Abstract
During normal sympathetic nervous system (SNS) development, cells of the ganglionic lineage can malignantly transform and develop into the childhood tumor neuroblastoma. Hypoxia-inducible transcription factors (HIFs) mediate cellular responses during normal development and are central in the adaptation to oxygen shortage. HIFs are also implicated in the progression of several cancer forms, and high HIF-2α expression correlates with disseminated disease and poor outcome in neuroblastoma. During normal SNS development, HIF2A is transiently expressed in neuroblasts and chromaffin cells. SNS cells can, during development, be distinguished by distinct gene expression patterns, and insulin-like growth factor 2 (IGF2) is a marker of sympathetic chromaffin cells, whereas sympathetic neuroblasts lack IGF2 expression. Despite the neuronal derivation of neuroblastomas, we show that neuroblastoma cell lines and specimens express IGF2 and that expression of HIF2A and IGF2 correlates, with the strongest correlation in high-stage tumors. In neuroblastoma, both IGF2 and HIF2A are hypoxia-driven and knocking down IGF2 at hypoxia resulted in downregulated HIF2A levels. HIF-2α and IGF2 were strongly expressed in subsets of immature neuroblastoma cells, suggesting that these two genes could be co-expressed also at early stages of SNS development. We show that IGF2 is indeed expressed in sympathetic chain ganglia at embryonic week 6.5, a developmental stage when HIF-2α is present. These findings provide a rationale for the unexpected IGF2 expression in neuroblastomas and might suggest that IGF2 and HIF2A positive neuroblastoma cells are arrested at an embryonic differentiation stage corresponding to the stage when sympathetic chain ganglia begins to coalesce.
Introduction
Neural crest-derived sympathoadrenal precursor cells give rise to three different lineages during development: the ganglionic, adrenal/extraadrenal chromaffin, and small intensely fluorescent (SIF) cell lineages [1]. The childhood tumor neuroblastoma is derived from immature cells of the ganglionic sympathetic nervous system (SNS) lineage, and tumors can arise at any site where sympathetic neuroblasts are present during normal development [2]. Neuroblastomas are clinically subdivided into stages 1 to 4, where the stage 4 tumors present with disseminated disease and are generally high-risk tumors with poor prognosis. There is also a strong correlation between high-risk tumors and an immature tumor cell phenotype [3].
Neuroblastoma cells cultured under hypoxic conditions lose their differentiated phenotype and acquire stem cell-like features [4], presumably contributing to the general finding that hypoxic solid tumors are more aggressive [5,6]. In particular, high expression of hypoxia-inducible factor 2α (HIF-2α) protein correlates to poor prognosis and high clinical stage in neuroblastoma [7,8]. In addition, perivascularly located neuroblastoma cells intensely staining for HIF-2α have an immature stem cell-like phenotype [9]. HIF2A is selectively and transiently expressed during discrete periods of normal murine and human SNS development [10,11] and deletion of HIF2A in 129/SvJ mice leads to severe impairment of the development of SNS structures [11]. Interestingly, in paraganglioma, another SNS-derived tumor, somatic HIF2A gain-of-function mutations were recently described [12], suggesting a role of HIF-2 in SNS cell tumorigenesis. The HIF-2 transcription factor is part of the cellular adaptation machinery activated at oxygen shortage. Mammalian cells adapt to hypoxia by activating a transcriptional program mainly orchestrated by the dimeric HIF-1, HIF-2, and HIF-3. The HIF-α subunits are typically degraded at physiological oxygen concentrations but are stabilized at hypoxia and can then form transcriptionally active complexes [13,14]. Thus, HIFs are generally not considered to be regulated at the transcriptional level by oxygen shortage. However, here we show that hypoxia transcriptionally upregulates HIF2A in neuroblastoma cells.
HIF transcription is known to be regulated by growth factors in presumably a cell context-dependent manner [15]. In search for growth factors that are upregulated by hypoxia in neuroblastoma, insulin-like growth factor II (IGF-II) was one candidate, as it is frequently induced in perinecrotic and hypoxic areas of neuroblastoma specimens [16] and in neuroblastoma cells exposed to long-term hypoxia [4]. Here, we show that IGF2 and HIF2A mRNA expression is induced in neuroblastoma cells during hypoxic conditions. We further demonstrate a positive correlation between IGF2 and HIF2A expression in a clinical neuroblastoma material, and knocking down IGF2 expression in hypoxic neuroblastoma cells results in diminished HIF2A expression.
