Abstract
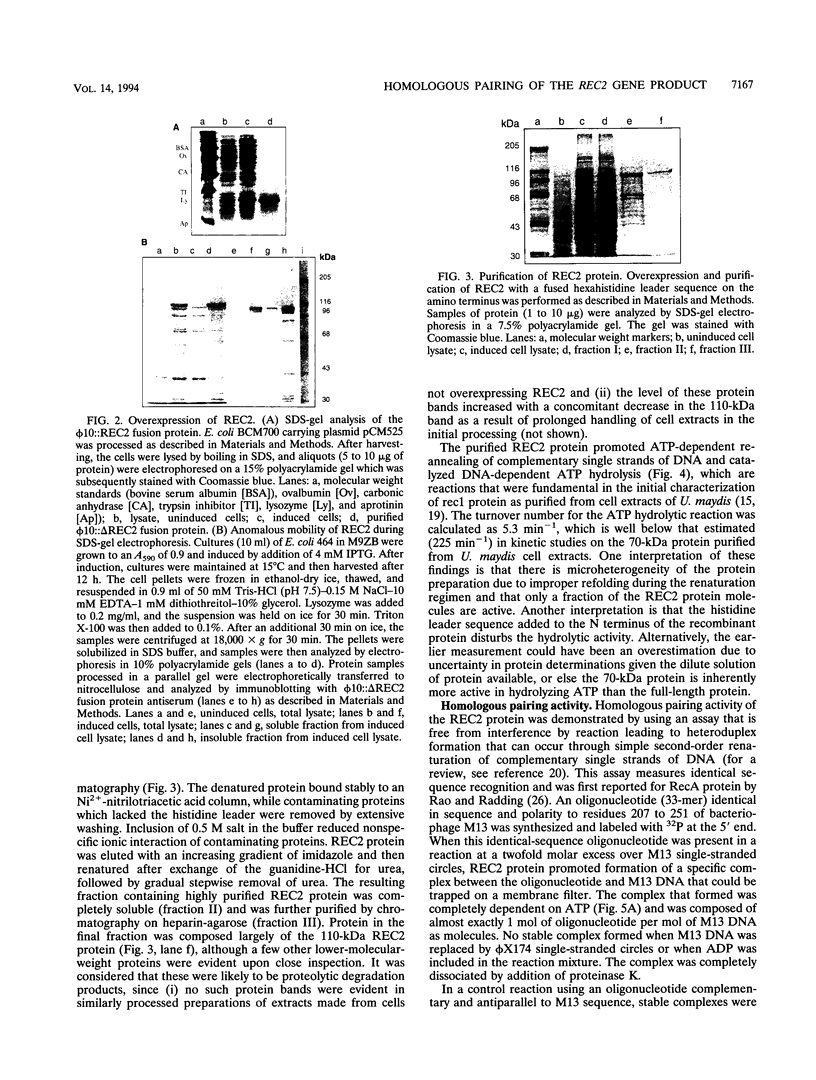
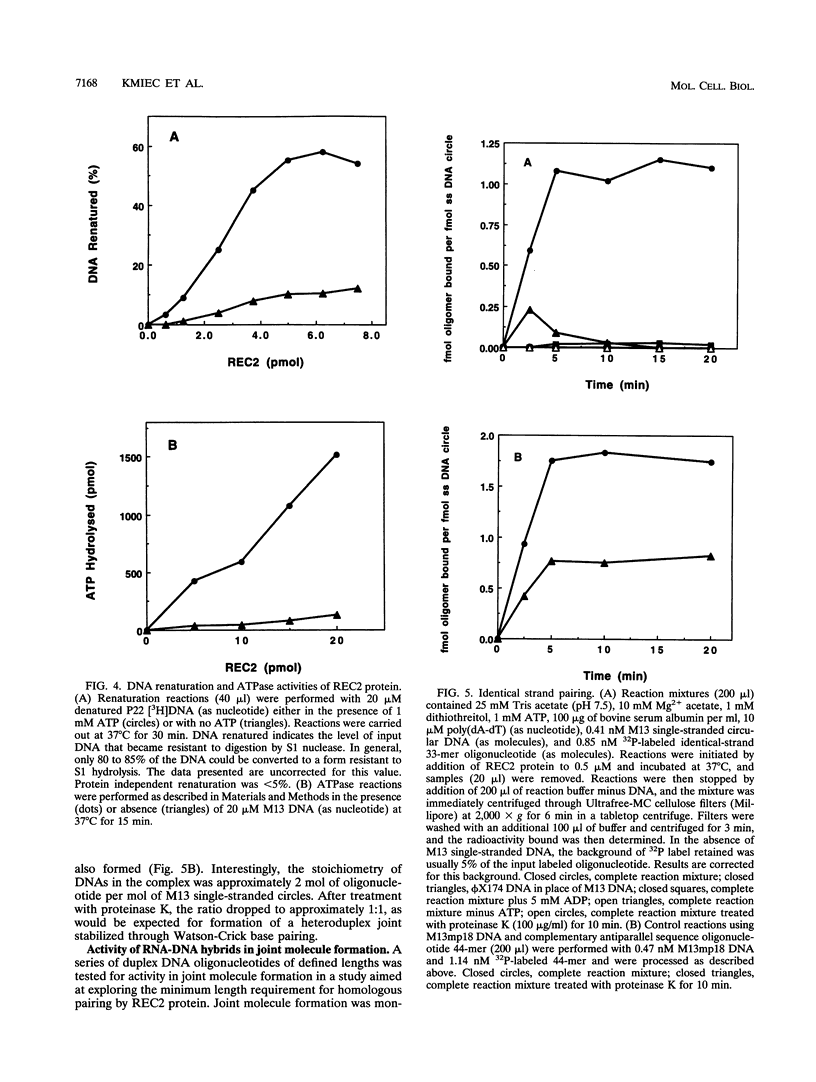
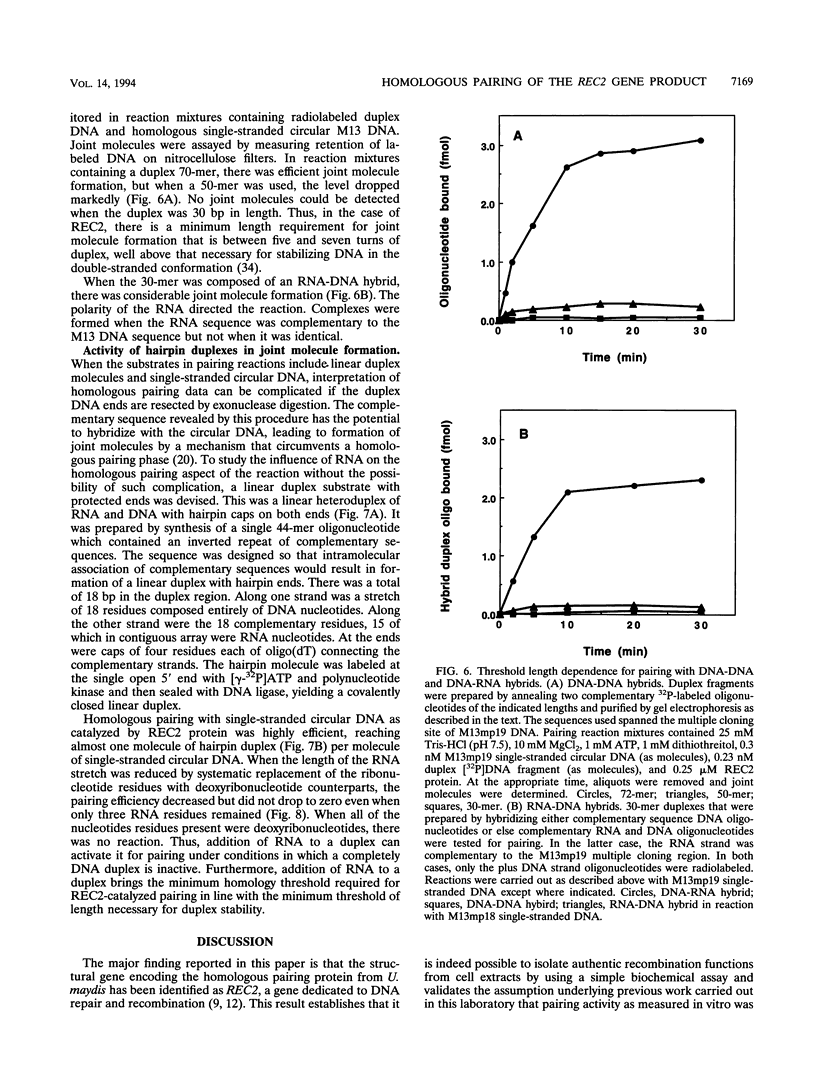
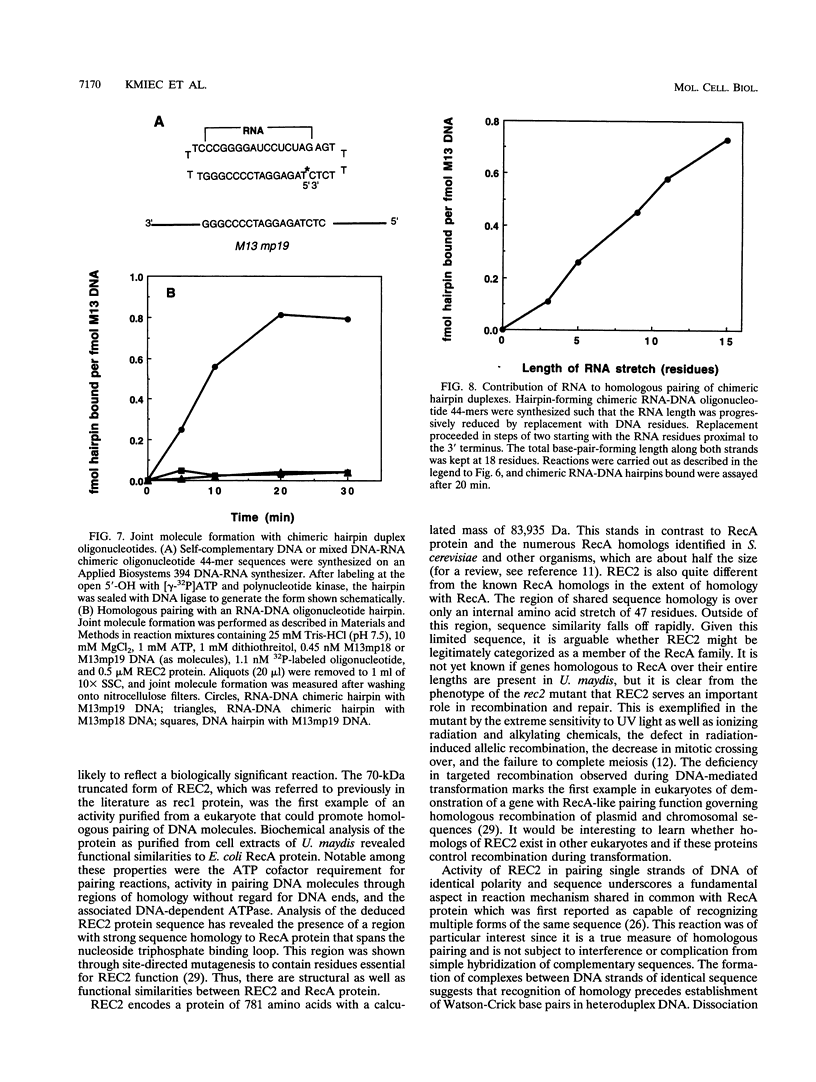
Amino acid sequence analysis has established that the homologous pairing protein of Ustilago maydis, known previously in the literature as rec1, is encoded by REC2, a gene essential for recombinational repair and meiosis with regional homology to Escherichia coli RecA. The 70-kDa rec1 protein is most likely a proteolytic degradation product of REC2, which has a predicted mass of 84 kDa but which runs anomalously during sodium dodecyl sulfate-gel electrophoresis with an apparent mass of 110 kDa. To facilitate purification of the protein product, the REC2 gene was overexpressed from a vector that fused a hexahistidine leader sequence onto the amino terminus, enabling isolation of the REC2 protein on an immobilized metal affinity column. The purified protein exhibits ATP-dependent DNA renaturation and DNA-dependent ATPase activities, which were reactions characteristic of the protein as purified from cell extracts of U. maydis. Homologous pairing activity was established in an assay that measures recognition via non-Watson-Crick bonds between identical DNA strands. A size threshold of about 50 bp was found to govern pairing between linear duplex molecules and homologous single-stranded circles. Joint molecule formation with duplex DNA well under the size threshold was efficiently catalyzed when one strand of the duplex was composed of RNA. Linear duplex molecules with hairpin caps also formed joint molecules when as few as three RNA residues were present.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Chanet R., Adjiri A., Fabre F. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol Cell Biol. 1992 Jul;12(7):3224–3234. doi: 10.1128/mcb.12.7.