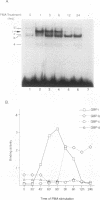
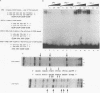
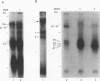
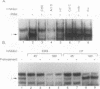
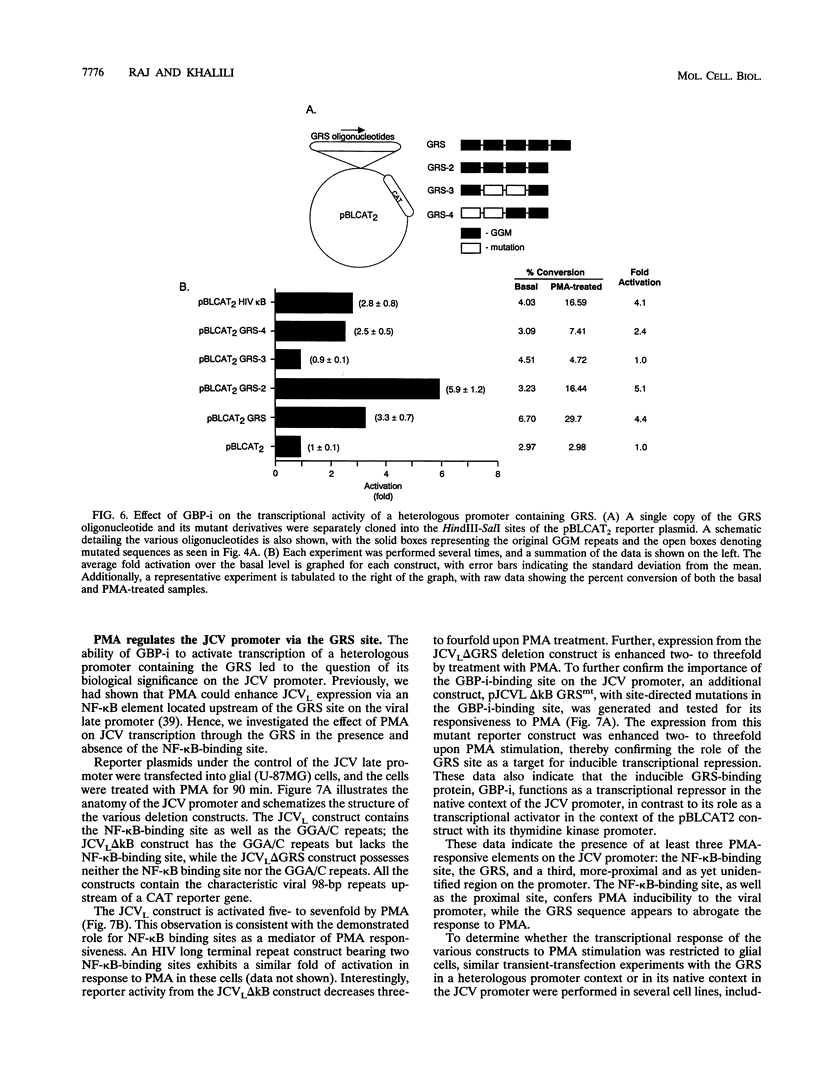
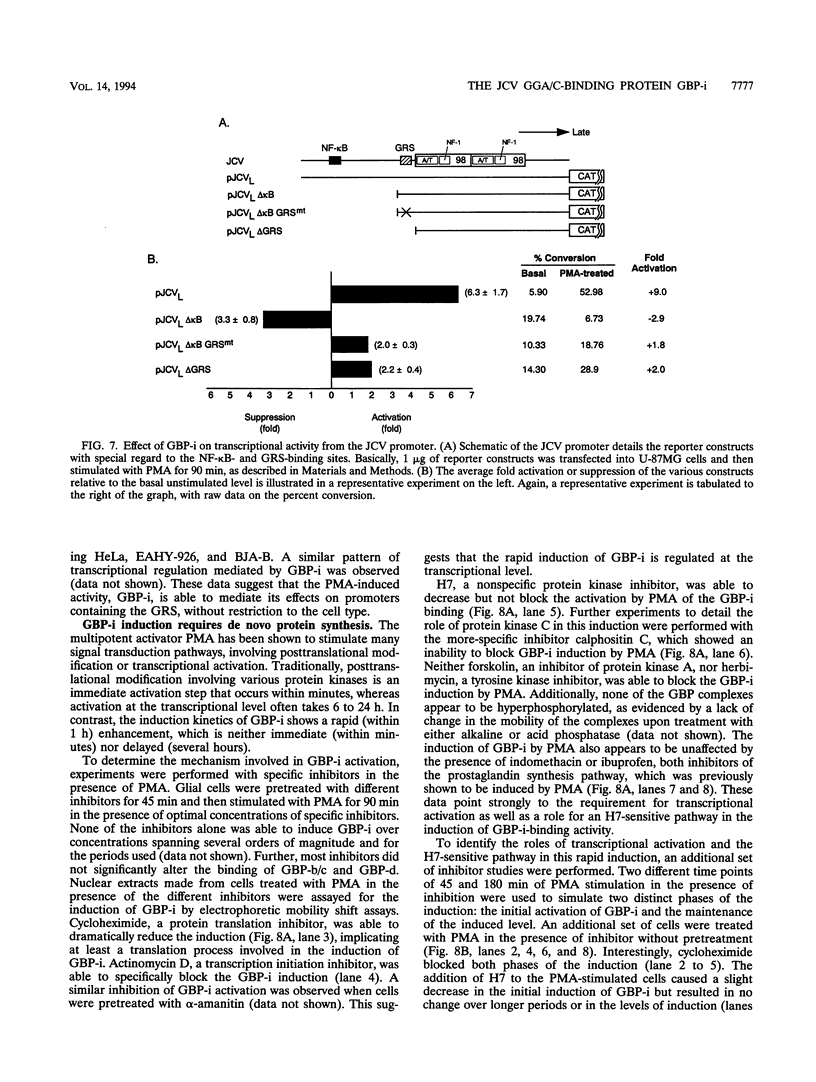
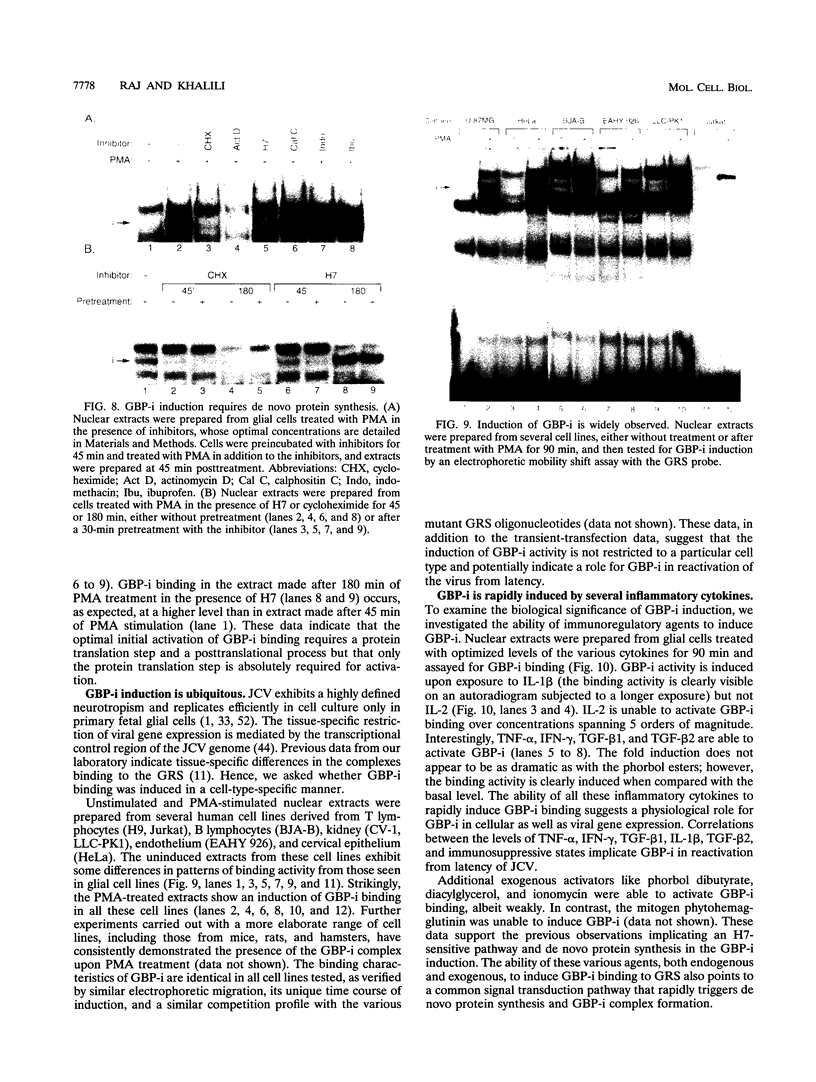
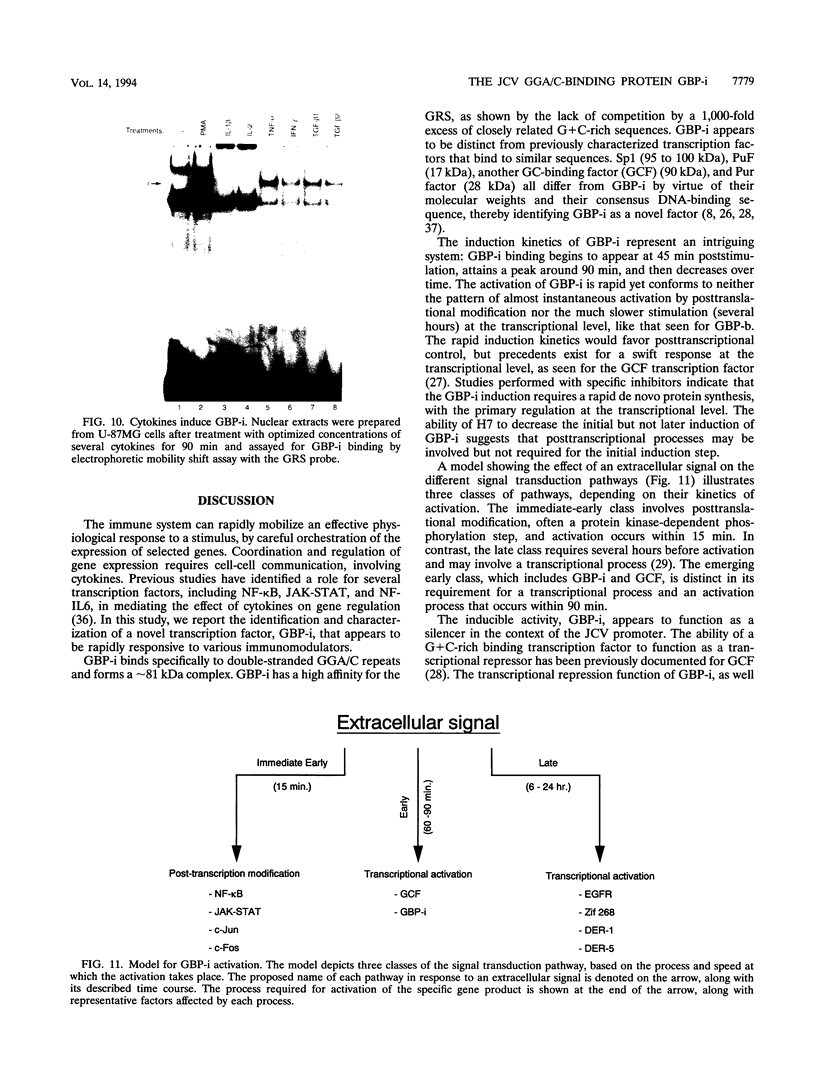
Abstract
Immunosuppressive states with accompanying alterations in cytokine profiles have been postulated to play a vital role in the reactivation of viruses from latency. Cytokines regulate gene expression by activating transcription factors via well-characterized signal transduction pathways. In this study, we report the identification of a novel inducible protein, GBP-i, that binds to a double-stranded GGA/C-rich region of the transcriptional control region of the human papovavirus JC virus (JCV), specifically within the origin of viral DNA replication. GBP-i is distinct from previously characterized GC-box-binding proteins with respect to both its sequence specificity and its electrophoretic mobility on native and denaturing gels. GBP-i responds within 90 min to phorbol myristate acetate stimulation; however, unlike typical phorbol myristate acetate-inducible factors, this rapid induction is regulated primarily at the transcriptional level. Further, the induction of GBP-i appears to be widespread and mediated by many inflammatory cytokines, including interleukin-1 beta, tumor necrosis factor alpha, gamma interferon, and transforming growth factor beta. Interestingly, the induced protein acts as a transcriptional repressor in its native context in the JCVL promoter. However, when its binding sequence is transposed to a heterologous promoter, GBP-i appears to function as a transcriptional activator. The data presented here suggest a role for GBP-i in cytokine-mediated induction of viral and cellular genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTROM K. E., MANCALL E. L., RICHARDSON E. P., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958 Mar;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Aksamit A. J., Gendelman H. E., Orenstein J. M., Pezeshkpour G. H. AIDS-associated progressive multifocal leukoencephalopathy (PML): comparison to non-AIDS PML with in situ hybridization and immunohistochemistry. Neurology. 1990 Jul;40(7):1073–1078. doi: 10.1212/wnl.40.7.1073. [DOI] [PubMed] [Google Scholar]
