feb;117(2):763–767. doi: 10.1242/dev.117.2.763. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Wei G., Oliver B., Mahowald A. P. Gonadal dysgenesis reveals sexual dimorphism in the embryonic germline of Drosophila. Genetics. 1991 Sep;129(1):203–210. doi: 10.1093/genetics/129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Oliver B., Pauli D., Mahowald A. P. Evidence for sex transformation of germline cells in ovarian tumor mutants of Drosophila. Dev Biol. 1994 Jan;161(1):318–320. doi: 10.1006/dbio.1994.1032. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]