Abstract
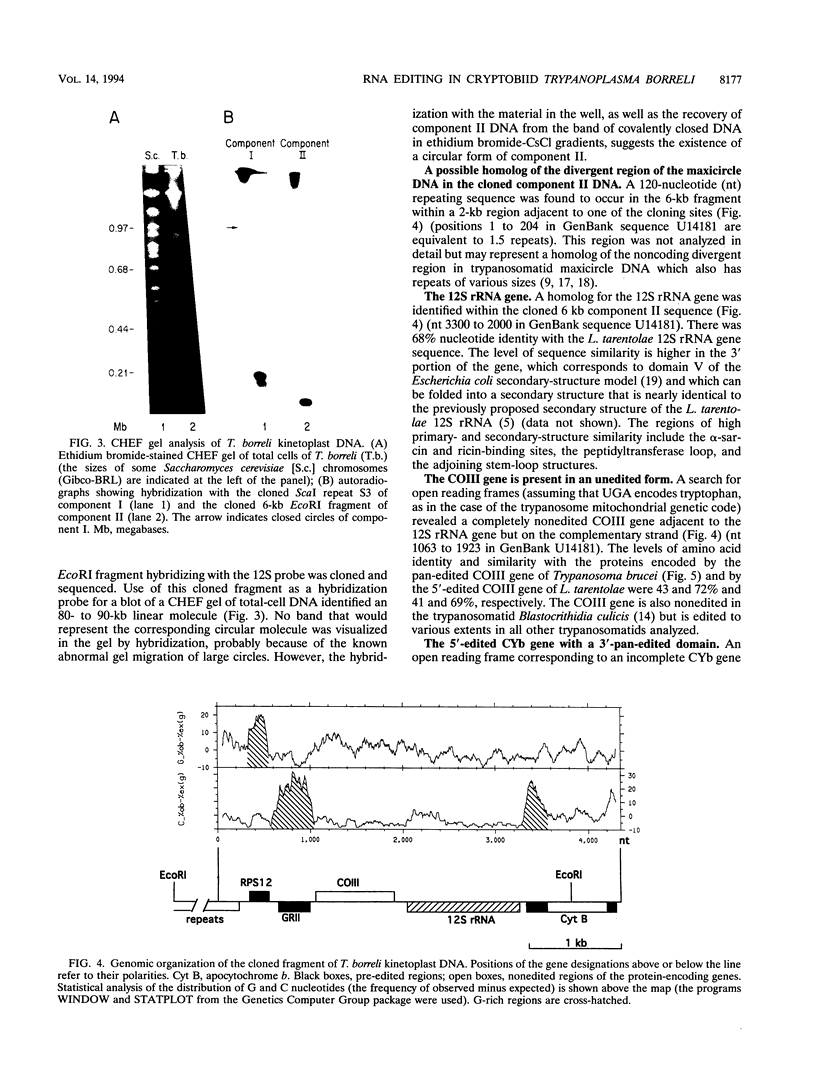
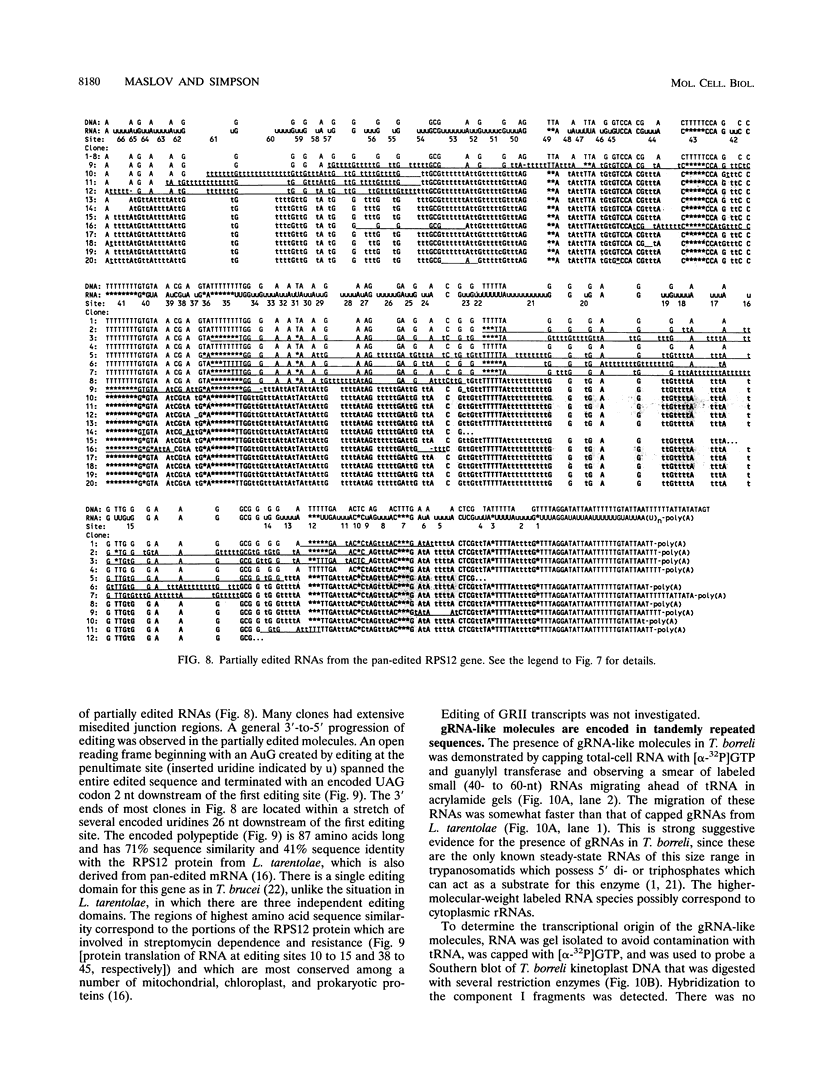
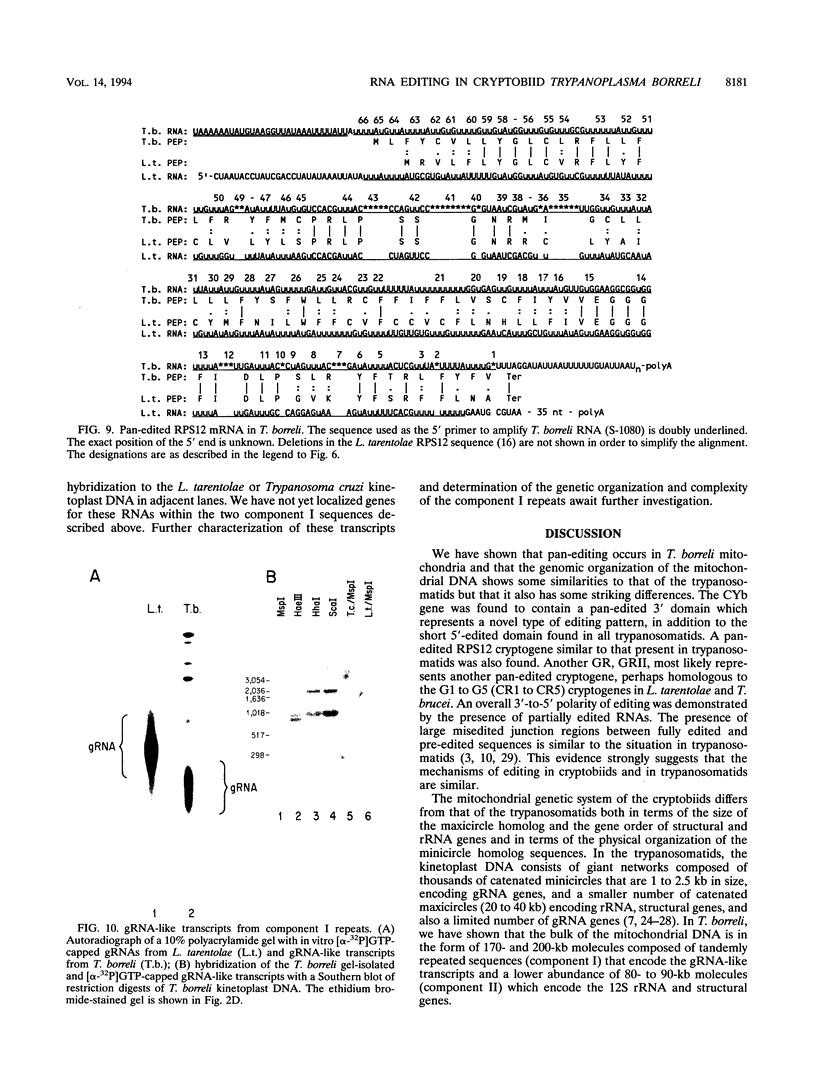
The bodonids and cryptobiids represent an early diverged sister group to the trypanosomatids among the kinetoplastid protozoa. The trypanosome type of uridine insertion-deletion RNA editing was found to occur in the cryptobiid fish parasite Trypanoplasma borreli. A pan-edited ribosomal protein, S12, and a novel 3'- and 5'-edited cytochrome b, in addition to an unedited cytochrome oxidase III gene and an apparently unedited 12S rRNA gene, were found in a 6-kb fragment of the 80- to 90-kb mitochondrial genome. The gene order differs from that in trypanosomatids, as does the organization of putative guide RNA genes; guide RNA-like molecules are transcribed from tandemly repeated 1-kb sequences organized in 200- and 170-kb molecules instead of minicircles. The presence of pan-editing in this lineage is consistent with an ancient evolutionary origin of this process.
Full text
PDF
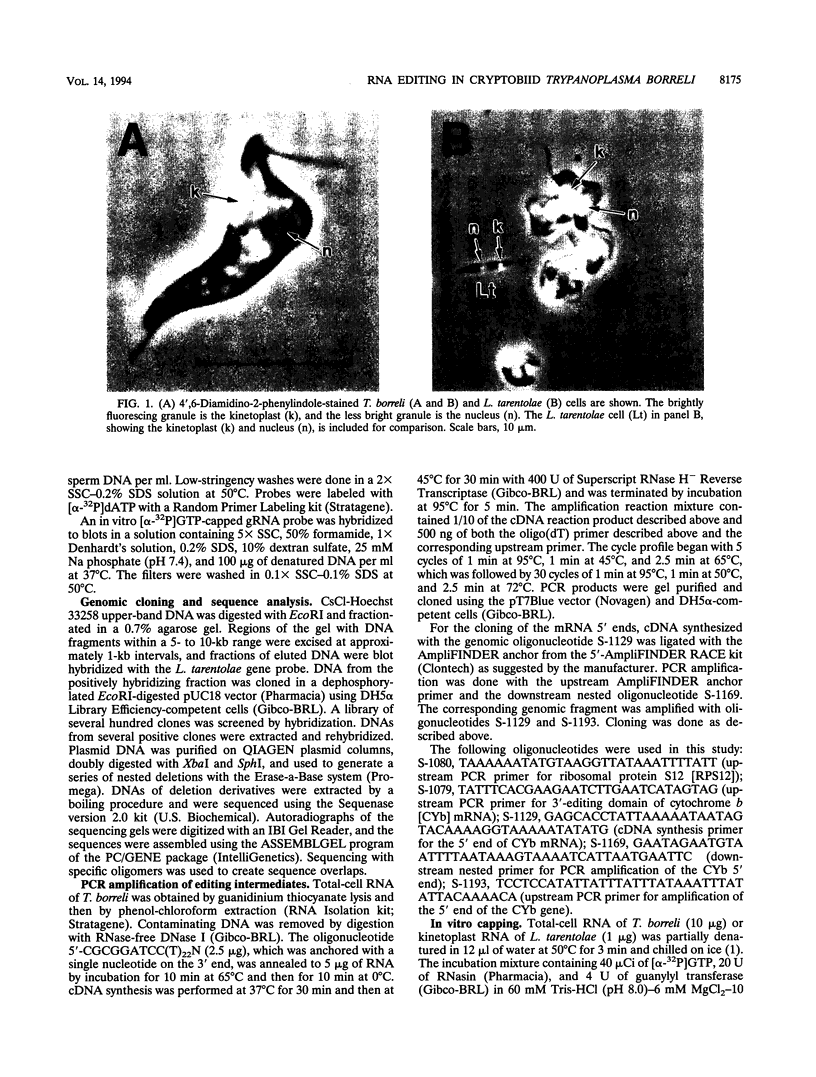




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum B., Simpson L. Guide RNAs in kinetoplastid mitochondria have a nonencoded 3' oligo(U) tail involved in recognition of the preedited region. Cell. 1990 Jul 27;62(2):391–397. doi: 10.1016/0092-8674(90)90375-o. [DOI] [PubMed] [Google Scholar]
