Abstract
Tyrosinase is a rate-limiting enzyme in melanin biosynthesis and is specifically expressed in differentiated melanocytes. We have identified the enhancer element in the 5'-flanking region of the human tyrosinase gene that is responsible for its pigment cell-specific transcription and have termed it tyrosinase distal element (TDE) (positions -1861 to -1842). Transient expression assays showed that TDE confers efficient expression of a firefly luciferase reporter gene linked to the tyrosinase gene promoter in MeWo pigmented melanoma cells but not in HeLa cells, which do not express tyrosinase. TDE was specifically bound by nuclear proteins of MeWo and HeLa cells, the binding properties of which were indistinguishable in gel mobility shift assays. TDE contains the CATGTG motif in its center, and mutation analysis indicates that the CA dinucleotides of this motif are crucial for protein binding and pigment cell-specific enhancer function. The CATGTG motif is consistent with the consensus sequence recognized by a large family of transcription factors with a basic helix-loop-helix structure, which prompted us to examine the possible involvement of a ubiquitous transcription factor, USF, and a novel factor, microphthalmia-associated transcription factor (MITF), recently cloned as the human homolog of the mouse microphthalmia (mi) gene product. The mi phenotype is associated with a mutant mi locus and characterized by small eyes and loss of melanin pigments. Both USF and MITF are predicted to contain a basic helix-loop-helix structure and a leucine zipper structure. We provide evidence that USF binds to TDE, whereas we were unable to detect the DNA-binding activity of MITF. Transient coexpression assays showed that MITF specifically transactivates the promoter activity of the tyrosinase gene through the CATGTG motif of TDE but not the promoter of the ubiquitously expressed heme oxygenase gene, while USF is able to activate both promoters. These results indicate that MITF is a cell-type-specific factor that is capable of activating transcription of the tyrosinase gene.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton D. E., Kwon B. S., Francke U. Human tyrosinase gene, mapped to chromosome 11 (q14----q21), defines second region of homology with mouse chromosome 7. Genomics. 1988 Jul;3(1):17–24. doi: 10.1016/0888-7543(88)90153-x. [DOI] [PubMed] [Google Scholar]
- Bean M. A., Bloom B. R., Herberman R. B., Old L. J., Oettgen H. F., Klein G., Terry W. D. Cell-mediated cytotoxicity for bladder carcinoma: evaluation of a workshop. Cancer Res. 1975 Oct;35(10):2902–2913. [PubMed] [Google Scholar]
- Bouchard B., Del Marmol V., Jackson I. J., Cherif D., Dubertret L. Molecular characterization of a human tyrosinase-related-protein-2 cDNA. Patterns of expression in melanocytic cells. Eur J Biochem. 1994 Jan 15;219(1-2):127–134. doi: 10.1111/j.1432-1033.1994.tb19922.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. The major late transcription factor binds to and activates the mouse metallothionein I promoter. Genes Dev. 1987 Nov;1(9):973–980. doi: 10.1101/gad.1.9.973. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Morgan J. G., Crabtree G. R., Sharp P. A. The adenovirus major late transcription factor activates the rat gamma-fibrinogen promoter. Science. 1987 Oct 30;238(4827):684–688. doi: 10.1126/science.3672119. [DOI] [PubMed] [Google Scholar]
