Abstract
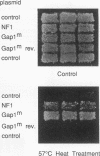
We have previously purified a novel GTPase-activating protein (GAP) for Ras which is immunologically distinct from the known Ras GAPs, p120GAP and neurofibromin (M. Maekawa, S. Nakamura, and S. Hattori, J. Biol. Chem. 268:22948-22952, 1993). On the basis of the partial amino acid sequence, we have obtained a cDNA which encodes the novel Ras GAP. The predicted protein consists of 847 amino acids whose calculated molecular mass, 96,369 Da, is close to the apparent molecular mass of the novel Ras GAP, 100 kDa. The amino acid sequence shows a high degree of similarity to the entire sequence of the Drosophila melanogaster Gap1 gene. When the catalytic domain of the novel GAP was compared with that of Drosophila Gap1, p120GAP, and neurofibromin, the highest degree of similarity was again observed with Gap1. Thus, we designated this gene Gap1m, a mammalian counterpart of the Drosophila Gap1 gene. Expression of Gap1m was relatively high in brain, placenta, and kidney tissues, and it was expressed at low levels in other tissues. A recombinant protein consisting of glutathione-S-transferase and the GAP-related domain of Gap1m stimulated GTPase of normal Ras but not that of Ras having valine at the 12th residue. Expression of the same region in Saccharomyces cerevisiae suppressed the ira2- phenotype. In addition to the GAP catalytic domain, Gap1m has two domains with sequence closely related to those of the phospholipid-binding domain of synaptotagmin and a region with similarity to the unique domain of Btk tyrosine kinase. These results clearly show that Gap1m is a novel Ras GAP molecule of mammalian cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Marchuk D., Boguski M., Saulino A., Letcher R., Wigler M., Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990 Nov 16;63(4):851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Bollag G., McCormick F., Clark R. Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. EMBO J. 1993 May;12(5):1923–1927. doi: 10.1002/j.1460-2075.1993.tb05841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul U., Mardon G., Rubin G. M. A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell. 1992 Mar 20;68(6):1007–1019. doi: 10.1016/0092-8674(92)90073-l. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Allard W. J., Sigal I. S., Scolnick E. M. Purification of ras GTPase activating protein from bovine brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5026–5030. doi: 10.1073/pnas.85.14.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Maekawa M., Nakamura S. Identification of neurofibromatosis type I gene product as an insoluble GTPase-activating protein toward ras p21. Oncogene. 1992 Mar;7(3):481–485. [PubMed] [Google Scholar]
- Iwamatsu A. S-carboxymethylation of proteins transferred onto polyvinylidene difluoride membranes followed by in situ protease digestion and amino acid microsequencing. Electrophoresis. 1992 Mar;13(3):142–147. doi: 10.1002/elps.1150130129. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. H., Weinstein I. B. Calcium-dependent activation of protein kinase C. The role of the C2 domain in divalent cation selectivity. J Biol Chem. 1993 Nov 5;268(31):23580–23584. [PubMed] [Google Scholar]
- Maekawa M., Nakamura S., Hattori S. Purification of a novel ras GTPase-activating protein from rat brain. J Biol Chem. 1993 Oct 25;268(30):22948–22952. [PubMed] [Google Scholar]
- Martin G. A., Viskochil D., Bollag G., McCabe P. C., Crosier W. J., Haubruck H., Conroy L., Clark R., O'Connell P., Cawthon R. M. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Ren R., Clark K. L., Baltimore D. A putative modular domain present in diverse signaling proteins. Cell. 1993 May 21;73(4):629–630. doi: 10.1016/0092-8674(93)90244-k. [DOI] [PubMed] [Google Scholar]
- McGlade J., Brunkhorst B., Anderson D., Mbamalu G., Settleman J., Dedhar S., Rozakis-Adcock M., Chen L. B., Pawson T. The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 1993 Aug;12(8):3073–3081. doi: 10.1002/j.1460-2075.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema R. H., de Laat W. L., Martin G. A., McCormick F., Bos J. L. GTPase-activating protein SH2-SH3 domains induce gene expression in a Ras-dependent fashion. Mol Cell Biol. 1992 Aug;12(8):3425–3430. doi: 10.1128/mcb.12.8.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Rice P., Thompson J., Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993 Sep;18(9):343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Igarashi K., Kikkawa U., Ogita K., Nishizuka Y. Nucleotide sequences of cDNAs for alpha and gamma subspecies of rat brain protein kinase C. Nucleic Acids Res. 1988 Jun 10;16(11):5199–5200. doi: 10.1093/nar/16.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J Biol Chem. 1991 Jan 5;266(1):623–629. [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Kaziro Y. Induction of neurite formation in PC12 cells by microinjection of proto-oncogenic Ha-ras protein preincubated with guanosine-5'-O-(3-thiotriphosphate). Mol Cell Biol. 1987 Dec;7(12):4553–4556. doi: 10.1128/mcb.7.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Nakafuku M., Kaziro Y. Studies on ras proteins. Catalytic properties of normal and activated ras proteins purified in the absence of protein denaturants. Biochim Biophys Acta. 1988 Jan 25;949(1):97–109. doi: 10.1016/0167-4781(88)90059-0. [DOI] [PubMed] [Google Scholar]
- Serth J., Weber W., Frech M., Wittinghofer A., Pingoud A. Binding of the H-ras p21 GTPase activating protein by the activated epidermal growth factor receptor leads to inhibition of the p21 GTPase activity in vitro. Biochemistry. 1992 Jul 21;31(28):6361–6365. doi: 10.1021/bi00143a001. [DOI] [PubMed] [Google Scholar]
- Settleman J., Narasimhan V., Foster L. C., Weinberg R. A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell. 1992 May 1;69(3):539–549. doi: 10.1016/0092-8674(92)90454-k. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993 Apr;13(4):2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Matsumoto K., Toh-E A. IRA1, an inhibitory regulator of the RAS-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):757–768. doi: 10.1128/mcb.9.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Satoh T., Marshall M. S., Gibbs J. B., Matsumoto K., Kaziro Y., Toh-e A. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell. 1990 Mar 9;60(5):803–807. doi: 10.1016/0092-8674(90)90094-u. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Tamanoi F., Kaziro Y., Matsumoto K., Toh-e A. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol Cell Biol. 1990 Aug;10(8):4303–4313. doi: 10.1128/mcb.10.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G. A., Ladner M., Long C. M., Crosier W. J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988 Dec 23;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Boguski M., Riggs M., Rodgers L., Wigler M. sar1, a gene from Schizosaccharomyces pombe encoding a protein that regulates ras1. Cell Regul. 1991 Jun;2(6):453–465. doi: 10.1091/mbc.2.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Müller O., Clark R., Conroy L., Moran M. F., Polakis P., McCormick F. Molecular cloning and nucleic acid binding properties of the GAP-associated tyrosine phosphoprotein p62. Cell. 1992 May 1;69(3):551–558. doi: 10.1016/0092-8674(92)90455-l. [DOI] [PubMed] [Google Scholar]
- Xu G. F., Lin B., Tanaka K., Dunn D., Wood D., Gesteland R., White R., Weiss R., Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990 Nov 16;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- Xu G. F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Shirataki H., Kishida S., Miyazaki M., Nishikawa J., Wada K., Numata S., Kaibuchi K., Takai Y. Two functionally different domains of rabphilin-3A, Rab3A p25/smg p25A-binding and phospholipid- and Ca(2+)-binding domains. J Biol Chem. 1993 Dec 25;268(36):27164–27170. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]