- Corell R. A., Feagin J. E., Riley G. R., Strickland T., Guderian J. A., Myler P. J., Stuart K. Trypanosoma brucei minicircles encode multiple guide RNAs which can direct editing of extensively overlapping sequences. Nucleic Acids Res. 1993 Sep 11;21(18):4313–4320. doi: 10.1093/nar/21.18.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C. J., Sollner-Webb B. RNA editing involves indiscriminate U changes throughout precisely defined editing domains. Cell. 1990 Jun 15;61(6):1001–1011. doi: 10.1016/0092-8674(90)90065-m. [DOI] [PubMed] [Google Scholar]
- Fernandes A. P., Nelson K., Beverley S. M. Evolution of nuclear ribosomal RNAs in kinetoplastid protozoa: perspectives on the age and origins of parasitism. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11608–11612. doi: 10.1073/pnas.90.24.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbat A., Maslov D. A., Peters L. S., Gaviernik P., Viustenkhagen T., Kolesnikov A. A. Analiz posledovatel'nosti povtorov v divergentnoi oblasti maksi- kol'tsevoi DNK iz kinetoplastov Crithidia oncopelti. Mol Biol (Mosk) 1990 Nov-Dec;24(6):1539–1548. [PubMed] [Google Scholar]
- Hajduk S. L., Harris M. E., Pollard V. W. RNA editing in kinetoplastid mitochondria. FASEB J. 1993 Jan;7(1):54–63. doi: 10.1096/fasebj.7.1.8422975. [DOI] [PubMed] [Google Scholar]
