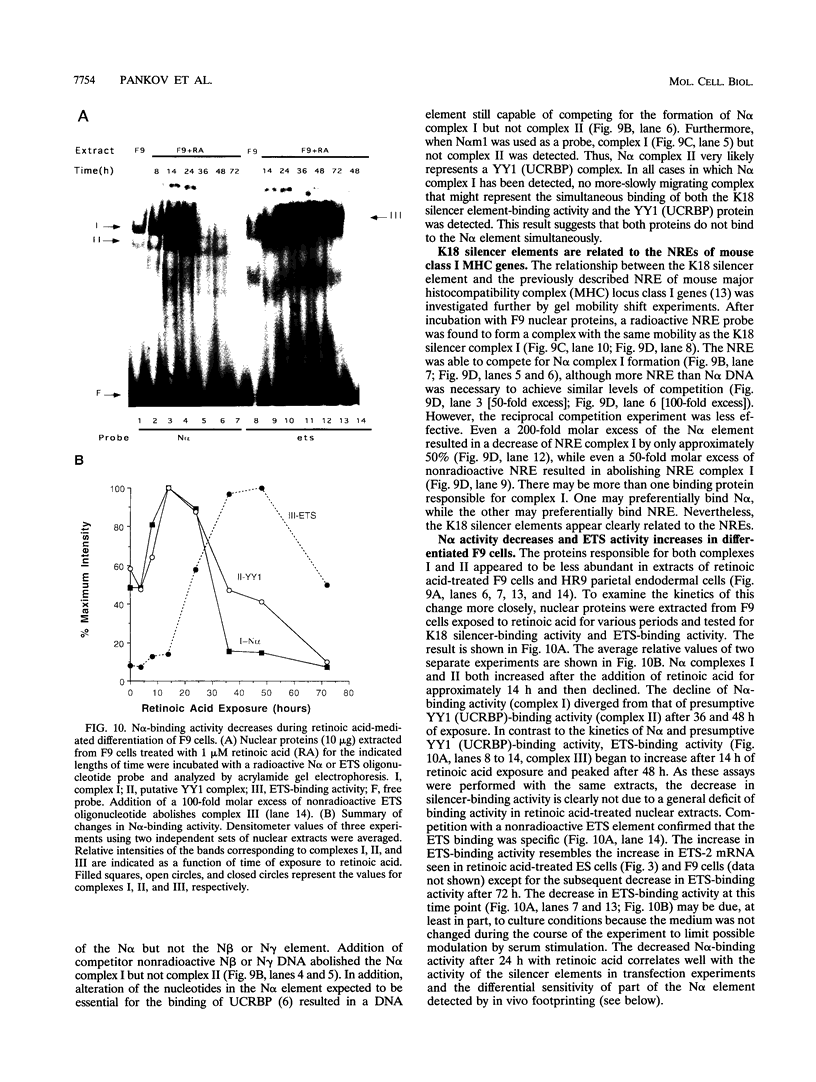
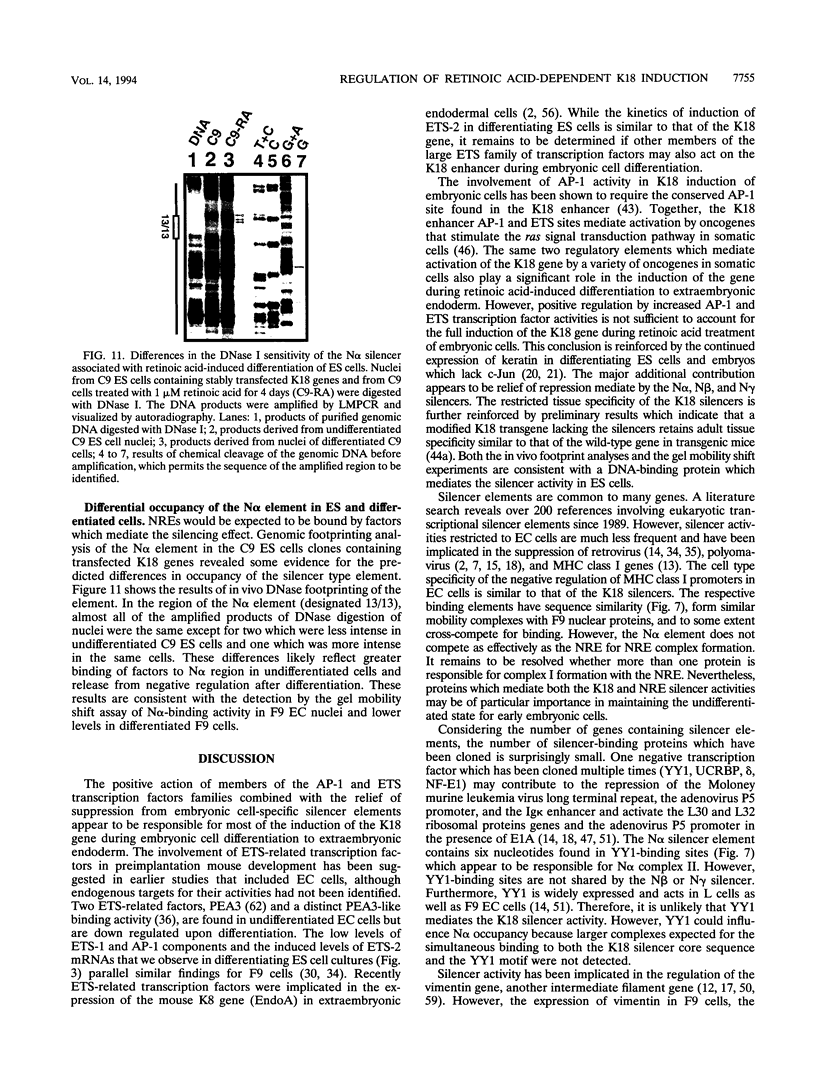
Abstract
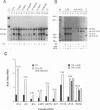
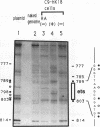
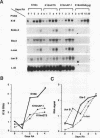
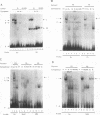
The differentiation of both embryonal carcinoma (EC) and embryonic stem (ES) cells can be triggered in culture by exposure to retinoic acid and results in the transcriptional induction of both the endogenous mouse keratin 18 (mK18) intermediate filament gene and an experimentally introduced human keratin 18 (K18) gene as well as a variety of other markers characteristic of extraembryonic endoderm. The induction of K18 in EC cells is limited, in part, by low levels of ETS and AP-1 transcription factor activities which bind to sites within a complex enhancer element located within the first intron of K18. RNA levels of ETS-2, c-Jun, and JunB increase upon the differentiation of ES cells and correlate with increased expression of K18. Occupancy of the ETS site, detected by in vivo footprinting methods, correlates with K18 induction in ES cells. In somatic cells, the ETS and AP-1 elements mediate induction by a variety of oncogenes associated with the ras signal transduction pathway. In EC cells, in addition to the induction by these limiting transcription factors, relief from negative regulation is mediated by three silencer elements located within the first intron of the K18 gene. These silencer elements function in F9 EC cells but not their differentiated derivatives, and their activity is correlated with proteins in F9 EC nuclei which bind to the silencers and are reduced in the nuclei of differentiated F9 cells. The induction of K18, associated with the differentiation of EC cells to extraembryonic endoderm, is due to a combination of relief from negative regulation and activation by members of the ETS and AP-1 transcription factor families.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Oshima R. G. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J Cell Biol. 1990 Sep;111(3):1197–1206. doi: 10.1083/jcb.111.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K., Takahashi H., Nakamura M., Ariga H. Effect of silencer on polyomavirus DNA replication. Mol Cell Biol. 1989 Sep;9(9):4026–4031. doi: 10.1128/mcb.9.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K., Takahashi H., Nakamura M., Ariga H. Negative transcriptional regulatory element that functions in embryonal carcinoma cells. Mol Cell Biol. 1989 Sep;9(9):4032–4037. doi: 10.1128/mcb.9.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribault H., Oshima R. G. Polarized and functional epithelia can form after the targeted inactivation of both mouse keratin 8 alleles. J Cell Biol. 1991 Dec;115(6):1675–1684. doi: 10.1083/jcb.115.