As IGF2 is a marker of sympathetic chromaffin and SIF cell differentiation and is not expressed in neuroblasts [2,16], we reinvestigated the expression of HIF-2α and IGF2 during normal human SNS development. We found that IGF2 indeed is expressed in embryonic (week 6.5) human sympathetic neuroblasts, a time point when HIF-2α expression still persists. This observation together with our in vitro data suggests that IGF-II regulates HIF2A expression in neuroblastoma and during discrete and short phases of SNS development.
Materials and Methods
Tissues and Cells
Human embryonic and fetal tissue was obtained at either elective (week 6.5 embryo) or spontaneous (week 12) abortions [Ethical approvals LU 389-98 (Lund University) and 93-216 (Karolinska Hospital, Stockholm, Sweden)]. Routinely fixed paraffin-embedded human neuroblastoma specimens were used (Ethical permit LU 389-98). Immunohistochemical staining and in situ hybridization were performed as described previously [2,16]. Antibodies used were mouse anti-HIF-2α (Novus Biologicals, Littleton, CO) and rabbit anti-tyrosine hydroxylase (TH; Abcam, Cambridge, United Kingdom). The human neuroblastoma cell lines SK-N-BE(2)c, IMR-32, and KCN-69n (kind gifts from Dr June Biedler, Memorial Sloan Kettering Cancer Institute and Dr Robert Ross, Fordham University) were cultured in minimal essential or RPMI 1640 (IMR-32) medium supplemented with FBS and antibiotics. As part of our laboratory routines, all cell lines were regularly screened for mycoplasma and replaced by low-passage cells on a trimonthly basis. Transfection of SK-N-BE(2)c cells was performed using three small interference RNAs (siRNAs) targeting IGF2 or a non-targeting control siRNA (all from Selleckchem, Munich, Germany) at concentrations of 50 nM with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as transfection reagent. KCN-69n cells were nucleofected with 2 µg of either siRNA using Amaxa Cell Line Nucleofector Kit V (Lonza, Köln, Germany) according to the manufacturer's instructions (Nucleofector Program A-023). Hypoxia was generated in a Whitley H35 Hypoxystation (Don Whitley Scientific, Shipley, United Kingdom).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) after homogenizing cells using the QIAshredder Kit (Qiagen) according to the manufacturer's recommendation. RNA was DNAse treated and washed (Millipore Amicon Ultra Centrifugal Filter Units). cDNA synthesis was performed using MultiScribe Reverse Transcriptase enzyme (Applied Biosystems, Foster City, CA) together with random primers. Quantitative polymerase chain reaction (PCR) analysis was performed in a 7300 Real-Time PCR System (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems), and the comparative Ct method to quantify relative mRNA was used. Expression levels of three housekeeping genes (YWHAZ, SDHA, and UBC) were used to normalize gene-of-interest expression [17]. Primer sequences are listed in Table W1.
Enzyme-Linked Immunosorbent Assay
Secreted (supernatant) and intracellular (whole-cell lysate) IGF-II protein was measured by ELISA (human IGF-II ELISA; Mediagnost, Reutlingen, Germany) according to the manufacturer's recommendation. Cells were cultured at 21% or 1% oxygen for 72 hours and supernatant was collected and kept at -20°C until ELISA was run. Samples were centrifuged at 1500 rpm before use to remove cell debris. For whole-cell lysates, cells were lysed in NP-40 buffer [150 mM NaCl, 50 mM Tris-Cl (pH 7.4), 1 mM EDTA (pH 8.0), 1% Igepal] supplemented with complete protease inhibitor (Roche, Indianapolis, IN), and whole protein content was determined using Bradford (Pierce, Rockford, IL). IGF-II protein concentrations were calculated using four-parameter non-linear regression.
