Abstract
Thymic atrophy has been described as a consequence of infection by several pathogens and shown to be induced through diverse mechanisms. Using the mouse model of Mycobacterium avium infection we show here that the production of nitric oxide from IFNγ-activated macrophages plays a major role in mycobacterial infection-induced thymic atrophy. Our results show that disseminated infection with a highly virulent strain of M. avium, but not with a low virulence strain, led to a progressive thymic atrophy. Thymic involution was prevented in genetically manipulated mice unable to produce IFNγ or the inducible nitric oxide synthase. In addition, mice with a selective impairment of IFNγ signaling in macrophages were similarly protected from infection-induced thymic atrophy. A slight increase in the concentration of corticosterone was found in mice infected with the highly virulent strain and thymocytes presented an increased susceptibility to dexamethasone-induced death during disseminated infection. The administration of an antagonist of glucocorticoid receptors partially reverted the infection-induced thymic atrophy. We observed a reduction in all thymocyte populations analyzed, including the earliest thymic precursors, suggesting a defect during thymic colonization by T cell precursors and/or during the differentiation of these cells in the bone-marrow in addition to local demise of thymic cells. Our data suggest a complex picture underlying thymic atrophy during infection by M. avium with the participation of locally produced nitric oxide, endogenous corticosteroid activity and reduced bone marrow seeding.
Keywords: infection, mycobacteria, thymus atrophy, nitric oxide, gamma interferon, corticosterone
Introduction
Several microorganisms have been shown to cause premature thymic involution (1). How much premature thymic dysfunction impacts on the immune system depends on factors like the age of the individual, the precise mechanisms that caused thymic atrophy, the consequent ability for thymic recovery after the resolution of the infection, and the concomitant existence or not of peripheral lymphocyte depletion (2). When thymic atrophy occurs in parallel with peripheral lymphopenia, the reconstitution of the immune system depends to a great extent on the ability of the thymus to recover its ability to generate new T cells as it has been extensively shown for AIDS patients (3–5). Since several mechanisms have been implicated in infection-induced thymic atrophy and their role varies depending on the microorganism causing infection, determining which pathways are responsible for thymic atrophy in distinct situations is essential to define strategies to prevent thymic atrophy and/or promote the recovery of thymic function once the infection is controlled.
Even though the thymus undergoes physiologic atrophy with age, T cells are generated and incorporated into the peripheral T cell pool in adulthood, albeit at slower rate than in children (6–9). Thymectomy or thymic dysfunction in early life has been shown to cause premature aging of the immune system in humans (10), as well as enhanced autoimmunity in mice (11). Moreover, loss of thymic activity in adults has been shown to reduce oral tolerance (12) and to hamper the affinity maturation of antibodies (13). Loss of thymic T cell output in adults has more obvious consequences when peripheral lymphopenia occurs concomitantly as it is the case in AIDS patients (14).
Several mechanisms have been implicated in infection-induced thymic atrophy. A major role was attributed to corticosteroids due to their ability to cause thymocyte death (15–18). The increased production of the pro-inflammatory cytokines TNF (16) and IFNα (19) has also been proposed. While this increased apoptosis of the thymocytes is one of the mechanisms leading to thymic atrophy, the increased export of immature T cells (20) and the disturbance of thymic epithelia (19) have also been suggested.
We have previously shown that infection of C57Bl/6 mice with a highly virulent strain of M. avium (strain ATCC 25291 SmT) induces profound peripheral T cell depletion and premature thymic atrophy (21, 22) and that M. avium is able to progressively infect the thymus (23, 24). Since IFNγ is a key molecule in peripheral lymphocyte loss we aimed at analyzing the role of this cytokine in mycobacterial infection-induced thymic atrophy. Moreover, IFNγ is known to cause the up-regulation of the inducible nitric oxide synthase (iNOS) in mycobacterial infection (25) and nitric oxide has been shown to synergize with corticosterone in the induction of apoptosis of thymocytes in in vitro experiments (26). Thus, we also aimed at determining if nitric oxide was leading to thymic atrophy during mycobacterial infection. IFNγ induced macrophage activation and the resulting increase in iNOS activity were identified as a major mechanism responsible for M. avium induced thymic involution. Moreover, we show that IFNγ-induced thymic atrophy synergized with corticosterone signaling. Interestingly, infection with a low virulence strain that also leads to iNOS up-regulation through IFNγ activation, did not lead to thymic atrophy suggesting that corticosterone up-regulation is essential for infection-induced thymic atrophy. Finally we provide evidence that the earliest steps of T cell differentiation might also be compromised.
Materials and Methods
Mice, infection and thymectomy
C57Bl/6 wild type (WT) mice were purchased from Charles River laboratories (Barcelona, Spain). IFNγ-deficient C57Bl/6 (IFNγ-KO) mice were bred in our facilities from a breeding pair purchased from Jackson Laboratories (Bar Harbor, ME). Transgenic mice with a selective impairment in IFNγ signaling in macrophages (MIIG) (27) were bred at the IBMC from a breeding pair provided by the Cincinnati Children's Hospital Medical Center and the University of Cincinnati College of Medicine (Cincinnati, Ohio, USA). iNOS-deficient C57BL/6 mice (iNOS KO) (28) were bred in our facilities after backcrossing the original strain (kindly provided by Drs J. Mudgett, J. D. MacMicking and C. Nathan, Cornell University, New York, NY, USA) onto a C57BL/6 background for seven generations.
Eight-to-ten weeks-old female mice were infected i.v. with 106 CFU of the highly virulent M. avium strain ATCC 25291 SmT (obtained from the American Type Culture Collection; Manassas, VA) or the low virulence strain 2447 (provided by Dr. F. Portaels, Institute of Tropical Medicine, Antwerp, Belgium) through a lateral tail vein. Bacterial inocula were prepared as described previously (21). The bacterial load in the organs was determined as previously described (23). While no signs of distress were observed for the first two months upon infection, some animals showed signs of deterioration of body condition in a non-synchronous way. To avoid excessive and unnecessary suffering of animals, humane end-points were applied. Mice were sacrificed when reaching 25% weight loss.
Thymectomy or sham thymectomy surgical procedures were performed on anesthetized 8-weeks-old mice 2 weeks prior to infection. The thymi were removed by aspiration through a supra-sternum incision.
All animal experiments were performed in accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123) and 86/609/EEC Directive and Portuguese rules (DL 129/92). The animal experimental protocol was approved by the competent national authority Direcção Geral de Veterinária (DGVet).