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribault H., Price J., Miyai K., Oshima R. G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993 Jul;7(7A):1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cremisi C., Babinet C. Negative regulation of early polyomavirus expression in mouse embryonal carcinoma cells. J Virol. 1986 Sep;59(3):761–763. doi: 10.1128/jvi.59.3.761-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dony C., Kessel M., Gruss P. Post-transcriptional control of myc and p53 expression during differentiation of the embryonal carcinoma cell line F9. Nature. 1985 Oct 17;317(6038):636–639. doi: 10.1038/317636a0. [DOI] [PubMed] [Google Scholar]
- Farrell F. X., Sax C. M., Zehner Z. E. A negative element involved in vimentin gene expression. Mol Cell Biol. 1990 May;10(5):2349–2358. doi: 10.1128/mcb.10.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Murata M., Burke P. A., Shirayoshi Y., Appella E., Sharp P. A., Ozato K. Negative regulation of the major histocompatibility complex class I promoter in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3145–3149. doi: 10.1073/pnas.88.8.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Yamaguchi Y., Ogawa E., Shigesada K., Satake M., Ito Y. A ubiquitous repressor interacting with an F9 cell-specific silencer and its functional suppression by differentiated cell-specific positive factors. Cell Growth Differ. 1990 Mar;1(3):135–147. [PubMed] [Google Scholar]
- Garzon R. J., Zehner Z. E. Multiple silencer elements are involved in regulating the chicken vimentin gene. Mol Cell Biol. 1994 Feb;14(2):934–943. doi: 10.1128/mcb.14.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Fromental C., Sassone-Corsi P., Chambon P. A mutated polyoma virus enhancer which is active in undifferentiated embryonal carcinoma cells is not repressed by adenovirus-2 E1A products. Nature. 1986 May 15;321(6067):249–251. doi: 10.1038/321249a0. [DOI] [PubMed] [Google Scholar]
- Hilberg F., Aguzzi A., Howells N., Wagner E. F. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993 Sep 9;365(6442):179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Hilberg F., Wagner E. F. Embryonic stem (ES) cells lacking functional c-jun: consequences for growth and differentiation, AP-1 activity and tumorigenicity. Oncogene. 1992 Dec;7(12):2371–2380. [PubMed] [Google Scholar]
- Hosler B. A., LaRosa G. J., Grippo J. F., Gudas L. J. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989 Dec;9(12):5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Makino K., Niwa H., Sugiyama H., Kimura S., Amemura M., Nakata A., Kakunaga T. Identification of the human beta-actin enhancer and its binding factor. Mol Cell Biol. 1988 Jan;8(1):267–272. doi: 10.1128/mcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Kitabayashi I., Kawakami Z., Chiu R., Ozawa K., Matsuoka T., Toyoshima S., Umesono K., Evans R. M., Gachelin G., Yokoyama K. Transcriptional regulation of the c-jun gene by retinoic acid and E1A during differentiation of F9 cells. EMBO J. 1992 Jan;11(1):167–175. doi: 10.1002/j.1460-2075.1992.tb05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., LaPointe J. W., Lorch Y. Preparation of nucleosomes and chromatin. Methods Enzymol. 