Animal Procedures
Female athymic mice (NMRI-Nu/Nu strain; Taconic, Lille Skensved, Denmark) were housed in a controlled environment and all procedures were approved by the regional ethics committee for animal research (Approval No. M299-10). KCN-69n cells (1 x 105 - 8 x 105) were prepared in 30 µl of Matrigel/Hank's balanced salt solution (2:1). Mice were anesthetized by isoflurane inhalation. Surgical exposure of the left adrenal gland was achieved by temporal displacement of the spleen. Using a 29-ga. needle, cells were injected into the left adrenal gland fat pad and the skin was closed using surgical clips [18]. Mice were sacrificed 4 weeks after injection and tumors were snap-frozen for further analysis.
Statistical Analyses
All values presented in this study are reported as means ± SEM from three independent experiments. Two-sided Student's unpaired t test was used for statistical analyses (*P < .05, **P < .01, and ***P < .001). A publicly available data set containing 88 neuroblastomas [R2: microarray analysis and visualization platform (http://r2.amc.nl)] was used to analyze Pearson's correlation between IGF2 and HIF2A expression.
Results
HIF2A and IGF2 Expression Correlates in Neuroblastoma Cells and Tumor Specimens
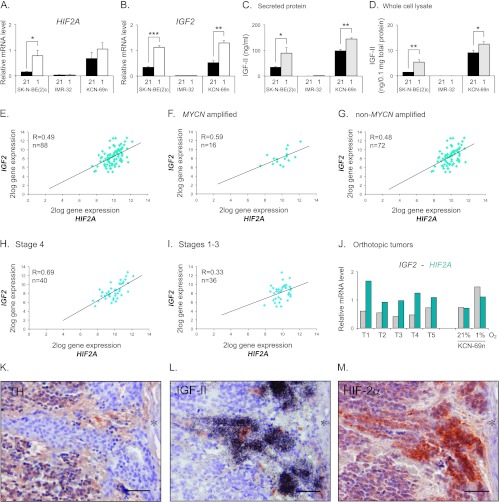
HIF2A is expressed in most neuroblastoma cell lines and mRNA levels are induced at hypoxia (1% O2) [7,19], as exemplified by SK-N-BE(2)c and KCN-69n cells (Figure 1A). The IMR-32 neuroblastoma cell line is an exception and lacks HIF2A mRNA and protein expression both at normoxia and hypoxia (Figure 1A and [4,20]). The mechanisms regulating normoxic and hypoxic HIF2A expression are poorly defined, but growth factors are known to be able to stimulate the expression and translation of both HIF1A and HIF2A (reviewed in [15]). A few of these factors are hypoxia-driven and IGF-II is one example in neuroblastoma. Interestingly, while the IGF2 expressing SK-N-BE(2)c and KCN-69n cells further increased their expression of IGF2 at hypoxia, IMR-32 cells had barely detectable expression independent of oxygen levels (Figure 1B). Thus, IMR-32 cells virtually lack expression of both HIF2A and IGF2. The expression pattern of IGF2 mRNA was reflected at the protein level as measured by ELISA (Figure 1, C and D). Notably, IMR-32 cells had extremely low (secreted protein) or even undetectable (cell lysates) IGF-II levels irrespective of oxygen pressure (Figure 1, C and D). The expression of HIF2A and IGF2 mRNA persisted when KCN-69n cells were grown as orthotopic xenografts (Figure 1J).
Figure 1.