- Hajduk S. L., Siqueira A. M., Vickerman K. Kinetoplast DNA of Bodo caudatus: a noncatenated structure. Mol Cell Biol. 1986 Dec;6(12):4372–4378. doi: 10.1128/mcb.6.12.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowsky D. J., Bhat G. J., Read L. K., Stuart K. Cycles of progressive realignment of gRNA with mRNA in RNA editing. Cell. 1991 Nov 1;67(3):537–546. doi: 10.1016/0092-8674(91)90528-7. [DOI] [PubMed] [Google Scholar]
- Landweber L. F., Gilbert W. Phylogenetic analysis of RNA editing: a primitive genetic phenomenon. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):918–921. doi: 10.1073/pnas.91.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landweber L. F., Gilbert W. RNA editing as a source of genetic variation. Nature. 1993 May 13;363(6425):179–182. doi: 10.1038/363179a0. [DOI] [PubMed] [Google Scholar]
- Landweber L. F. The evolution of RNA editing in kinetoplastid protozoa. Biosystems. 1992;28(1-3):41–45. doi: 10.1016/0303-2647(92)90006-k. [DOI] [PubMed] [Google Scholar]
- Maslov D. A., Avila H. A., Lake J. A., Simpson L. Evolution of RNA editing in kinetoplastid protozoa. Nature. 1994 Mar 24;368(6469):345–348. doi: 10.1038/368345a0. [DOI] [PubMed] [Google Scholar]
- Maslov D. A., Elgort M. G., Wong S., Pecková H., Lom J., Simpson L., Campbell D. A. Organization of mini-exon and 5S rRNA genes in the kinetoplastid Trypanoplasma borreli. Mol Biochem Parasitol. 1993 Sep;61(1):127–135. doi: 10.1016/0166-6851(93)90165-t. [DOI] [PubMed] [Google Scholar]