- Atwood W. J., Berger J. R., Kaderman R., Tornatore C. S., Major E. O. Human immunodeficiency virus type 1 infection of the brain. Clin Microbiol Rev. 1993 Oct;6(4):339–366. doi: 10.1128/cmr.6.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Johnson E. M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992 Mar;12(3):1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. R., Kaszovitz B., Post M. J., Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987 Jul;107(1):78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- Buonaguro L., Barillari G., Chang H. K., Bohan C. A., Kao V., Morgan R., Gallo R. C., Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992 Dec;66(12):7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M., Kundu M., Khalili K. GA/GC-rich sequence confers Tat responsiveness to human neurotropic virus promoter, JCVL, in cells derived from central nervous system. Oncogene. 1993 Apr;8(4):887–892. [PubMed] [Google Scholar]
- Chowdhury M., Taylor J. P., Chang C. F., Rappaport J., Khalili K. Evidence that a sequence similar to TAR is important for induction of the JC virus late promoter by human immunodeficiency virus type 1 Tat. J Virol. 1992 Dec;66(12):7355–7361. doi: 10.1128/jvi.66.12.7355-7361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M., Taylor J. P., Tada H., Rappaport J., Wong-Staal F., Amini S., Khalili K. Regulation of the human neurotropic virus promoter by JCV-T antigen and HIV-1 tat protein. Oncogene. 1990 Dec;5(12):1737–1742. [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp C., Taylor J. P., Khalili K., Amini S. Evidence for stimulation of the transforming growth factor beta 1 promoter by HIV-1 Tat in cells derived from CNS. Oncogene. 1993 Aug;8(8):2231–2236. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First annual meeting of the Society for Experimental Neuropathology. October 1, 1988, Philadelphia, PA. Abstracts. Ann Neurol. 