HIF2A and IGF2 correlate and are co-expressed in neuroblastoma specimen. (A) Quantitative real-time-PCR (qRT-PCR) analysis of HIF2A mRNA expression in SK-N-BE(2)c, KCN-69n, and IMR-32 cells cultured for 72 hours at 21% or 1% oxygen. (B) qRT-PCR analysis of IGF2 mRNA expression in cells cultured for 72 hours at 21% or 1% oxygen. (C) Secreted IGF-II protein levels detected in conditioned medium by human IGF-II ELISA. (D) Intracellular IGF-II protein levels detected by human IGF-II ELISA. (A–D) Data are presented as means ± SEM from three independent experiments; P values were calculated using Student's t test (*P < .05, **P < .01, and ***P < .001). (E) HIF2A and IGF2 gene expression correlation in a data set containing 88 neuroblastoma tumors (Versteeg-88-MAS5.0-u133p2). (F) HIF2A and IGF2 gene expression correlation in the subgroup of MYCN-amplified tumors from the data set used in D. (G) HIF2A and IGF2 gene expression correlation in the subgroup of non-MYCN-amplified tumors from the data set used in D. (H) HIF2A and IGF2 gene expression correlation in the subgroup of International Neuroblastoma Staging System (INSS) stage 4 tumors from the data set used in D. (I) HIF2A and IGF2 gene expression correlation in the joint subgroup of INSS stage 1 to 3 tumors from the data set used in D. (E–I) R value was calculated using Pearson correlation and n is equal to the number of tumors in each subset. (J) Levels of IGF2 and HIF2A mRNA expression in orthotopic neuroblastoma tumors as measured by qRT-PCR. KCN-69n neuroblastoma cells were grown at 21% O2 for 24 hours before transplantation into the adrenal gland fat pad of NMRI-Nu/Nu mice. Tumors were excised 4 weeks after cell implantation. Gene expression levels in KCN-69n cells cultured in vitro at 21% or 1% O2 for 72 hours were used as comparison. (K–M) Immunohistochemical TH (K) and HIF-2α (M) staining and IGF-II in situ hybridization (L) of a neuroblastoma specimen. Asterisk indicates a blood vessel. Scale bars represent 50 µm.
Mining a publicly available clinical neuroblastoma expression data set [R2: microarray analysis and visualization platform (http://r2.amc.nl)] further revealed a strong correlation between IGF2 and HIF2A expression in a clinical material of 88 well-defined neuroblastoma specimens (R = 0.49; Figure 1E). In this material, 87 of 88 tumors presented with an IGF2 signal, while all 88 tumors had robust HIF2A expression. In light of the recent report by Qing et al. [21], proposing that HIF2A is not expressed in MYCN-amplified neuroblastoma cells, we subdivided the expression data into an MYCN-amplified and anon-MYCN-amplified group. As shown in Figure 1, F and G, MYCN-amplified tumors express HIF2A and the correlation between IGF2 and HIF2A expression persists and is even stronger in MYCN-amplified compared to non-amplified tumors (R = 0.59 and R = 0.48, respectively). To test the potential association between HIF2A and IGF2 mRNA expression and aggressive disease, the data set was divided into stage 4 versus stage 1 to 3 tumors. This revealed a strong correlation between HIF2A and IGF2 expression in stage 4 tumors (R = 0.69; Figure 1H), while the correlation was substantially weaker in stage 1 to 3 tumors (R = 0.33; Figure 1I).
Collectively, our data indicate that the expression of IGF2 and HIF2A is interrelated. This is further supported by our previous observation [9] that human neuroblastoma tumors often contain immature, stem cell-like neuroblastoma cells that have high HIF-2α as well as high IGF2 expression as exemplified in Figure 1, L and M. These cells typically lack expression of neuronal sympathetic differentiation marker genes such as TH (Figure 1K) and neuron-specific enolase and are often located in perivascular niches [9]. These and published in vivo data [10,11] further indicate that HIF-2α protein in developing and malignant sympathetic structures is not efficiently degraded under oxygenated conditions.
HIF2A Is Transcriptionally Regulated by IGF-II in Neuroblastoma Cells
HIF-2 is a transcription factor normally stabilized and activated under hypoxic conditions, but in vivo data suggest that HIF-2α, the oxygen sensitive and degradable HIF-2 subunit, might be active also in contexts of physiological oxygen tensions [4,7,10]. Under in vivo conditions, HIF2A expression is distinctly and transiently regulated during SNS development. To test if IGF-II could be one factor regulating the transcription of HIF2A in neuroblastoma cells, IGF2 expression was downregulated using three different siRNAs (Figure 2, A and B). At normoxia, a slight decrease of HIF2A mRNA was noted, whereas expression of the HIF-2α-driven gene SERPINB9 was virtually unaffected by IGF2 knockdown (Figure 2A). At hypoxia however, HIF2A and SERPINB9 expression significantly decreased in the IGF2 knocked down cells (Figure 2B), indicating that IGF-II regulates HIF2A expression at conditions of low oxygen pressures.