1989;170:3–14. doi: 10.1016/0076-6879(89)70039-2. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Complete structure of the gene for human keratin 18. Genomics. 1989 Apr;4(3):339–347. doi: 10.1016/0888-7543(89)90340-6. [DOI] [PubMed] [Google Scholar]
- Kwon M., Oshima R. G. JunB does not inhibit the induction of c-Jun during the retinoic acid induced differentiation of F9 cells. Dev Dyn. 1992 Feb;193(2):193–198. doi: 10.1002/aja.1001930211. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston A. W., Gudas L. J. Identification of a retinoic acid responsive enhancer 3' of the murine homeobox gene Hox-1.6. Mech Dev. 1992 Sep;38(3):217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Evidence for a stem cell-specific repressor of Moloney murine leukemia virus expression in embryonal carcinoma cells. Mol Cell Biol. 1990 Aug;10(8):4045–4057. doi: 10.1128/mcb.10.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Proviral sequences that restrict retroviral expression in mouse embryonal carcinoma cells. Mol Cell Biol. 1987 Oct;7(10):3775–3784. doi: 10.1128/mcb.7.10.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Yang X. Y., Folk W. R. Expression of a 91-kilodalton PEA3-binding protein is down-regulated during differentiation of F9 embryonal carcinoma cells. Mol Cell Biol. 1992 May;12(5):2213–2221. doi: 10.1128/mcb.12.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Neznanov N. S., Oshima R. G. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Mar;13(3):1815–1823. doi: 10.1128/mcb.13.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N., Thorey I. S., Ceceña G., Oshima R. G. Transcriptional insulation of the human keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Apr;13(4):2214–2223. doi: 10.1128/mcb.13.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Identification and immunoprecipitation of cytoskeletal proteins from murine extra-embryonic endodermal cells. J Biol Chem. 1981 Aug 10;256(15):8124–8133. [PubMed] [Google Scholar]
- Oshima R. G. Intermediate filament molecular biology. Curr Opin Cell Biol. 1992 Feb;4(1):110–116. doi: 10.1016/0955-0674(92)90067-m. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Millán J. L., Ceceña G. Comparison of mouse and human keratin 18: a component of intermediate filaments expressed prior to implantation. Differentiation. 1986;33(1):61–68. doi: 10.1111/j.1432-0436.1986.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Pankov R., Umezawa A., Maki R., Der C. J., Hauser C. A., Oshima R. G. Oncogene activation of human keratin 18 transcription via the Ras signal transduction pathway. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):873–877. doi: 10.1073/pnas.91.