Cell preparation
Spleens and thymi were collected and processed individually. Cell-suspensions were obtained by gentle mechanical dissociation in PBS containing 3% FCS. Red blood cells were lysed using a hemolytic solution (155 mM NH4Cl, 10 mM KHCO3, pH 7.2) for 4 min at room temperature, and cells were resuspended in DMEM supplemented with 10% heat inactivated FCS, 10 mM HEPES, 1mM sodium pyruvate, 2 mM L-glutamine, 50 μg/ml streptomycin and 50 U/ml penicillin (all from Invitrogen Life Technologies). The number of viable cells was counted by trypan blue exclusion using a hemocytometer.
Histology
Thymi were fixed on paraformaldehyde (4% in PBS) and embedded in paraffin. Thymic sections (2–3 μm) were stained by the Ziehl-Neelsen technique. Slides were visualized using an epifluorescence microscope (BX61 microscope) and photographed with an Olympus DP70 camera.
Flow cytometry
For the flow cytometry analysis of splenocytes 106 cells were stained with FITC-conjugated anti-CD4 (RM4-5) or anti-CD19 (6D5) and PE-conjugated anti-CD8 (53–6.7). Propidium iodide (PI, Sigma-Aldrich) was added at a final concentration of 1 μg/mL to allow the exclusion of dead cells. For thymocyte analysis 106 thymic cells were labeled with distinct combinations of the following antibodies: FITC or APC-conjugated anti-CD3 (145-2C11), PE-Cy5.5 or V450-conjugated anti-CD4 (RM4-5), PE or V500-conjugated anti-CD8 (53–6.7), PerCP-Cy5.5-conjugated anti-CD24 (M1/69), APC-Cy7-conjugated anti-CD25 (PC61), Pe-Cy7-conjugated anti-CD44 (IM7) and Pe-conjugated anti-c-Kit (2B8). For Lin the following antibodies were used conjugated with the same fluorochrome (FITC): anti-CD19 (6D5), anti-B220 (RA3-6B2), anti-CD11b (M1/70), anti-CD11c (N418), anti-NK1.1 (PK136), anti-Gr1 (RB6-8C5), anti-TER119 (TER119). All antibodies were purchased from BioLegend (San Diego, CA) except the V450-conjugated anti-CD4 (RM4-5) and the V500-conjugated anti-CD8 (53–6.7) that were obtained from BD Biosciences. The acquisition of the spleen or thymus cell populations was performed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences); on a LSRII or on a FACSCantoII flow cytometer using BD FACSDiva software (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Blockage of glucocorticoid receptors
Mifepristone (RU486; Sigma-Aldrich), a synthetic glucocorticoid receptor antagonist, was dissolved in sterile polyethylene glycol 400 (PEG 400, Sigma-Aldrich) at a concentration of 5 mg/ml. Treatment was initiated by daily subcutaneous injections of either RU486 (25 mg/Kg; 0.1 ml volume) or an equivalent volume of PEG 400 (vehicle-treated mice) 1 day prior to infection until day 12 post-infection. This dose was previously shown to be effective in the reversion of corticosterone dependent lymph node atrophy in stressed and influenza infected mice (29, 30). From day 13 to 74 post-infection, mice were treated daily by i.p. injection with either RU486 (2.5 mg/Kg; 0.2 ml volume) or an equivalent volume of vehicle. The RU486 solution for i.p. injections was obtained by dissolving RU486 in DMSO (Sigma-Aldrich) at a concentration of 50 mg/ml. This DMSO stock solution was diluted 1/10 with 1 M acetic acid just prior to use and then diluted again in PBS/BSA to a final concentration of 250 μg/ml.
Determination of serum corticosterone level
To obtain the serum concentration of corticosterone at basal levels blood samples were obtained at 9 a.m. from a venous incision at the tip of the tail during a period not exceeding 2 min for each mouse, to avoid corticosterone sera level increase due to handling. Sera were collected and stored at −80 °C until assayed for corticosterone by radioimmunoassay using a 125I RIA Kit (MP Biomedicals). Corticosterone levels were determined from a standard curve and expressed in ng/ml.
Dexamethasone Sensitivity Test
Control or infected mice were treated i.p. with dexamethasone (Oradexon, N. V. Organon Oss Holand) at 0.1, 1.0 and 4.0 mg/Kg or PBS (PBS-treated animals) 24 h before sacrifice. Mice were sacrificed at day 50 post-infection and organs were removed for analysis. Thymocyte suspensions were obtained and analyzed by flow cytometry as described above.
Statistical analysis
Results were expressed as mean + standard deviation (SD). Statistical significance was calculated by using the two-tailed Student's t test for data presented on figure 2 A and the One-way ANOVA on all the other figures. Values of p<0.05 were considered statistically significant.
Figure 2.
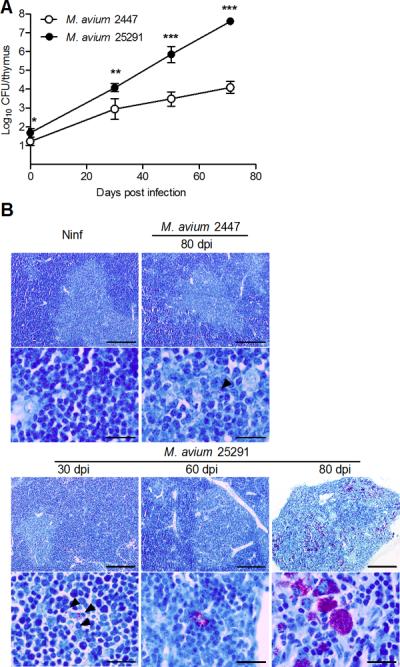
Infection with M. avium strain 25291 leads to higher bacterial loads in the thymus as compared with strain 2447.
A. Representative kinetics of M. avium infection of the thymus from WT mice inoculated with strains 2447 or 25291. Data represent the mean and standard deviation of CFU from five mice per group from one out of three experiments. Statistically significant differences between mice infected with strains 2447 and 25291 are labeled as *p<0.05, **p<0.01, *** p<0.001. B. Representative thymic sections from non-infected mice (Ninf) and mice infected with strains 2447 or 25291 stained for acid-fast bacteria (Ziehl-Neelsen method). Scale bars represent 200 μm in the low-power views and 20 μm in the high magnification pictures. Arrowheads indicate acid fast bacteria in cases of low infection burden.