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile G., Aker M., Mortimer R. K. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992 Jul;12(7):3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchwitz R., Holloman W. K. Isolation of the REC2 gene controlling recombination in Ustilago maydis. Gene. 1990 Dec 15;96(2):285–288. doi: 10.1016/0378-1119(90)90265-s. [DOI] [PubMed] [Google Scholar]
- Bianchi M., DasGupta C., Radding C. M. Synapsis and the formation of paranemic joints by E. coli RecA protein. Cell. 1983 Oct;34(3):931–939. doi: 10.1016/0092-8674(83)90550-0. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Park D., Xu L., Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992 May 1;69(3):439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M., Usman N., Rich A. Conformational influence of the ribose 2'-hydroxyl group: crystal structures of DNA-RNA chimeric duplexes. Biochemistry. 1993 Apr 6;32(13):3221–3237. [PubMed] [Google Scholar]
- Fedoroff OYu, Salazar M., Reid B. R. Structure of a DNA:RNA hybrid duplex. Why RNase H does not cleave pure RNA. J Mol Biol. 1993 Oct 5;233(3):509–523. doi: 10.1006/jmbi.1993.1528. [DOI] [PubMed] [Google Scholar]
- Fotheringham S., Holloman W. K. Extrachromosomal recombination is deranged in the rec2 mutant of Ustilago maydis. Genetics. 1991 Dec;129(4):1052–1060. [PMC free article] [PubMed] [Google Scholar]
- Gonda D. K., Radding C. M. By searching processively RecA protein pairs DNA molecules that share a limited stretch of homology. Cell. 1983 Sep;34(2):647–654. doi: 10.1016/0092-8674(83)90397-5. [DOI] [PubMed] [Google Scholar]
- Heyer W. D. The search for the right partner: homologous pairing and DNA strand exchange proteins in eukaryotes. Experientia. 1994 Mar 15;50(3):223–233. doi: 10.1007/BF01924005. [DOI] [PubMed] [Google Scholar]