- Cohen T., Muller R. M., Tomita Y., Shibahara S. Nucleotide sequence of the cDNA encoding human tyrosinase-related protein. Nucleic Acids Res. 1990 May 11;18(9):2807–2808. doi: 10.1093/nar/18.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Hasumi K., Sakamoto G., Sugano H., Kasuga T., Masubuchi K. Primary malignant melanoma of the vagina: study of four autopsy cases with ultrastructural findings. Cancer. 1978 Dec;42(6):2675–2686. doi: 10.1002/1097-0142(197812)42:6<2675::aid-cncr2820420624>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902–2909. [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrímsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul 30;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hughes M. J., Lingrel J. B., Krakowsky J. M., Anderson K. P. A helix-loop-helix transcription factor-like gene is located at the mi locus. J Biol Chem. 1993 Oct 5;268(28):20687–20690. [PubMed] [Google Scholar]
- Inoue S., Ito S., Imai Y., Kasuga T., Fujita K. Growth inhibition of melanoma cells by N-protected dopa derivatives. Biochem Pharmacol. 1987 Oct 15;36(20):3537–3540. doi: 10.1016/0006-2952(87)90340-6. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Cervantes C., Solano F., Kobayashi T., Urabe K., Hearing V. J., Lozano J. A., García-Borrón J. C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J Biol Chem. 1994 Jul 8;269(27):17993–18000. [PubMed] [Google Scholar]
- Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993 Jan;4(1):49–55. [PubMed] [Google Scholar]
- Kikuchi H., Miura H., Yamamoto H., Takeuchi T., Dei T., Watanabe M. Characteristic sequences in the upstream region of the human tyrosinase gene. Biochim Biophys Acta. 1989 Dec 22;1009(3):283–286. doi: 10.1016/0167-4781(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Klüppel M., Beermann F., Ruppert S., Schmid E., Hummler E., Schütz G. The mouse tyrosinase promoter is sufficient for expression in melanocytes and in the pigmented epithelium of the retina. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3777–3781. doi: 10.1073/pnas.88.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaigre F. P., Courtois S. J., Lafontaine D. A., Rousseau G. G. Evidence that the upstream stimulatory factor and the Sp1 transcription factor bind in vitro to the promoter of the human-growth-hormone gene. Eur J Biochem. 1989 May 15;181(3):555–561. doi: 10.1111/j.1432-1033.1989.tb14760.x. [DOI] [PubMed] [Google Scholar]
- Lowings P., Yavuzer U., Goding C. R. Positive and negative elements regulate a melanocyte-specific promoter. Mol Cell Biol. 1992 Aug;12(8):3653–3662. doi: 10.1128/mcb.12.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraosa Y., Shibahara S. Identification of a cis-regulatory element and putative trans-acting factors responsible for 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated induction of heme oxygenase expression in myelomonocytic cell lines. Mol Cell Biol. 1993 Dec;13(12):7881–7891. doi: 10.1128/mcb.13.12.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Roeder R. G. Recombinant 43-kDa USF binds to DNA and activates transcription in a manner indistinguishable from that of natural 43/44-kDa USF. Mol Cell Biol. 1991 Oct;11(10):5125–5136. doi: 10.1128/mcb.11.10.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnazhagan S., Hou L., Kwon B. S. Structural organization of the human tyrosinase gene and sequence analysis and characterization of its promoter region. J Invest Dermatol. 1994 May;102(5):744–748. doi: 10.1111/1523-1747.ep12376924. [DOI] [PubMed] [Google Scholar]