Results
Severe thymic atrophy occurs in mice infected with the highly virulent (25291) but not with the low virulence M. avium strain (2447)
We compared the fate of the thymus in WT mice intravenously infected with a low virulence strain of M. avium (strain 2447) with that in mice infected with the highly virulent strain of M. avium 25291. As shown in Figure 1 A–C, the size, weight and cellularity of thymi from mice infected with M. avium 2447 did not differ from those of non-infected animals up to 80 days post-infection (dpi). Infection with strain 25291 led to a progressive shrinking of the organ, accompanied by a drastic reduction of its weight and cellularity. Flow cytometry analysis of the major thymocyte populations revealed no differences in mice infected with the low virulence strain as compared with thymi from non-infected controls (Figure 1 D–F). Infection with the highly virulent strain led to changes in the relative proportions and total counts of double-negative (DN, CD4−CD8−), double positive (DP, CD4+CD8+) and single-positive (SP, CD4+CD8− or CD4−CD8+) populations. Thymic involution was somehow asynchronous with some mice exhibiting initial signs of thymic dysfunction sooner than others, as revealed by some variability in the pattern of thymocyte flow cytometry 60 dpi, illustrated in Figure 1D. On average, the cells exhibiting a greater relative depletion were the DP populations (Figure 1E) although all populations underwent massive loss from day 60 up to day 80 post-infection (Figure 1F). This thymic atrophy was associated with increased bacterial loads within the thymus (Figure 2A) in mice infected with the highly virulent strain, in agreement with what has been described for other organs (21, 22). Histologically, increasing numbers of acid fast bacilli were detected and progressive loss of the thymic structure was observed in samples taken from mice infected with the highly virulent strain but no significant alterations were found in thymi from mice infected with the low virulence strain up to 80 dpi (Figure 2B). As previously shown, mice infected with the highly virulent strain developed peripheral lymphopenia which was not observed in the case of infection by strain 2447 (supplementary figure 1A). Removal of the thymus prior to infection with strain 25291 caused a slight increase in the peripheral depletion of T cells (supplementary figure 1B) suggesting that thymic atrophy may have minor additive effects on the development of peripheral lymphopenia.
Figure 1.
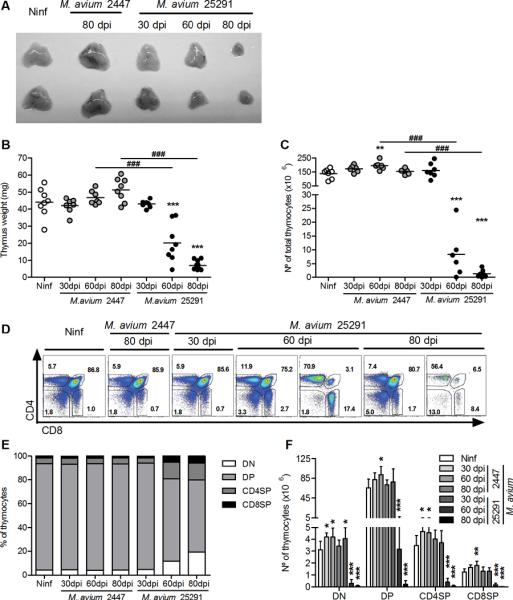
Infection with the highly virulent M. avium strain 25291 but not with the low virulence strain 2447 induces strong thymus atrophy.
A. Photographs of thymi from uninfected WT mice (Ninf) and WT mice infected with M. avium strains 2447 or 25291 at the indicated time-points (in days post-infection, dpi). B, C. Thymus weight and number of thymocytes from control non-infected (Ninf) mice and animals infected with strains 2447 or 25291. D. Representative plots of anti-CD4 and anti-CD8 staining of thymi from control non-infected (Ninf) mice and mice infected with strains 2447 or 25291. The numbers indicate the percentage of cells in each region. E, F. Percentage and number of thymic populations from control non-infected (Ninf) mice and animals infected with strains 2447 or 25291. Data represent the mean of the percentages or the mean and standard deviation of the numbers of cells. Each group of non-infected mice comprised three to four animals and each group of infected mice comprised seven animals analysed individually and data presented represents one experiment out of three. Statistically significant differences between infected and non-infected (Ninf) mice are labeled as *p<0.05, **p<0.01, ***p<0.001. Statistically significant differences between infected groups are labeled as ###p<0.001.
Thymic atrophy induced by M. avium infection is IFNγ-dependent and mediated by nitric oxide
IFNγ is a key cytokine in the protective immune response against mycobacterial infection (31, 32) but has also been shown to participate in the induction of peripheral lymphopenia associated with M. avium 25291 infection (21, 22). To determine if IFNγ was also implicated in M. avium infection thymic atrophy we analyzed the thymi of IFNγ knock-out (KO) mice during infection by strain 25291. As shown in Fig. 3A, mice that are unable to produce IFNγ exhibited no thymic atrophy despite having similar mycobacterial burdens (7.9±0.5 and 8.0±0.4 log10CFU/thymus in WT and IFNγ-KO mice, respectively, at 70 dpi). This observation shows that IFNγ is essential for thymic atrophy during M. avium 25291 infection. Although essential for the depletion of lymphocytes at the periphery IFNγ was shown not to act directly on T cells as the loss of these latter cells occurred in chimeric mice whose T cells lacked the receptor for IFNγ (22). The most likely candidate for an IFNγ-responsive effector cell mediating lymphocyte or thymocyte loss is the macrophage. Thus, we infected “macrophage insensitive to IFNγ” (MIIG) mice, whose CD68-expressing cells (mostly macrophages) do not respond to IFNγ (27). In four independent experiments, control WT mice showed consistent thymic atrophy, ranging from 86 to 98% depletion of thymic cells during infection with strain 25291 whereas MIIG mice showed partial protection with 66% (p<0.05), 27% (not statistically significant, NSS), 29% (NSS), and 74% (NSS) depletion of thymic cells. Figure 3B shows the data of one of the experiments where sub-populations were analysed and where no statistically significant differences were found. These data show that the macrophage is one of the cell types mediating thymocyte loss. One of the major consequences of the activation of macrophages by IFNγ is the increased production of nitric oxide due to the increased expression of the inducible (type 2) nitric oxide synthase (iNOS). To assess the possible role of this reactive species in the induction of thymic atrophy we infected iNOS-deficient animals with strain 25291. As shown in Figure 3C, iNOS-KO mice did not develop atrophy of the thymus. Interestingly the bacterial load within the thymus was almost the same between wild type and iNOS-deficient mice (7.7 and 7.5 log10CFU/thymus in WT and iNOS-KO mice, respectively, at 80 dpi).
Figure 3.