Figure 2.
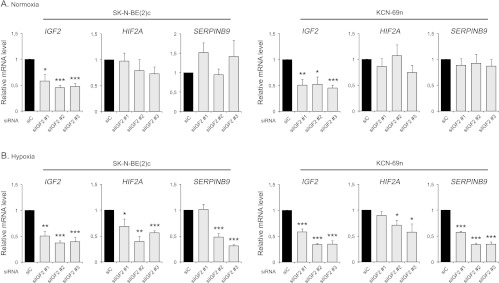
IGF-II regulates HIF2A mRNA expression at hypoxia. (A) Expression of HIF2A and HIF-2-driven gene SERPINB9 mRNA after down-regulation of IGF2 in SK-N-BE(2)c and KCN-69n cells using three different siRNAs targeting the IGF2 gene at normoxia (21% O2), as measured by qRT-PCR. (B) Expression of HIF2A and SERPINB9 mRNA after down-regulation of IGF2 in SK-N-BE(2)c and KCN-69n cells using three different siRNAs targeting the IGF2 gene at hypoxia (1% O2), as measured by qRT-PCR. Data are presented as means ± SEM from three independent experiments, and mRNA expression after IGF2 knockdown is normalized against siC within each experiment. Statistical significance is calculated compared to siC using Student's t test (*P < .05, **P < .01, and ***P < .001).
HIF-2α and IGF2 Are Co-expressed during Normal Human Embryonic SNS Development
IGF2 is abundantly expressed during human embryogenesis and fetal development. However, in the developing SNS, fetal neuroblasts (defined by, for instance, GAP43 and SCG10 expression; Figure 3, A and B and [2]) of SNS ganglia proper and the adrenal gland do not express IGF2 (Figure 3, A and B). In contrast, IGF2 is highly expressed in chromaffin cells of the fetal sympathetic paraganglia and adrenal gland (Figure 3, A and B, respectively) and in SIF cells [16]. Neuroblastoma cells derive from immature or precursor cells of the SNS ganglionic lineage and should not express IGF2. Hence, coexpression of IGF2 and HIF-2α in a subpopulation of immature neuroblastoma cells in vivo and in neuroblastoma cell lines is in apparent disagreement with an IGF2 negative ganglionic phenotype of the developing SNS.
Figure 3.
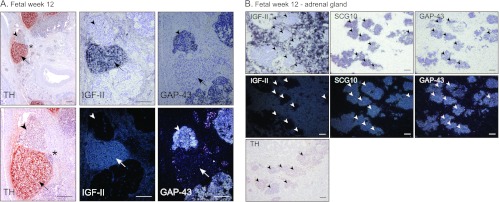
IGF-II expression is absent in human fetal sympathetic ganglia. (A) Immunohistochemical staining of TH and in situ hybridization of IGF-II and GAP43 (bright field and dark field) in human fetus at week 12. Arrowheads indicate sympathetic ganglia; arrows indicate paraganglia. *Area of magnification. Scale bars represent 200 µm. (B) IGF-II, SCG10, and GAP43 in situ hybridization (bright field and dark field) and TH immunostaining in a 12-week-old fetal adrenal gland. Arrowheads indicate sympathetic ganglia. Scale bars represent 100 µm.