- Ponnazhagan S., Kwon B. S. A cis-acting element involved in mouse tyrosinase gene expression and partial purification of its binding protein. Pigment Cell Res. 1992 Oct;5(4):155–161. doi: 10.1111/j.1600-0749.1992.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Potter J. J., Cheneval D., Dang C. V., Resar L. M., Mezey E., Yang V. W. The upstream stimulatory factor binds to and activates the promoter of the rat class I alcohol dehydrogenase gene. J Biol Chem. 1991 Aug 15;266(23):15457–15463. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Ishizawa S., Yoshida T., Shibahara S. Interaction of upstream stimulatory factor with the human heme oxygenase gene promoter. Eur J Biochem. 1990 Mar 10;188(2):231–237. doi: 10.1111/j.1432-1033.1990.tb15394.x. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Kaulen H., Roeder R. G. Positive and negative regulation of the gene for transcription factor IIIA in Xenopus laevis oocytes. Genes Dev. 1989 May;3(5):651–662. doi: 10.1101/gad.3.5.651. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Sato M., Muller R. M., Yoshida T. Structural organization of the human heme oxygenase gene and the function of its promoter. Eur J Biochem. 1989 Feb 15;179(3):557–563. doi: 10.1111/j.1432-1033.1989.tb14583.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Taguchi H., Muller R. M., Shibata K., Cohen T., Tomita Y., Tagami H. Structural organization of the pigment cell-specific gene located at the brown locus in mouse. Its promoter activity and alternatively spliced transcript. J Biol Chem. 1991 Aug 25;266(24):15895–15901. [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Tagami H., Müller R. M., Cohen T. Molecular basis for the heterogeneity of human tyrosinase. Tohoku J Exp Med. 1988 Dec;156(4):403–414. doi: 10.1620/tjem.156.403. [DOI] [PubMed] [Google Scholar]
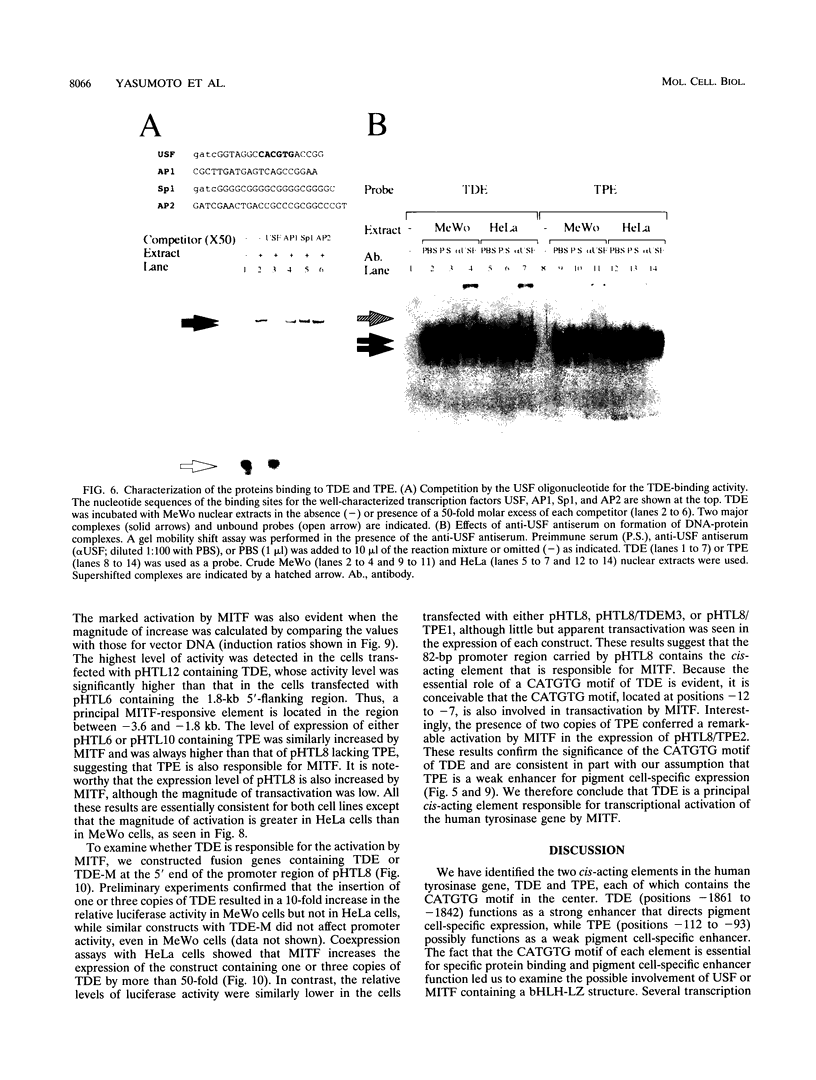
- Shibata K., Muraosa Y., Tomita Y., Tagami H., Shibahara S. Identification of a cis-acting element that enhances the pigment cell-specific expression of the human tyrosinase gene. J Biol Chem. 1992 Oct 15;267(29):20584–20588. [PubMed] [Google Scholar]
- Shibata K., Takeda K., Tomita Y., Tagami H., Shibahara S. Downstream region of the human tyrosinase-related protein gene enhances its promoter activity. Biochem Biophys Res Commun. 1992 Apr 30;184(2):568–575. doi: 10.1016/0006-291x(92)90627-w. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Perez-Jurado L. A., Nakayama A., Hodgkinson C. A., Li X., Schneider M., Miki T., Fex J., Francke U., Arnheiter H. Cloning of MITF, the human homolog of the mouse microphthalmia gene and assignment to chromosome 3p14.1-p12.3. Hum Mol Genet. 1994 Apr;3(4):553–557. doi: 10.1093/hmg/3.4.553. [DOI] [PubMed] [Google Scholar]
- Takeda A., Tomita Y., Matsunaga J., Tagami H., Shibahara S. Molecular basis of tyrosinase-negative oculocutaneous albinism. A single base mutation in the tyrosinase gene causing arginine to glutamine substitution at position 59. J Biol Chem. 1990 Oct 15;265(29):17792–17797. [PubMed] [Google Scholar]
- Tanaka M., Tanaka S., Miura H., Yamamoto H., Kikuchi H., Takeuchi T. Conserved regulatory mechanisms of tyrosinase genes in mice and humans. Pigment Cell Res. 1992 Nov;5(5 Pt 2):304–311. doi: 10.1111/j.1600-0749.1992.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Takeda A., Okinaga S., Tagami H., Shibahara S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989 Nov 15;164(3):990–996. doi: 10.1016/0006-291x(89)91767-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasaradhi S., Bouchard B., Houghton A. N. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med. 1990 Apr 1;171(4):1375–1380. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuzer U., Goding C. R. Melanocyte-specific gene expression: role of repression and identification of a melanocyte-specific factor, MSF. Mol Cell Biol. 1994 May;14(5):3494–3503. doi: 10.1128/mcb.14.5.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Suzuki H., Yasumoto K., Tomita Y., Shibahara S. Molecular cloning and functional analysis of a cDNA coding for human DOPAchrome tautomerase/tyrosinase-related protein-2. Biochim Biophys Acta. 1994 Apr 6;1217(3):317–321. doi: 10.1016/0167-4781(94)90292-5. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]