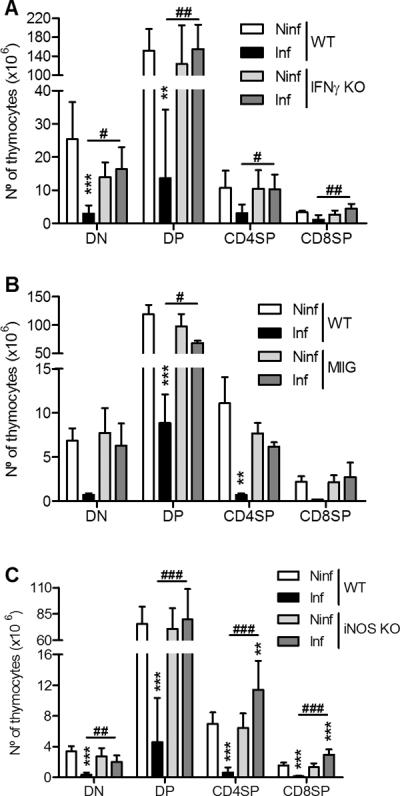
Thymus atrophy depends on IFNγ production, IFNγ-dependent macrophage activation and nitric oxide production.
A. Number of cells of the thymic populations from WT or IFNγ-KO mice, either non-infected (Ninf) or infected with M. avium 25291 at 75 dpi. B. Number of cells of the thymic populations from WT or MIIG transgenic mice, either non-infected (Ninf) or infected with M. avium 25291 at 80 dpi. C. Number of cells of the thymic populations from WT or iNOS-KO mice, either non-infected (Ninf) or infected with M. avium 25291 at 80 dpi. Data represent the mean and standard deviation of the numbers of cells. Each group of mice comprised three to seven animals analyzed individually. Data shown represents one out of three experiments. Statistically significant differences between infected and non-infected mice are labeled as **p<0.01, ***p<0.001. Statistically significant differences between infected groups are labeled as #p<0.05, ##p<0.01, ###p<0.001.
Glucocorticoids cooperate with nitric oxide in the induction of thymus atrophy
As increased levels of glucocorticoids are widely recognized as a common cause for increased thymocyte apoptosis during infection (16–18) we questioned whether corticosteroids were necessary for M. avium-induced thymic atrophy. Mice infected with strain 25291 but not those infected by strain 2447 showed slightly increased serum levels of corticosterone at 60 and 80 dpi (Figure 4A). The increase in corticosterone induced by the highly virulent strain of M. avium was observed both in control WT mice and in IFNγ-deficient mice showing that corticosteroids on their own were not sufficient to induce the atrophy of the thymus. To assess the biological role of this hormone in thymic atrophy, mice were infected with strain 25291 and treated daily with a steroid receptor type II antagonist (RU486) throughout the period of M. avium infection. In one experiment, the RU486 treatment of infected mice partially reverted the atrophy of the thymus whereas in the second experiment, such reversion was complete (Fig. 4B) as compared to vehicle-treated controls. No effect of RU486 on peripheral lymphopenia was observed in either experiment (data not shown).
Figure 4.
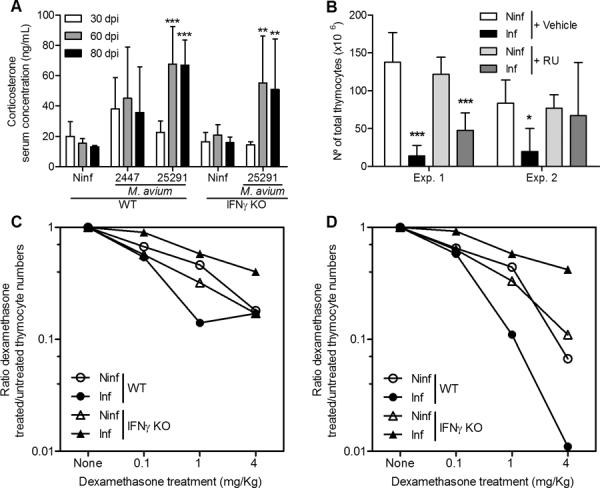
Glucocorticoids are involved in the induction of thymus atrophy during infection with M. avium strain 25291.
A. Representative kinetics of serum corticosterone levels in WT mice infected with M. avium 25291 or 2447 as compared to non-infected (Ninf) mice or of IFNγ-KO mice infected with strain 25291 versus non-infected (Ninf). B. Number of thymocytes from non-infected (Ninf) mice and mice infected for 75 days with M. avium 25291 and treated with either vehicle or RU486 (RU). Data represent the mean and standard deviation of values obtained from six to eight mice in each group analyzed individually and represents two independent experiments. Statistically significant differences between infected and non-infected mice are labeled as *p <0.05, **p <0.01 ***p <0.001. C, D. Decrease of total (C) and DP (D) thymocytes after dexamethasone treatment of non-infected (Ninf) or M. avium 25291-infected animals of either WT or IFNγ-KO strains. Data represent the ratio between the cell numbers in dexamethasone-treated animals and the cell numbers in PBS-treated animals, i.e. the fraction of cells remaining after dexamethasone treatment.
Given that corticosterone blood levels showed only minor increases between M. avium 25291-infected and control non infected mice and that, in contrast, RU486 treatment was quite active in preventing thymic atrophy in infected animals we next investigated if thymocytes became more sensitive to apoptosis-inducing stimuli during infection by the highly virulent strain of M. avium. To that purpose, control and infected mice were administered with dexamethasone, known to induce extensive cell death via corticosteroid receptor activation (33). Since IFNγ-KO mice do not exhibit thymus atrophy, we compared the effects of dexamethasone in both IFNγ-KO and WT animals. Mice were given increasing doses of dexamethasone (0.1, 1.0 and 4.0 mg/Kg) 24 h before sacrifice at 50 dpi and compared to non-infected controls treated in a similar way. We observed a dose-dependent decrease of total and DP cells in both infected and non-infected WT and IFNγ-KO mice (Fig. 4C and D). Thymocyte loss was exacerbated by infection in WT mice but not in IFNγ-KO animals showing that IFNγ production during infection increases thymocyte susceptibility to dexamethasone-induced cell death.