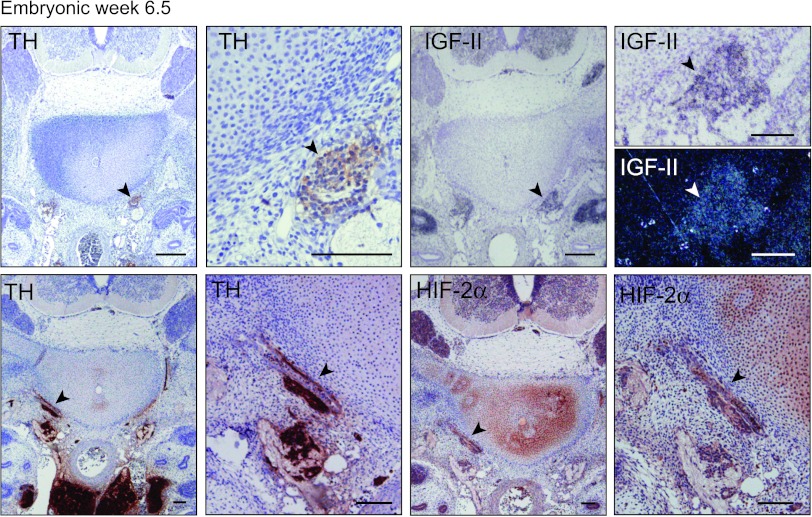
In mouse, HIF2A/Epas1 is expressed in SNS neuroblasts during early stages (E10.5–12.5) of murine development [11], corresponding to human embryonic weeks 4 to 6.5 [22]. Staining of a cross section of the trunk region of a week 6.5 human embryo (the earliest human embryonic tissue we have access to) revealed a faint HIF-2α signal in the SNS chain ganglia defined by location and TH positivity (Figure 4). Interestingly, at this developmental stage, the SNS chain ganglia were IGF2 positive as well (Figure 4). Thus, in keeping with the co-expression of these two genes in immature neuroblastoma cells, both IGF2 and HIF-2α are expressed in neuroblasts at the 6.5-week stage. Later during human development, HIF-2α expression disappears in neuroblasts and IGF2 expression becomes restricted to chromaffin cells (Figures 3, A and B, and 5 and [2,11,16]). In fetal sympathetic paraganglia, however, HIF-2α expression recurs transiently under conditions when IGF2 is persistently expressed (Figures 3, A and B, and 5 and [10,11]).
Figure 4.
Embryonic neuroblasts express HIF-2α and IGF-II. Immunohistochemical staining of TH and HIF-2α and in situ hybridization of IGF-II (bright field and dark field) in a week 6.5 human embryo at different magnifications. Arrowheads indicate sympathetic ganglia. Scale bars represent 100 µm.
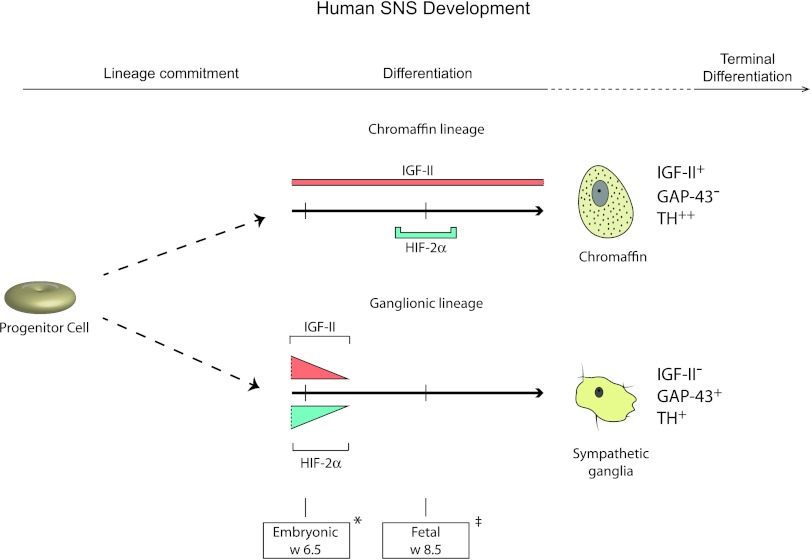
Figure 5.
HIF-2α and IGF-II are co-expressed in sympathetic neuroblasts during early human development. Sympathoadrenal progenitor cells commit to either of three lineages, the sympathetic ganglionic, chromaffin, or SIF (not shown) cell lineages. IGF-II is expressed in both chromaffin and sympathetic ganglionic cells during late embryogenesis, but expression becomes restricted to cells of the chromaffin lineage during fetal development. HIF-2α can be detected in sympathetic neuroblasts of the ganglionic lineage only during late embryo-genesis, whereas it is transiently expressed around fetal week 8.5 in chromaffin cells. Hence, IGF-II and HIF-2α are co-expressed at early developmental stages before sympathetic ganglionic cells lose expression of both proteins. Marker gene expression for chromaffin cells (IGF-II+, GAP-43-,TH++) and sympathetic ganglionic cells (IGF-II-, GAP-43+,TH+) as depicted. Two distinct time points are shown: *embryonic week 6.5 and ‡fetal week 8.5.
