Abstract
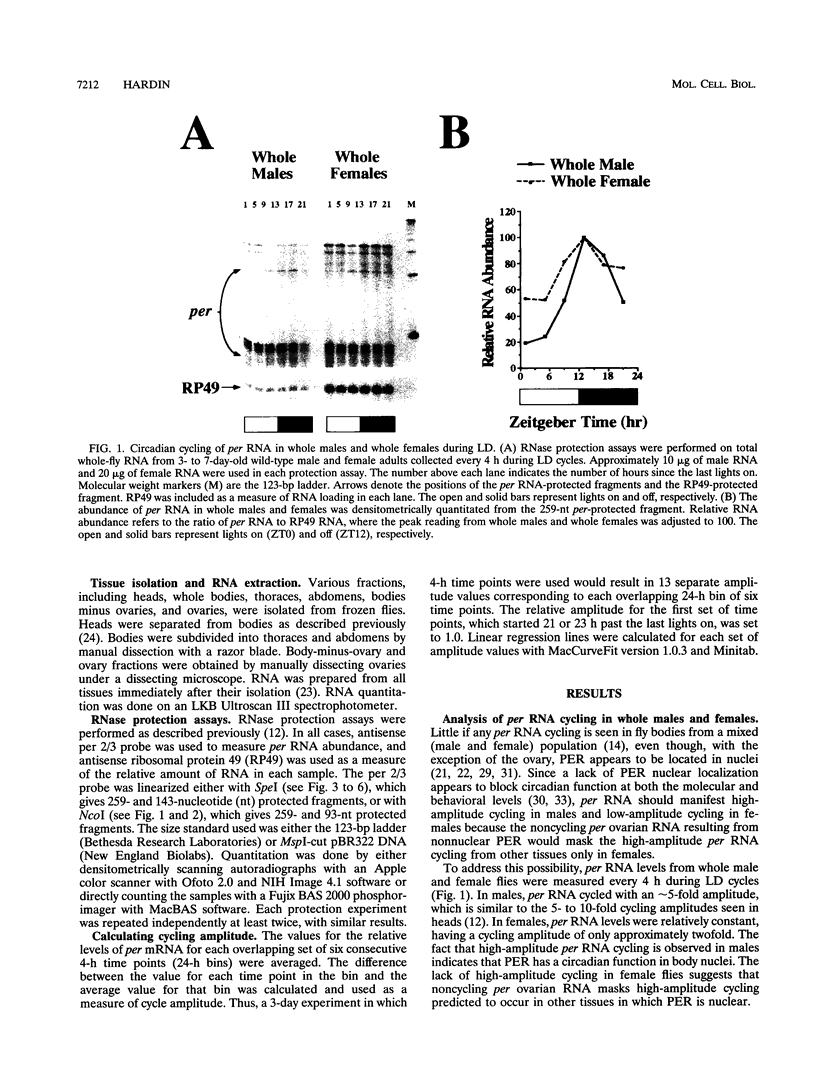
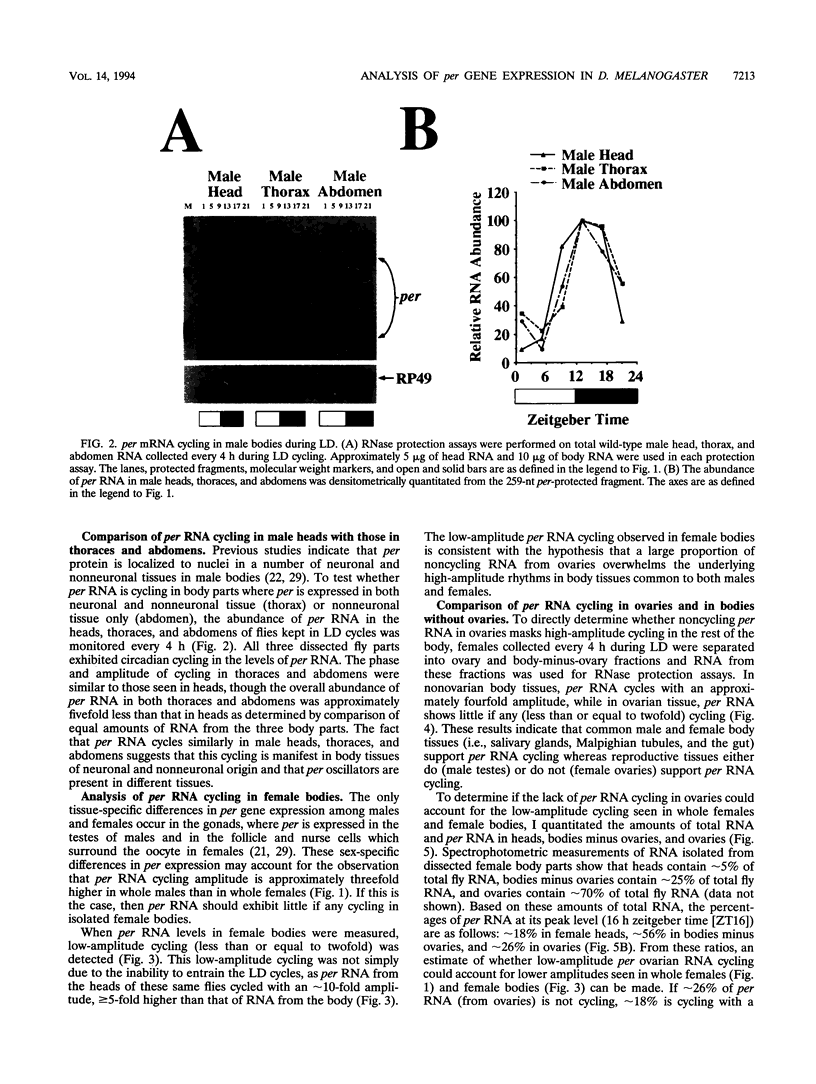
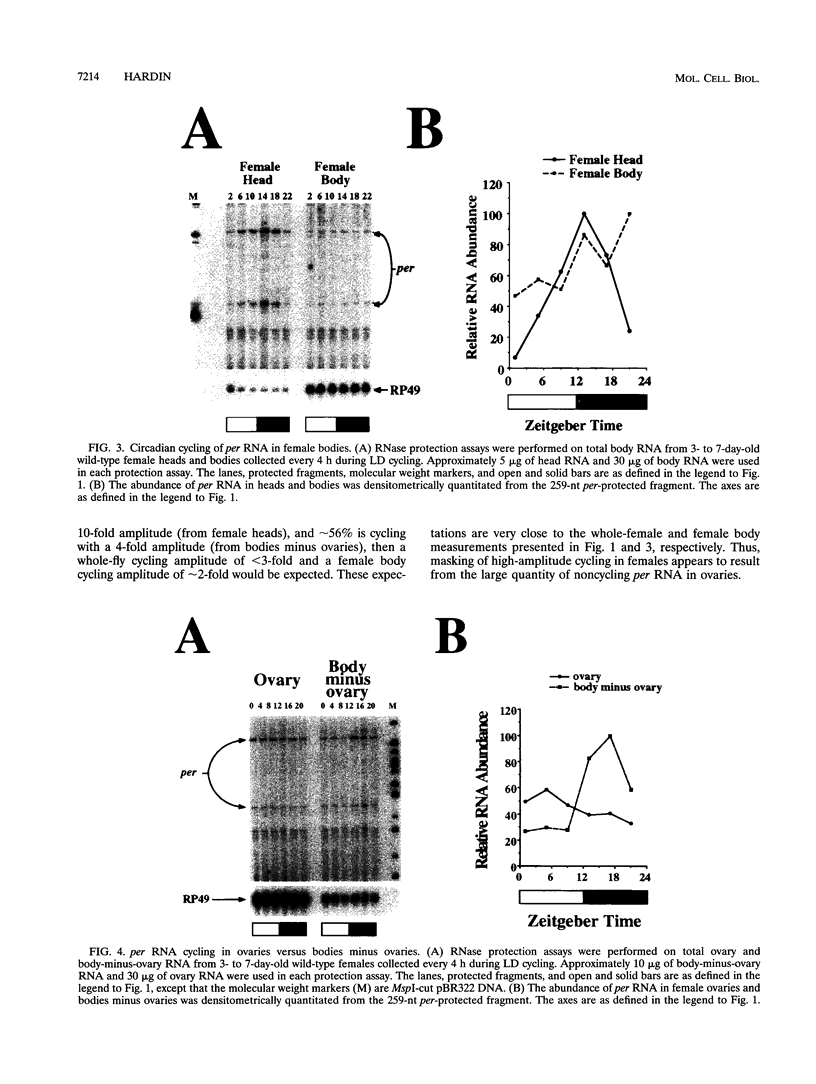
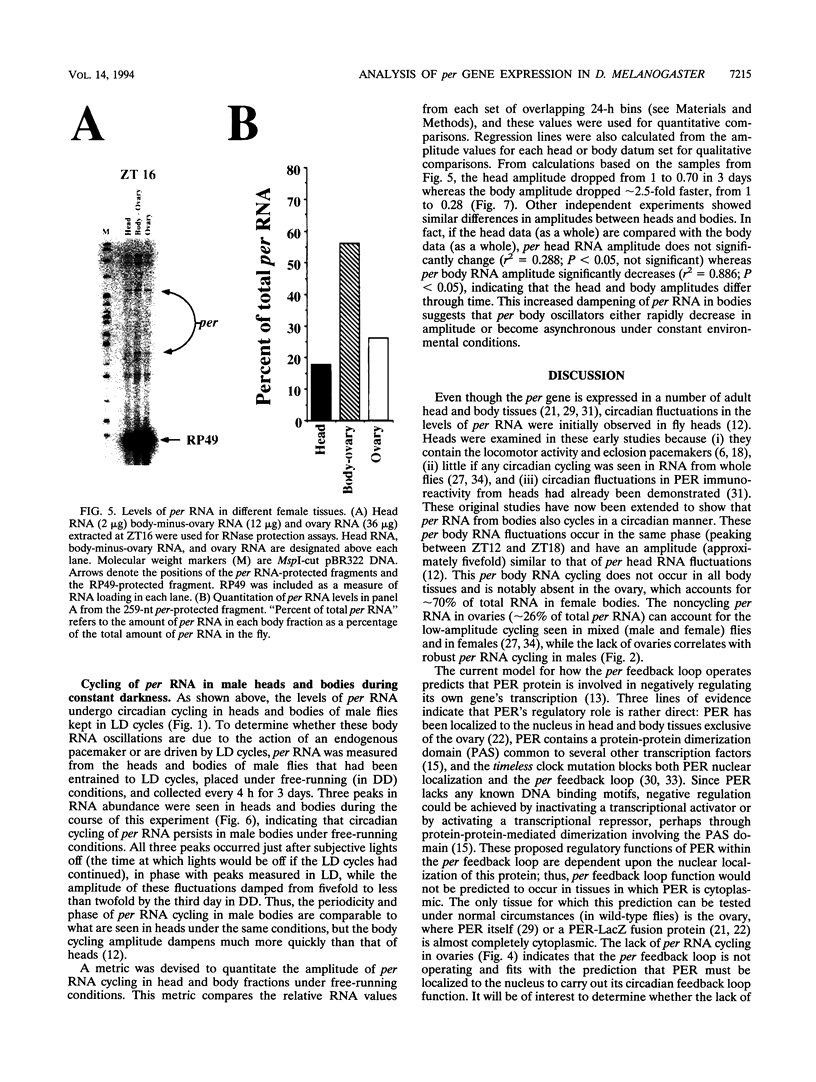
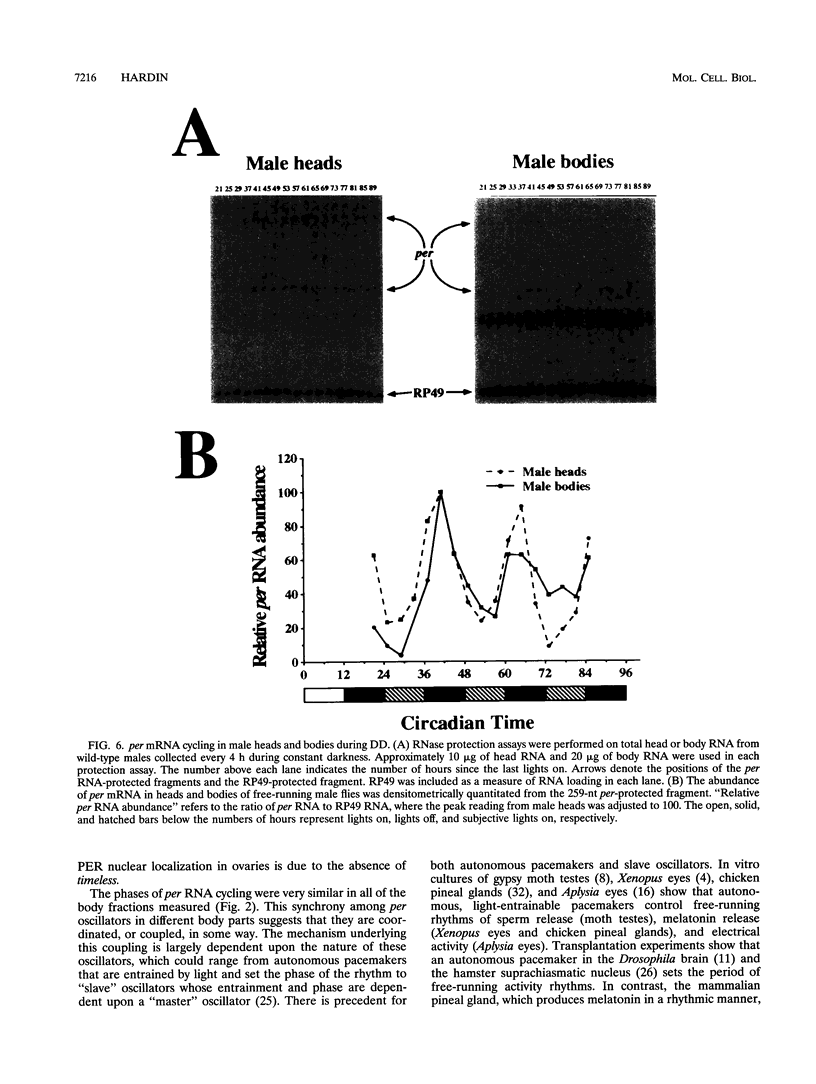
The period (per) gene is thought to be part of the Drosophila circadian pacemaker. The circadian fluctuations in per RNA and protein that constitute the per feedback loop appear to be required for pacemaker function, and have been measured in head neuronal tissues that are necessary for locomotor activity and eclosion rhythms. The per gene is also expressed in a number of neuronal and nonneuronal body tissues for which no known circadian phenomena have been described. To determine whether per might affect some circadian function in these body tissues, per RNA cycling was examined. These studies show that per RNA cycles in the same phase and amplitude in head and body tissues during light-dark cycles. One exception to this is the lack of per RNA cycling in the ovary, which also appears to be the only tissue in which PER protein is primarily cytoplasmic. In constant darkness, however, the amplitude of per RNA cycling dampens much more quickly in bodies than in heads. Taken together, these results indicate that circadian oscillators are present in head and body tissues in which PER protein is nuclear and that these oscillators behave differently.