- Maslov D. A., Sturm N. R., Niner B. M., Gruszynski E. S., Peris M., Simpson L. An intergenic G-rich region in Leishmania tarentolae kinetoplast maxicircle DNA is a pan-edited cryptogene encoding ribosomal protein S12. Mol Cell Biol. 1992 Jan;12(1):56–67. doi: 10.1128/mcb.12.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Neckelmann N., Simpson L. The divergent region of the Leishmania tarentolae kinetoplast maxicircle DNA contains a diverse set of repetitive sequences. Nucleic Acids Res. 1985 May 10;13(9):3241–3260. doi: 10.1093/nar/13.9.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myler P. J., Glick D., Feagin J. E., Morales T. H., Stuart K. D. Structural organization of the maxicircle variable region of Trypanosoma brucei: identification of potential replication origins and topoisomerase II binding sites. Nucleic Acids Res. 1993 Feb 11;21(3):687–694. doi: 10.1093/nar/21.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Pecková H., Lom J. Growth, morphology and division of flagellates of the genus Trypanoplasma (Protozoa, Kinetoplastida) in vitro. Parasitol Res. 1990;76(7):553–558. doi: 10.1007/BF00932559. [DOI] [PubMed] [Google Scholar]
- Pollard V. W., Hajduk S. L. Trypanosoma equiperdum minicircles encode three distinct primary transcripts which exhibit guide RNA characteristics. Mol Cell Biol. 1991 Mar;11(3):1668–1675. doi: 10.1128/mcb.11.3.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read L. K., Myler P. J., Stuart K. Extensive editing of both processed and preprocessed maxicircle CR6 transcripts in Trypanosoma brucei. J Biol Chem. 1992 Jan 15;267(2):1123–1128. [PubMed] [Google Scholar]