Discussion
A cornerstone in surgical pathology is the concept that differentiation stage and phenotype of a given subset of tumor cells are reflections of the tissue and cell type that these tumor cells are derived from. Neuroblastoma is one example of this practice [23]. In old literature, this tumor is also denoted sympathicoblastoma [24], and on the basis of morphology, tumor location, and tumor cell phenotype, neuroblastomas are generally considered to derive from sympathetic neuroblasts or their precursors. In previous work, we defined a set of marker genes for the ganglionic (neuroblasts), chromaffin (paraganglionic and adrenal chromaffin cells), and SIF cell differentiation lineages of the SNS [2]. A key marker in this scheme is IGF2, which during human development is expressed in the chromaffin and SIF cells but not in the sympathetic neuroblasts [16]. Unless one argues that IGF2 is aberrantly expressed as a consequence of malignant transformation, expression of IGF2 in neuroblastoma cells apparently contradicts a derivation from immature neuroblasts. In this report, we present data supporting an alternative model in which IGF2 is expressed in sympathetic chain ganglia neuroblasts at an early developmental stage (Figure 5). The IGF2 expression in neuroblastoma cells can thus be explained by them being arrested at such early stages of differentiation. Our data further show that endogenous IGF-II drives expression of HIF2A in neuroblastoma cells and possibly also in embryonic SNS neuroblasts during human SNS development (Figure 5). We have previously shown that high HIF-2α protein levels are linked to unfavorable outcome in neuroblastoma. Here, we demonstrate that expression of HIF2A and IGF2 in neuroblastoma tumors correlates and that this correlation is even stronger in stage 4 and MYCN-amplified tumors, the most aggressive neuroblastoma subtypes.
The decreased HIF2A expression seen most overtly in hypoxic cells with downregulated IGF2 suggests that endogenously produced IGF-II activates an autocrine stimulation mechanism leading to enhanced HIF2A transcription. These in vitro observations are corroborated by the strong correlation between HIF2A and IGF2 expression in a well-defined clinical neuroblastoma material, by the co-expression of these two genes in immature neuroblastoma cells in vivo and in neuroblasts of 6.5-week-old human embryonic SNS chain ganglia. Thus, co-expression of HIF2A and IGF2 in neuroblastoma cells appears to be associated with the immature, stem cell-like phenotype previously described in neuroblastoma specimens and in cultured hypoxic neuroblastoma cells [4,9].
Numerous studies have demonstrated that HIF-2α, like HIF-1α, protein becomes efficiently degraded in cells cultured at normoxia. The prevailing model is that the transcriptional activity of HIF-1 and HIF-2 is mainly dependent on the availability of the oxygen-sensitive α subunits. Less well documented are the molecular mechanisms regulating the abundant expression of HIF2A mRNA and protein in ganglia and paraganglia during discrete and transient periods of SNS development [4,10,11]. Emerging data highlight the fact that HIF2A expression is distinctly regulated during normal development and suggest that the HIF-2α protein does not become instantly degraded under these conditions. One explanation could be that the HIF-2α positive SNS structures are hypoxic. However, the HIF-1α protein, which appears to be a more specific marker of hypoxic cells than HIF-2α, is not detected within these HIF-2α positive regions [10]. A similar situation seems to exist in neuroblastoma specimens where a small subset of immature tumor cells located at proximity to blood vessels expresses high levels of HIF-2α but not HIF-1α [9]. In vitro, HIF2A mRNA levels are only modestly affected by IGF2 knockdown at normoxia. This finding indicates that additional mechanisms regulate basal HIF2A expression in oxygenated neuroblastoma cells as well as during the discrete periods of normal SNS development when the HIF2A gene is expressed. The specific mechanisms that allow the HIF-2α protein to escape degradation under non-hypoxic conditions are yet unknown, but understanding such mechanisms might facilitate the targeting of HIF-2 transcription for therapeutic purposes. Our data suggest that interfering with the IGF-II signaling pathway could be one strategy for targeting the HIF-2α- or pseudohypoxia-driven aggressive phenotype in neuroblastoma.