1988 Sep;24(3):471–482. doi: 10.1002/ana.410240330. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P., Frei K., Rordorf C., Lazdins J., Tavolato B., Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989 Jul;23(2):109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., MacKenzie E. F., Smith C., Porter A. A. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984 May;37(5):578–586. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Herschbach B. M., Johnson A. D. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- Houff S. A., Major E. O., Katz D. A., Kufta C. V., Sever J. L., Pittaluga S., Roberts J. R., Gitt J., Saini N., Lux W. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988 Feb 4;318(5):301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- Janson L., Bark C., Pettersson U. Identification of proteins interacting with the enhancer of human U2 small nuclear RNA genes. Nucleic Acids Res. 1987 Jul 10;15(13):4997–5016. doi: 10.1093/nar/15.13.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. C., Kageyama R., Popescu N. C., Pastan I. Expression and chromosomal localization of the gene for the human transcriptional repressor GCF. J Biol Chem. 1992 Jan 25;267(3):1689–1694. [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Tornatore C. S., Houff S. A., Berger J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992 Jan;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Kurata H., Tajima M., Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Ann Neurol. 1991 Apr;29(4):428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. Cytokine modulation of HIV expression. Semin Immunol. 1993 Jun;5(3):165–173. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Berberich S. J., Flint S. J., Ferrone C. A. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science. 1993 Jul 23;261(5120):478–480. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Ranganathan P. N., Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993 Apr 25;21(8):1959–1964. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Sturm R., Baumruker T., Franza B. R., Jr, Herr W. A 100-kD HeLa cell octamer binding protein (OBP100) interacts differently with two separate octamer-related sequences within the SV40 enhancer. Genes Dev. 1987 Dec;1(10):1147–1160. doi: 10.1101/gad.1.10.1147. [DOI] [PubMed] [Google Scholar]