Impairment of the earliest stages of thymocyte development
To further dissect at what levels of the thymocyte development the infection impacts on cell viability, we analysed in detail DN thymocytes of mice infected with each M. avium strain. Whereas in mice infected with the low virulence strain no differences were observed in the DN1 to DN4 populations, animals infected with M. avium 25291 showed progressive reduction in cell numbers at all stages (Figure 5A and B). The relative reduction in all stages was similar such that the proportions remained almost unchanged (Figure 5A). We next analyzed what changes were found in M. avium 25291-infected mice that showed no thymic atrophy either because they were unable to produce IFNγ, their macrophages did not respond to IFNγ or they do not express iNOS. No major alterations were induced by infection in IFNγ-KO or MIIG mice that exhibited normal or only slightly reduced numbers of DN1 to DN4 subpopulations (Figure 5C and D). Likewise, iNOS-deficient mice also maintained most of these subpopulations unchanged during infection except for DN2 (Figure 5E). Finally, we analyzed the most immature thymocytes, the early thymic precursors (ETP), defined in the DN population as lineage-negative, CD44+, CD25− and c-Kit+ cells (see Supplementary figure 2 for a schematic representation of the gating strategy used to select this population). These cells were almost lost during infection of WT mice with the highly virulent strain of M. avium but not during infection with the strain 2447 (Figure 6A and B). No loss was found in IFNγ-KO or MIIG mice infected with the highly virulent strain 25291 (Figure 6C to F) but a statistically significant reduction was found in iNOS-KO animals (Figure 6G and H).
Figure 5.
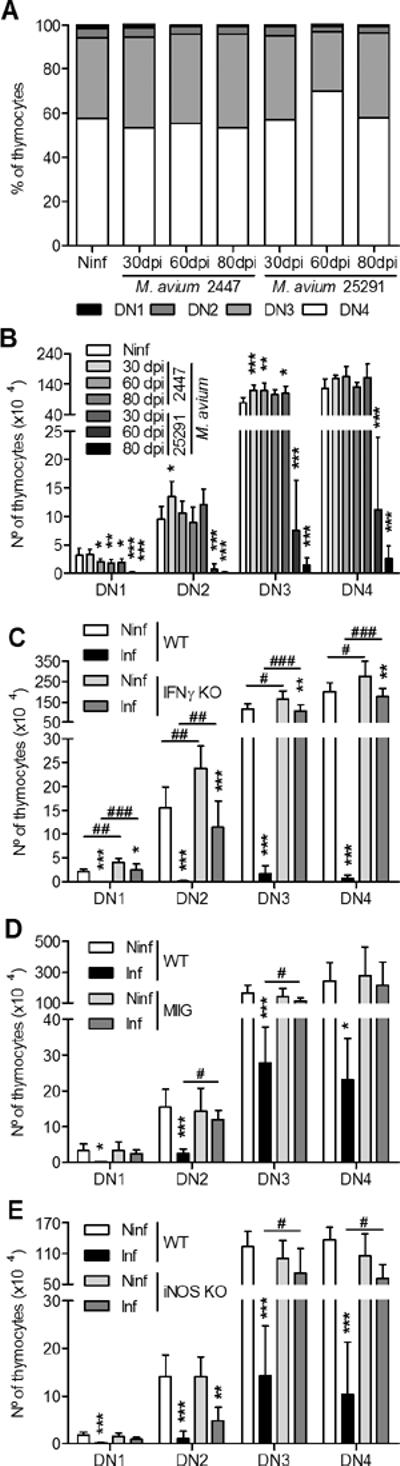
Infection with M. avium strain 25291 induces depletion of all subpopulations of DN thymocytes which depends on IFNγ production, IFNγ-dependent macrophage activation and NO production.
A, B. Percentage and number of DN thymic subpopulations from non-infected (Ninf) mice and mice infected with strains 2447 or 25291. C. Number of DN thymic subpopulations from WT and IFNγ-KO mice, either non-infected (Ninf) or infected with M. avium 25291 at 80 dpi. D. Number of DN thymic subpopulations from WT and MIIG mice, either non-infected (Ninf) or infected with M. avium 25291 at 80 dpi. E. Number of DN thymic subpopulations from WT and iNOS-KO mice, either non-infected (Ninf) or infected with M. avium 25291 at 80 dpi. Data represent the mean and standard deviation of the number of cells. Each group of mice comprised three to six animals analyzed individually. Data shown represents one experiment out of three. Statistically significant differences between infected and control mice are labeled as *p<0.05, **p<0.01, ***p<0.001. Statistically significant differences between infected groups are labeled as #p<0.05, ##p<0.01, ###p<0.001.
Figure 6.
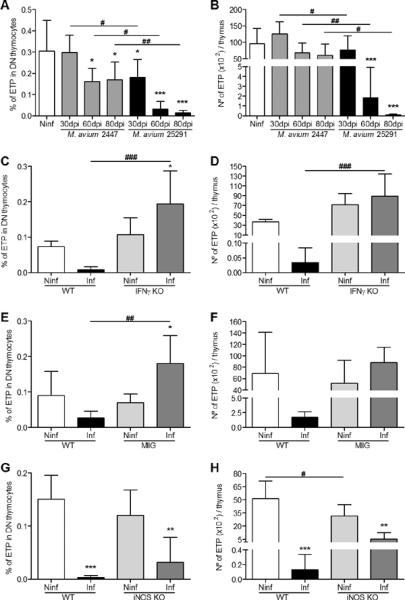
Infection with M. avium 25291 induces depletion of the early thymocyte precursors and depends on IFNγ production, IFNγ-dependent macrophage activation and NO production.
A, B. Percentage (A) and number (B) of ETP from non-infected (Ninf) thymi and from thymi of WT mice infected with M. avium strains 2447 or 25291. C, D. Percentage (C) and number (D) of ETP from non-infected (Ninf) thymi and from thymi of WT or IFNγ-KO mice infected with M. avium 25291 at 80 dpi. E, F. Percentage (E) and number (F) of ETP from non-infected (Ninf) thymi and from thymi of WT or MIIG mice infected with M. avium 25291 at 80 dpi. G, H. Percentage (G) and number (H) of ETP from non-infected (Ninf) thymi and from thymi of WT or iNOS-KO mice infected with M. avium 25291 at 80 dpi. Data represent the mean of the percentages or the mean and standard deviation of the number of cells. Each group of mice comprised three to six animals analysed individually. Data shown represents one experiment out of three. Statistically significant differences between infected and non-infected control mice are labeled as *p<0.05, **p<0.01, ***p<0.001. Statistically significant differences between infected groups are labeled as #p<0.05, ##p<0.01, ###p<0.001.