Supplementary Material
Abbreviations
- HIF
hypoxia-inducible factor
- IGF
insulin-like growth factor
- SIF
small intensely fluorescent
- SNS
sympathetic nervous system
Footnotes
This work was supported by the Swedish Cancer Society, the Children's Cancer Foundation of Sweden, the Swedish Research Council, the Stiftelsen för Strategisk Forskning Strategic Center for Translational Cancer Research (CREATE Health), VINNOVA, BioCARE, a Strategic Research Program at Lund University, Hans von Kantzows Stiftelse, Gunnar Nilsson's Cancer Foundation, and the research funds of Malmö University Hospital. The authors declare no conflict of interest.
This article refers to supplementary material, which is designated by Table W1 and is available online at www.neoplasia.com.
References
- 1.Anderson DJ. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- 2.Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Påhlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors' progress along an extra-adrenal chromaffin lineage. Lab Invest. 1996;75:659–675. [PubMed] [Google Scholar]
- 3.Fredlund E, Ringner M, Maris JM, Påhlman S. High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci USA. 2008;105:14094–14099. doi: 10.1073/pnas.0804455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jögi A, Øra I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Påhlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 6.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg Å, Gradin K, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Noguera R, Fredlund E, Piqueras M, Pietras A, Beckman S, Navarro S, Påhlman S. HIF-1α and HIF-2α are differentially regulated in vivo in neuroblastoma: high HIF-1α correlates negatively to advanced clinical stage and tumor vascularization. Clin Cancer Res. 2009;15:7130–7136. doi: 10.1158/1078-0432.CCR-09-0223. [DOI] [PubMed] [Google Scholar]
- 9.Pietras A, Gisselsson D, Øra I, Noguera R, Beckman S, Navarro S, Påhlman S. High levels of HIF-2α highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson H, Jögi A, Beckman S, Harris AL, Poellinger L, Påhlman S. HIF-2α expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res. 2005;303:447–456. doi: 10.1016/j.yexcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 15.Löfstedt T, Fredlund E, Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L, Påhlman S. Hypoxia inducible factor-2α in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 16.Hedborg F, Ohlsson R, Sandstedt B, Grimelius L, Hoehner JC, Påhlman S. IGF2 expression is a marker for paraganglionic/SIF cell differentiation in neuroblastoma. Am J Pathol. 1995;146:833–847. [PMC free article] [PubMed] [Google Scholar]
- 17.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna C, Jaboin JJ, Drakos E, Tsokos M, Thiele CJ. Biologically relevant orthotopic neuroblastoma xenograft models: primary adrenal tumor growth and spontaneous distant metastasis. In Vivo. 2002;16:77–85. [PubMed] [Google Scholar]
- 19.Fredlund E, Ovenberger M, Borg K, Påhlman S. Transcriptional adaptation of neuroblastoma cells to hypoxia. Biochem Biophys Res Commun. 2008;366:1054–1060. doi: 10.1016/j.bbrc.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 20.Lin Q, Cong X, Yun Z. Differential hypoxic regulation of hypoxia-inducible factors 1α and 2α. Mol Cancer Res. 2011;9:757–765. doi: 10.1158/1541-7786.MCR-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qing G, Skuli N, Mayes PA, Pawel B, Martinez D, Maris JM, Simon MC. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1α. Cancer Res. 2010;70:10351–10361. doi: 10.1158/0008-5472.CAN-10-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strachan T, Lindsay S, Wilson DI. Molecular Genetics of Early Human Development. Oxford, United Kingdom: BIOS Scientific Publishers Ltd; 1997. [Google Scholar]
- 23.Hoehner JC, Hedborg F, Eriksson L, Sandstedt B, Grimelius L, Olsen L, Pahlman S. Developmental gene expression of sympathetic nervous system tumors reflects their histogenesis. Lab Invest. 1998;78:29–45. [PubMed] [Google Scholar]
- 24.Cushing H, Wolbach SB. The transformation of a malignant para-vertebral sympathicoblastoma into a benign ganglioneuroma. Am J Pathol. 1927;3:203–216. 7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.