- Tada H., Lashgari M., Rappaport J., Khalili K. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J Virol. 1989 Jan;63(1):463–466. doi: 10.1128/jvi.63.1.463-466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Rappaport J., Lashgari M., Amini S., Wong-Staal F., Khalili K. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3479–3483. doi: 10.1073/pnas.87.9.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Kajioka J., Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26(11):1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. P., Pomerantz R. J., Raj G. V., Kashanchi F., Brady J. N., Amini S., Khalili K. Central nervous system-derived cells express a kappa B-binding activity that enhances human immunodeficiency virus type 1 transcription in vitro and facilitates TAR-independent transactivation by Tat. J Virol. 1994 Jun;68(6):3971–3981. doi: 10.1128/jvi.68.6.3971-3981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C., Berger J. R., Houff S. A., Curfman B., Meyers K., Winfield D., Major E. O. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992 Apr;31(4):454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Nath A., Amemiya K., Major E. O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991 Nov;65(11):6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux R., Cumont M., Girard P. M., Nassif X., Trotot P., Marche C., Matthiessen L., Vedrenne C., Mikol J., Henin D. Severe encephalitis resulting from coinfections with HIV and JC virus. Neurology. 1990 Jun;40(6):944–948. doi: 10.1212/wnl.40.6.944. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L. The epidemiology of human polyomaviruses. Prog Clin Biol Res. 1983;105:99–106. [PubMed] [Google Scholar]
- Wroblewska Z., Wellish M., Gilden D. Growth of JC virus in adult human brain cell cultures. Arch Virol. 1980;65(2):141–148. doi: 10.1007/BF01317325. [DOI] [PubMed] [Google Scholar]