Discussion
We examined here the mechanisms underlying the emergence of progressive thymic atrophy in mice infected with M. avium 25291. We showed that IFNγ-deficient animals are resistant to the M. avium-induced thymic atrophy consistent with the fact that albeit essential for protective immunity against mycobacteria (31, 32) production of IFNγ by T cells may not always be correlated with protection during infection by HIV or M. tuberculosis (34–37). IFNγ has been shown to be involved in the development of peripheral lymphopenia in mice infected with M. avium 25291 (22), to limit the exacerbated T cell activation responsible for immune pathology associated with granulomatous lesions (38) and to increase apoptosis of DP thymocytes (39). Furthermore, IFNγ produced during measles virus infection not only inhibited cell proliferation but also disrupted the thymic epithelium responsible for thymocyte survival and differentiation into mature T cells, leading to thymic atrophy (40). Since IFNγ does not act directly on lymphocytes in the periphery to induce their demise (22), and macrophages accumulate in large numbers in tissue granulomas and in the thymus of M. avium-infected mice (23, 24) we assessed the involvement of these phagocytes in the IFNγ-mediated atrophy of the thymus. MIIG mice have macrophages (defined as CD68-expressing cells) that do not respond to IFNγ and were found here not to develop thymic atrophy. This shows that macrophages respond to IFNγ and mediate to a great extent the involution of the organ. Since IFNγ-activated macrophages secrete important quantities of nitric oxide, we next evaluated the fate of the thymus in iNOS-deficient animals infected with strain 25291. These mice showed no evidence of thymic atrophy. Thus, a major pathway leading to thymic atrophy is dependent on the induction of iNOS expression in macrophages by IFNγ produced in response to infection. Nitric oxide has already been shown to be important in limiting T cell activity and survival at the periphery during mycobacterial infections (38) but this is the first demonstration of its activity in the thymus of an infected animal.
The mycobacterial loads in the thymus of mice infected with strain 25291 were significantly higher than those of mice infected with strain 2447 raising the possibility that the mycobacteria might themselves be toxic for the thymic tissue. However, this is not supported by the fact that the bacterial loads in the thymi of infected WT mice which undergo atrophy were very similar to those found in IFNγ- or iNOS-deficient animals which did not develop such atrophy. Moreover, M. avium is known to be relatively non-toxic as evidenced by the very high microbial burdens that can be observed in infected hosts and which are still compatible with their survival (41–43).
The most frequent mechanism implicated in infection induced thymic atrophy is increased thymocyte apoptosis due to increased production of GCs (15–17, 44–46). For example, the severe thymic atrophy found in mice infected with Trypanosoma cruzi is caused by an increased production of GCs (17, 47). This can also be seen during M. tuberculosis infection (48) or in mice given an injection of M. tuberculosis cord factor (trehalose 6,6'-dimycolate) (49). The activity of GCs might also synergize with that of IFNγ. IFNγ is known to increase serum levels of TNF which in turn induces thymocyte apoptosis and augments the susceptibility of thymocytes to GCs (50). Given the importance of GCs in thymic involution, we studied whether GCs were implicated in the depletion of thymocytes during M. avium infection. Indeed, the infection with the highly virulent strain led to an increase, albeit small, of serum corticosterone levels. To determine if this increase affected thymocyte numbers we performed an in vivo functional assay, using an antagonist of the GC receptor (RU486). In two independent experiments RU486 partially or completely reversed M. avium-induced thymus atrophy. Furthermore, infection increased thymocyte depletion by dexamethasone with cells from WT animals being more susceptible than those of IFNγ-deficient mice. These data show an increase in susceptibility to GC-mediated thymocyte elimination. Although it is unclear to what extent GCs are required for M. avium-induced thymic atrophy we suggest that they do play at least a partial role. It is clear, however, that GCs on their own are not sufficient to induce M. avium-induced thymic atrophy and that they need to synergize with IFNγ or IFNγ-induced pathways. The source of the GCs that participate in thymic involution is unclear. It has been shown that the murine thymus itself is capable of producing GCs (52) and it was suggested that thymic epithelial cells may be able to produce GCs that would act directly in thymocytes (53, 54). The mechanisms that underlie the IFNγ dependence to GC sensitivity are also not known, although changes in the levels of Notch and Bcl-2 expression have been suggested (55). Further work is needed to clarify this issue.
Since thymocytes expand several orders of magnitude from incoming bone marrow-derived precursors, a reduction in early thymic precursors could impact on thymic cellularity. The progressive reduction in thymocyte numbers observed here was seen among the DN1 to the DN4 stages to an extent which was proportional to the relative abundance of these cells in a normal thymus. Furthermore, the most immature thymocytes (c-Kit+, lin− DN1 cells) were found to be severely deficient in advanced stages of thymic atrophy. These observations are consistent with several scenarios of thymocyte depletion: 1) it might occur predominantly in the thymus and affect all cell types, 2) there might be a predominant effect on the recently arrived precursors impacting on the subsequent ability to expand inside the thymic tissue or, 3) a reduction in the release of precursors from the bone marrow might reduce thymus seeding. In this context, it has been shown that early lymphoid progenitors in mouse and man are highly sensitive to GCs (56) and alterations in chemokine expression and bone marrow retention phenomena are observed in HIV infected patients (57). Our data with iNOS-deficient mice suggest that at least some of the reduction in thymocyte numbers is occurring locally in the thymus. iNOS-deficient mice showed a statistically significant reduction in the number of early thymocyte precursors suggesting that nitric oxide-independent mechanisms lead to the failure of the bone marrow to seed the thymus. Yet, despite the reduced seeding of the thymus by early thymocyte precursors iNOS-deficient animals did not develop thymic atrophy indicating that nitric oxide may be required for local thymocyte death in the thymic tissue. The lower production of thymic precursors by the bone marrow might be due to the direct action of IFNγ on haematopoietic stem cells or multipotent progenitors as has already been proposed (58–60). The fact that MIIG mice are not as well protected from thymocyte loss as IFNγ-deficient mice suggests that IFNγ may also act on cells other than the macrophage, namely the progenitor cells in the bone marrow. Although thymic emigrants are important for the maintenance of the peripheral lymphocyte pool (61–63) and the loss of thymic output may accelerate the loss of splenic T cells, as shown here in thymectomized mice (supplementary figure 1B) we found that peripheral lymphopenia still occurs in situations where the thymus remains apparently intact such as in iNOS-deficient animals or when GC activity has been blocked by the receptor antagonist RU486 (data not shown).
In summary, the present report identifies the role of IFNγ-activated macrophages and their ability to produce nitric oxide in the development of thymic atrophy most likely co-adjuvanted by the increased susceptibility to GC-mediated thymocyte apoptosis that develops during infection with the highly virulent strain of M. avium and suggest the possibility of a failure of the bone marrow to seed the thymus with precursors.