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allemand R. Les rythmes de vitellogenese et d'ovulation en photopériode LD 12:12 de Drosophila melanogaster. J Insect Physiol. 1976;22(7):1031–1035. doi: 10.1016/0022-1910(76)90088-3. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Iuvone P. M. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983 Sep 8;305(5930):133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Boulos Z., Terman M. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 1980 Summer;4(2):119–131. doi: 10.1016/0149-7634(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Ewer J., Frisch B., Hamblen-Coyle M. J., Rosbash M., Hall J. C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J Neurosci. 1992 Sep;12(9):3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J., Hamblen-Coyle M., Rosbash M., Hall J. C. Requirement for period gene expression in the adult and not during development for locomotor activity rhythms of imaginal Drosophila melanogaster. J Neurogenet. 1990 Nov;7(1):31–73. doi: 10.3109/01677069009084151. [DOI] [PubMed] [Google Scholar]
- Giebultowicz J. M., Riemann J. G., Raina A. K., Ridgway R. L. Circadian system controlling release of sperm in the insect testes. Science. 1989 Sep 8;245(4922):1098–1100. doi: 10.1126/science.245.4922.1098. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Mutations and molecules influencing biological rhythms. Annu Rev Neurosci. 1988;11:373–393. doi: 10.1146/annurev.ne.11.030188.002105. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Oscillating molecules and how they move circadian clocks across evolutionary boundaries. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5382–5383. doi: 10.1073/pnas.90.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler A. M., Konopka R. J. Transplantation of a circadian pacemaker in Drosophila. Nature. 1979 May 17;279(5710):236–238. doi: 10.1038/279236a0. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W. Circadian rhythm of optic nerve impulses recorded in darkness from isolated eye of Aplysia. Science. 1969 May 2;164(3879):562–563. doi: 10.1126/science.164.3879.562. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou C. P., Hall J. C. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male's courtship song. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6729–6733. doi: 10.1073/pnas.77.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou C. P., Oldroyd M., Wood J., Sharp M., Hill M. Clock mutations alter developmental timing in Drosophila. Heredity (Edinb) 1990 Jun;64(Pt 3):395–401. doi: 10.1038/hdy.1990.50. [DOI] [PubMed] [Google Scholar]
- Liu X., Lorenz L., Yu Q. N., Hall J. C., Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988 Feb;2(2):228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- Liu X., Zwiebel L. J., Hinton D., Benzer S., Hall J. C., Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992 Jul;12(7):2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz L. J., Hall J. C., Rosbash M. Expression of a Drosophila mRNA is under circadian clock control during pupation. Development. 1989 Dec;107(4):869–880. doi: 10.1242/dev.107.4.869. [DOI] [PubMed] [Google Scholar]
- Ralph M. R., Foster R. G., Davis F. C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990 Feb 23;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reddy P., Zehring W. A., Wheeler D. A., Pirrotta V., Hadfield C., Hall J. C., Rosbash M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984 Oct;38(3):701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Saez L., Young M. W. In situ localization of the per clock protein during development of Drosophila melanogaster. Mol Cell Biol. 1988 Dec;8(12):5378–5385. doi: 10.1128/mcb.8.12.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Price J. L., Man B., Young M. W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994 Mar 18;263(5153):1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., Hamm H., Menaker M. Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2319–2322. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L. B., Price J. L., Sehgal A., Saez L., Young M. W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994 Mar 18;263(5153):1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- Young M. W., Jackson F. R., Shin H. S., Bargiello T. A. A biological clock in Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:865–875. doi: 10.1101/sqb.1985.050.01.104. [DOI] [PubMed] [Google Scholar]
- Zeng H., Hardin P. E., Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994 Aug 1;13(15):3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]