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. H., Gregg R. G., Smithies O., Koller B. H. Regulatory elements in the introns of the human HPRT gene are necessary for its expression in embryonic stem cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4299–4303. doi: 10.1073/pnas.87.11.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Differentiation-responsive elements in the 5' region of the mouse tissue plasminogen activator gene confer two-stage regulation by retinoic acid and cyclic AMP in teratocarcinoma cells. Mol Cell Biol. 1989 Apr;9(4):1691–1704. doi: 10.1128/mcb.9.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti A., Lilienbaum A., Li Z., Paulin D., Gazzolo L. Identification of a negative element in the human vimentin promoter: modulation by the human T-cell leukemia virus type I Tax protein. Mol Cell Biol. 1993 Jan;13(1):89–97. doi: 10.1128/mcb.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. Differentiation and proliferation in mouse embryonal carcinoma cells. Bioessays. 1992 Nov;14(11):769–775. doi: 10.1002/bies.950141109. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto Y., Fujimura Y., Matsumoto M., Tamai Y., Morita T., Matsushiro A., Nozaki M. The promoter of the endo A cytokeratin gene is activated by a 3' downstream enhancer. Nucleic Acids Res. 1991 May 25;19(10):2761–2765. doi: 10.1093/nar/19.10.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thorey I. S., Ceceña G., Reynolds W., Oshima R. G. Alu sequence involvement in transcriptional insulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Nov;13(11):6742–6751. doi: 10.1128/mcb.13.11.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G., Mader S., Gold J. D., Leid M., Lutz Y., Gaub M. P., Chambon P., Gudas L. The late retinoic acid induction of laminin B1 gene transcription involves RAR binding to the responsive element. EMBO J. 1991 May;10(5):1149–1158. doi: 10.1002/j.1460-2075.1991.tb08055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler-Guettler H., Yu K., Soff G., Gudas L. J., Rosenberg R. D. Thrombomodulin gene regulation by cAMP and retinoic acid in F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2155–2159. doi: 10.1073/pnas.89.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure at the SV40 major late promoter: melted and wrapped DNA flank the start site. Genes Dev. 1989 Nov;3(11):1814–1822. doi: 10.1101/gad.3.11.1814. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Pals C., Kruijer W. Transcriptional control of c-jun by retinoic acid. Nucleic Acids Res. 1991 Apr 11;19(7):1585–1591. doi: 10.1093/nar/19.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Klundert F. A., van Eldik G. J., Pieper F. R., Jansen H. J., Bloemendal H. Identification of two silencers flanking an AP-1 enhancer in the vimentin promoter. Gene. 1992 Dec 15;122(2):337–343. doi: 10.1016/0378-1119(92)90223-c. [DOI] [PubMed] [Google Scholar]