Supplementary Material
Acknowledgments
This work was funded by project FCOMP-01-0124-FEDER-011142, reference PTDC/SAU-MII/099102/2008 and PTDC/SAU-MII/101663/2008 from the Fundação para a Ciência e a Tecnologia (FCT, Lisbon, Portugal). PBS was granted a PhD fellowship by FCT. MBJ was funded through the NIH grant R01HL091769.
References
- 1.Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathogens. 2006;2:472–83. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–73. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigano A, Vella S, Saresella M, Vanzulli A, Bricalli D, Di Fabio S, Ferrante P, Andreotti M, Pirillo M, Dally LG, Clerici M, Principi N. Early immune reconstitution after potent antiretroviral therapy in HIV-infected children correlates with the increase in thymus volume. AIDS. 2000;14:251–61. doi: 10.1097/00002030-200002180-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kalayjian RC, Spritzler J, Pu M, Landay A, Pollard RB, Stocker V, Harthi LA, Gross BH, Francis IR, Fiscus SA, Tebas P, Bosch RJ, Valcour V, Lederman MM. Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J Infect Dis. 2005;192:1577–87. doi: 10.1086/466527. [DOI] [PubMed] [Google Scholar]
- 5.Smith KY, Valdez H, Landay A, Spritzler J, Kessler HA, Connick E, Kuritzkes D, Gross B, Francis I, McCune JM, Lederman MM. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181:141–7. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 6.Eysteinsdottir JH, Freysdottir J, Haraldsson A, Stefansdottir J, Skaftadottir I, Helgason H, Ogmundsdottir HM. The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life. Clin Exp Immunol. 2004;136:349–55. doi: 10.1111/j.1365-2249.2004.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prelog M, Keller M, Geiger R, Brandstatter A, Wurzner R, Schweigmann U, Zlamy M, Zimmerhackl LB, Grubeck-Loebenstein B. Thymectomy in early childhood: significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin Immunol. 2009;130:123–32. doi: 10.1016/j.clim.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142(Suppl 1):S111–5. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–9. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauce D, Appay V. Altered thymic activity in early life: how does it affect the immune system in young adults? Curr Opin Immunol. 2011;23:543–8. doi: 10.1016/j.coi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Gagnerault MC, Lanvin O, Pasquier V, Garcia C, Damotte D, Lucas B, Lepault F. Autoimmunity during thymectomy-induced lymphopenia: role of thymus ablation and initial effector T cell activation timing in nonobese diabetic mice. J Immunol. 2009;183:4913–20. doi: 10.4049/jimmunol.0901954. [DOI] [PubMed] [Google Scholar]
- 12.Song F, Guan Z, Gienapp IE, Shawler T, Benson J, Whitacre CC. The thymus plays a role in oral tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2006;177:1500–9. doi: 10.4049/jimmunol.177.3.1500. [DOI] [PubMed] [Google Scholar]
- 13.Abuattieh M, Bender D, Liu E, Wettstein P, Platt JL, Cascalho M. Affinity maturation of antibodies requires integrity of the adult thymus. Eur J Immunol. 2012;42:500–10. doi: 10.1002/eji.201141889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–90. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Muller N, McPherson KG, Reichardt HM. Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J Immunol. 2006;176:1695–702. doi: 10.4049/jimmunol.176.3.1695. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Kuolee R, Austin JW, Shen H, Che Y, Conlan JW. Low dose aerosol infection of mice with virulent type A Francisella tularensis induces severe thymus atrophy and CD4+CD8+ thymocyte depletion. Microb Pathog. 2005;39:189–96. doi: 10.1016/j.micpath.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez AR, Roggero E, Nicora A, Palazzi J, Besedovsky HO, Del Rey A, Bottasso OA. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun. 2007;21:890–900. doi: 10.1016/j.bbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Leite de Moraes MC, Hontebeyrie-Joskowicz M, Leboulenger F, Savino W, Dardenne M, Lepault F. Studies on the thymus in Chagas' disease. II. Thymocyte subset fluctuations in Trypanosoma cruzi-infected mice: relationship to stress. Scand J Immunol. 1991;33:267–75. doi: 10.1111/j.1365-3083.1991.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulou AS, Dooley J, Linterman MA, Pierson W, Ucar O, Kyewski B, Zuklys S, Hollander GA, Matthys P, Gray DH, De Strooper B, Liston A. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-alpha receptor. Nat Immunol. 2011;13:181–7. doi: 10.1038/ni.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrot A, Terra-Granado E, Perez AR, Silva-Barbosa SD, Milicevic NM, Farias-de-Oliveira DA, Berbert LR, De Meis J, Takiya CM, Beloscar J, Wang X, Kont V, Peterson P, Bottasso O, Savino W. Chagasic thymic atrophy does not affect negative selection but results in the export of activated CD4+CD8+ T cells in severe forms of human disease. PLoS Negl Trop Dis. 2011;5:e1268. doi: 10.1371/journal.pntd.0001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florido M, Goncalves AS, Silva RA, Ehlers S, Cooper AM, Appelberg R. Resistance of virulent Mycobacterium avium to gamma interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect Immun. 1999;67:3610–8. doi: 10.1128/iai.67.7.3610-3618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florido M, Pearl JE, Solache A, Borges M, Haynes L, Cooper AM, Appelberg R. Gamma interferon-induced T-cell loss in virulent Mycobacterium avium infection. Infect Immun. 2005;73:3577–86. doi: 10.1128/IAI.73.6.3577-3586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobrega C, Cardona PJ, Roque S, Pinto do OP, Appelberg R, Correia-Neves M. The thymus as a target for mycobacterial infections. Microbes Infect. 2007;9:1521–9. doi: 10.1016/j.micinf.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Nobrega C, Roque S, Nunes-Alves C, Coelho A, Medeiros I, Castro AG, Appelberg R, Correia-Neves M. Dissemination of mycobacteria to the thymus renders newly generated T cells tolerant to the invading pathogen. J Immunol. 2010;184:351–8. doi: 10.4049/jimmunol.0902152. [DOI] [PubMed] [Google Scholar]
- 25.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–7. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen O, Kfir-Erenfeld S, Spokoini R, Zilberman Y, Yefenof E, Sionov RV. Nitric oxide cooperates with glucocorticoids in thymic epithelial cell-mediated apoptosis of double positive thymocytes. Int Immunol. 2009;21:1113–23. doi: 10.1093/intimm/dxp079. [DOI] [PubMed] [Google Scholar]