- Holliday R. Altered recombination frequencies in radiation sensitivie strains of Ustilago. Mutat Res. 1967 May-Jun;4(3):275–288. doi: 10.1016/0027-5107(67)90022-x. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Camerini-Otero C. S., Camerini-Otero R. D. The synapsis event in the homologous pairing of DNAs: RecA recognizes and pairs less than one helical repeat of DNA. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6492–6496. doi: 10.1073/pnas.89.14.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kans J. A., Mortimer R. K. Nucleotide sequence of the RAD57 gene of Saccharomyces cerevisiae. Gene. 1991 Aug 30;105(1):139–140. doi: 10.1016/0378-1119(91)90527-i. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. ATP-dependent DNA renaturation and DNA-dependent ATPase reactions catalyzed by the Ustilago maydis homologous pairing protein. Eur J Biochem. 1994 Feb 1;219(3):865–875. doi: 10.1111/j.1432-1033.1994.tb18568.x. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. DNA strand exchange in the absence of homologous pairing. J Biol Chem. 1994 Apr 1;269(13):10163–10168. [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Heteroduplex formation and polarity during strand transfer promoted by Ustilago rec 1 protein. Cell. 1983 Jul;33(3):857–864. doi: 10.1016/0092-8674(83)90028-4. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Homologous pairing of DNA molecules by Ustilago rec1 protein is promoted by sequences of Z-DNA. Cell. 1986 Feb 28;44(4):545–554. doi: 10.1016/0092-8674(86)90264-3. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Kroeger P. E., Brougham M. J., Holloman W. K. Topological linkage of circular DNA molecules promoted by Ustilago rec 1 protein and topoisomerase. Cell. 1983 Oct;34(3):919–929. doi: 10.1016/0092-8674(83)90549-4. [DOI] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu Rev Biophys Biophys Chem. 1991;20:539–575. doi: 10.1146/annurev.bb.20.060191.002543. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical RecA nucleoprotein filaments mediate homologous pairing and strand exchange. Biochim Biophys Acta. 1989 Jul 7;1008(2):131–145. doi: 10.1016/0167-4781(80)90001-9. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Rao B. J., Radding C. M. Homologous recognition promoted by RecA protein via non-Watson-Crick bonds between identical DNA strands. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6646–6650. doi: 10.1073/pnas.90.14.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Rubin B. P., Ferguson D. O., Holloman W. K. Structure of REC2, a recombinational repair gene of Ustilago maydis, and its function in homologous recombination between plasmid and chromosomal sequences. Mol Cell Biol. 1994 Sep;14(9):6287–6296. doi: 10.1128/mcb.14.9.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Thelen M. P., Onel K., Holloman W. K. The REC1 gene of Ustilago maydis involved in the cellular response to DNA damage encodes an exonuclease. J Biol Chem. 1994 Jan 7;269(1):747–754. [PubMed] [Google Scholar]
- Thomas C. A., Jr Recombination of DNA molecules. Prog Nucleic Acid Res Mol Biol. 1966;5:315–337. doi: 10.1016/s0079-6603(08)60237-8. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., van der Marel G. A., van Boeckel S. A., Rich A. Molecular structure of r(GCG)d(TATACGC): a DNA--RNA hybrid helix joined to double helical DNA. Nature. 1982 Oct 14;299(5884):601–604. doi: 10.1038/299601a0. [DOI] [PubMed] [Google Scholar]
- West S. C. Enzymes and molecular mechanisms of genetic recombination. Annu Rev Biochem. 1992;61:603–640. doi: 10.1146/annurev.bi.61.070192.003131. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]