- Rovai L., Tripp C., Stuart K., Simpson L. Recurrent polymorphisms in small chromosomes of Leishmania tarentolae after nutrient stress or subcloning. Mol Biochem Parasitol. 1992 Jan;50(1):115–125. doi: 10.1016/0166-6851(92)90249-j. [DOI] [PubMed] [Google Scholar]
- Simpson L. Isolation of maxicircle component of kinetoplast DNA from hemoflagellate protozoa. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1585–1588. doi: 10.1073/pnas.76.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Sloof P., Benne R. RNA editing in trypanosome mitochondria: guidelines for models. FEBS Lett. 1993 Jun 28;325(1-2):146–151. doi: 10.1016/0014-5793(93)81431-x. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Maslov D. A., Blum B., Simpson L. Generation of unexpected editing patterns in Leishmania tarentolae mitochondrial mRNAs: misediting produced by misguiding. Cell. 1992 Aug 7;70(3):469–476. doi: 10.1016/0092-8674(92)90171-8. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]
- de la Cruz V. F., Simpson A. M., Lake J. A., Simpson L. Primary sequence and partial secondary structure of the 12S kinetoplast (mitochondrial) ribosomal RNA from Leishmania tarentolae: conservation of peptidyl-transferase structural elements. Nucleic Acids Res. 1985 Apr 11;13(7):2337–2356. doi: 10.1093/nar/13.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]