- 27.Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, Karp CL, Aliberti J, Flick MJ, Jordan MB. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol. 2010;184:877–85. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 29.Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48:151–60. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs CM, Feng N, Beck FM, Sheridan JF. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: effects of restraint stress-induced elevation in endogenous corticosterone. J Immunol. 1996;157:1870–7. [PubMed] [Google Scholar]
- 31.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purton JF, Monk JA, Liddicoat DR, Kyparissoudis K, Sakkal S, Richardson SJ, Godfrey DI, Cole TJ. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J Immunol. 2004;173:3816–24. doi: 10.4049/jimmunol.173.6.3816. [DOI] [PubMed] [Google Scholar]
- 34.Draenert R, Verrill CL, Tang Y, Allen TM, Wurcel AG, Boczanowski M, Lechner A, Kim AY, Suscovich T, Brown NV, Addo MM, Walker BD. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J Virol. 2004;78:630–41. doi: 10.1128/JVI.78.2.630-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leal IS, Smedegard B, Andersen P, Appelberg R. Failure to induce enhanced protection against tuberculosis by increasing T-cell-dependent interferon-gamma generation. Immunology. 2001;104:157–61. doi: 10.1046/j.0019-2805.2001.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias D, Akuffo H, Britton S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:363–8. doi: 10.1016/j.trstmh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, Nouze C, Ladant D, Cole ST, Sebo P, Leclerc C. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74:2128–37. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN-gamma and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10:221–6. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y, Morikawa A, Sugiyama T, Koide N, Jiang GZ, Lwin T, Yoshida T, Yokochi T. Augmentation of lipopolysaccharide-induced thymocyte apoptosis by interferon-gamma. Cell Immunol. 1997;177:103–8. doi: 10.1006/cimm.1997.1103. [DOI] [PubMed] [Google Scholar]
- 40.Vidalain PO, Laine D, Zaffran Y, Azocar O, Servet-Delprat C, Wild TF, Rabourdin-Combe C, Valentin H. Interferons mediate terminal differentiation of human cortical thymic epithelial cells. J Virol. 2002;76:6415–24. doi: 10.1128/JVI.76.13.6415-6424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong B, Edwards FF, Kiehn TE, Whimbey E, Donnelly H, Bernard EM, Gold JW, Armstrong D. Continuous high-grade mycobacterium avium intracellulare bacteremia in patients with the acquired immune deficiency syndrome. Am J Med. 1985;78:35–40. doi: 10.1016/0002-9343(85)90458-9. [DOI] [PubMed] [Google Scholar]
- 42.Rathbun RC, Martin ES, 3rd, Eaton VE, Matthew EB. Current and investigational therapies for AIDS-associated Mycobacterium avium complex disease. Clin Pharm. 1991;10:280–91. [PubMed] [Google Scholar]
- 43.Griffith DE. Emergence of nontuberculous mycobacteria as pathogens in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:810–2. doi: 10.1164/rccm.2301001. [DOI] [PubMed] [Google Scholar]
- 44.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cell Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartzman RA, Cidlowski JA. Glucocorticoid-induced apoptosis of lymphoid cells. Int Arch Allergy Immunol. 1994;105:347–54. doi: 10.1159/000236781. [DOI] [PubMed] [Google Scholar]
- 46.Chung HT, Samlowski WE, Daynes RA. Modification of the murine immune system by glucocorticosteroids: alterations of the tissue localization properties of circulating lymphocytes. Cell Immunol. 1986;101:571–85. doi: 10.1016/0008-8749(86)90167-x. [DOI] [PubMed] [Google Scholar]
- 47.Roggero E, Perez AR, Tamae-Kakazu M, Piazzon I, Nepomnaschy I, Besedovsky HO, Bottasso OA, del Rey A. Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J Endocrinol. 2006;190:495–503. doi: 10.1677/joe.1.06642. [DOI] [PubMed] [Google Scholar]
- 48.Bottasso O, Bay ML, Besedovsky H, del Rey A. The immuno-endocrine component in the pathogenesis of tuberculosis. Scand J Immunol. 2007;66:166–75. doi: 10.1111/j.1365-3083.2007.01962.x. [DOI] [PubMed] [Google Scholar]
- 49.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6'-dimycolate) Infect Immun. 1997;65:1793–9. doi: 10.1128/iai.65.5.1793-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brouckaert P, Everaerdt B, Fiers W. The glucocorticoid antagonist RU38486 mimics interleukin-1 in its sensitization to the lethal and interleukin-6-inducing properties of tumor necrosis factor. Eur J Immunol. 1992;22:981–6. doi: 10.1002/eji.1830220416. [DOI] [PubMed] [Google Scholar]
- 51.Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 2002;16:727–9. doi: 10.1096/fj.01-0891fje. [DOI] [PubMed] [Google Scholar]
- 52.Lechner O, Wiegers GJ, Oliveira-Dos-Santos AJ, Dietrich H, Recheis H, Waterman M, Boyd R, Wick G. Glucocorticoid production in the murine thymus. Eur J Immunol. 2000;30:337–46. doi: 10.1002/1521-4141(200002)30:2<337::AID-IMMU337>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 53.Pazirandeh A, Xue Y, Rafter I, Sjovall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J. 1999;13:893–901. doi: 10.1096/fasebj.13.8.893. [DOI] [PubMed] [Google Scholar]
- 54.Zilberman Y, Zafrir E, Ovadia H, Yefenof E, Guy R, Sionov RV. The glucocorticoid receptor mediates the thymic epithelial cell-induced apoptosis of CD4+8+ thymic lymphoma cells. Cell Immunol. 2004;227:12–23. doi: 10.1016/j.cellimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Kong FK, Chen CL, Cooper MD. Reversible disruption of thymic function by steroid treatment. J Immunol. 2002;168:6500–5. doi: 10.4049/jimmunol.168.12.6500. [DOI] [PubMed] [Google Scholar]
- 56.Igarashi H, Medina KL, Yokota T, Rossi MI, Sakaguchi N, Comp PC, Kincade PW. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int Immunol. 2005;17:501–11. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- 57.Sauce D, Larsen M, Fastenackels S, Pauchard M, Ait-Mohand H, Schneider L, Guihot A, Boufassa F, Zaunders J, Iguertsira M, Bailey M, Gorochov G, Duvivier C, Carcelain G, Kelleher AD, Simon A, Meyer L, Costagliola D, Deeks SG, Lambotte O, Autran B, Hunt PW, Katlama C, Appay V. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142–51. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacNamara KC, Jones M, Martin O, Winslow GM. Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PLoS One. 2011;6:e28669. doi: 10.1371/journal.pone.0028669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–54. doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- 61.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berzins SP, Godfrey DI, Miller JF, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci U S A. 1999;96:9787–91. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, Godfrey DI, Boyd RL. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;8:469–76. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


