Abstract
Background.
Lower ambulatory performance with aging may be related to a reduced oxidative capacity within skeletal muscle. This study examined the associations between skeletal muscle mitochondrial capacity and efficiency with walking performance in a group of older adults.
Methods.
Thirty-seven older adults (mean age 78 years; 21 men and 16 women) completed an aerobic capacity (VO2 peak) test and measurement of preferred walking speed over 400 m. Maximal coupled (State 3; St3) mitochondrial respiration was determined by high-resolution respirometry in saponin-permeabilized myofibers obtained from percutanous biopsies of vastus lateralis (n = 22). Maximal phosphorylation capacity (ATPmax) of vastus lateralis was determined in vivo by 31P magnetic resonance spectroscopy (n = 30). Quadriceps contractile volume was determined by magnetic resonance imaging. Mitochondrial efficiency (max ATP production/max O2 consumption) was characterized using ATPmax per St3 respiration (ATPmax/St3).
Results.
In vitro St3 respiration was significantly correlated with in vivo ATPmax (r 2 = .47, p = .004). Total oxidative capacity of the quadriceps (St3*quadriceps contractile volume) was a determinant of VO2 peak (r 2 = .33, p = .006). ATPmax (r 2 = .158, p = .03) and VO2 peak (r 2 = .475, p < .0001) were correlated with preferred walking speed. Inclusion of both ATPmax/St3 and VO2 peak in a multiple linear regression model improved the prediction of preferred walking speed (r 2 = .647, p < .0001), suggesting that mitochondrial efficiency is an important determinant for preferred walking speed.
Conclusions.
Lower mitochondrial capacity and efficiency were both associated with slower walking speed within a group of older participants with a wide range of function. In addition to aerobic capacity, lower mitochondrial capacity and efficiency likely play roles in slowing gait speed with age.
Key Words: Muscle, Mitochondria, Aging, Walking speed.
Aging is associated with reduced mitochondrial capa city (1,2) and loss of muscle mass and strength (3,4), which could potentially predispose individuals to frailty and a slower preferential walking speed (5,6). Preferred walking speed is slower in older adults (7,8), and is a strong, independent predictor of disability, health care utilization, nursing home admission, and mortality (9–11). A number of studies have found a close relationship between VO2 peak and walking speed in older adults (12,13), which suggests that the decline in aerobic capacity contributes to, and may be predictive of, slower walking speed with age. A reduced efficiency of locomotion is also apparent in older adults, which leads to an increased metabolic cost of walking (14).
Aging is also associated with declines in both the capacity and efficiency of energy supply in muscle. Several studies using a variety of techniques have reported reduced capacity to generate ATP with age (1,15,16). The age-related changes in skeletal muscle mitochondrial function apparent in these studies are consistent with their likely role in the parallel loss of aerobic capacity (1,16). In addition, reduced mitochondrial efficiency (energy conversion of O2 uptake into ATP generation) has been reported in a variety of tissues in vitro and in vivo (17–19). The importance of mitochondrial efficiency is that it could affect the ability to generate ATP during ambulation (16) as well as movement efficiency (20–22). One study found that walking speed in patients with peripheral arterial disease was related to their capacity for ATP generation assessed by phosphorus magnetic resonance spectroscopy (31P MRS) (23). Although the loss of muscle mitochondrial function has been widely hypothesized to contribute to the decline in VO2 max and slowing of locomotion with age, there currently is insufficient published evidence to show that reductions in available energy results in a decline in customary walking speed with aging and disease (11). Furthermore, the potential role of mitochondrial efficiencey in energy availablity for walking has not been examined.
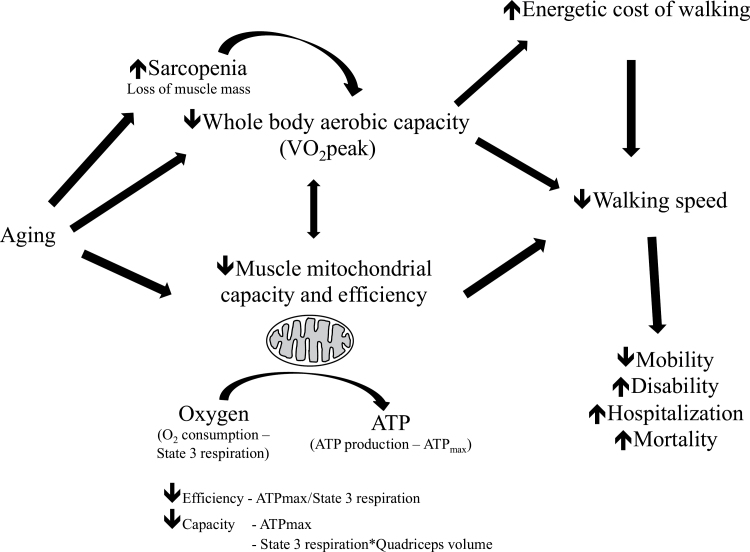
The goal of this study was to test the hypothesis that reduced mitochondrial capacity and efficiency are associated with slower walking speed in older adults. In vitro (respirometry) and in vivo (31P MRS) measurements of mitochondrial function were combined and related to whole-body aerobic capacity (VO2 peak) and preferred walking speed in a group of older men and women. High-resolution respirometry of permeabilized fibers isolated from muscle biopsy specimens yielded mitochondrial oxidative capacity (State 3 or St3 respiration). These measures of mitochondrial capacity at cellular level were extended to the muscle tissue level by assessing quadriceps muscle volume with magnetic resonance imaging (MRI). 31P MRS was used to determine the maximum mitochondrial ATP production (ATPmax) in vivo, which was combined with St3 respiration to yield an index of mitochondrial efficiency (ATPmax/St3). This study tested the paradigm that muscle mitochondrial properties affect walking speed and that mitochondria capacity and efficiency may be associated with the decline in mobility with age (Figure 1).
Figure 1.
Concept map illustrating age-related changes in muscle physiology and how they contribute to reduced walking speed in older adults. This study examined the relationships between muscle mitochondrial capacity/efficiency, aerobic capacity, and walking speed in older adults. VO2 peak = maximal oxygen consumption during maximal dynamic exercise. This is an index of whole-body aerobic capacity.
Methods
Recruitment
Participants were community-dwelling, ambulatory men and women aged 70–89 years from the Pittsburgh, Pennsylvania area. A telephone interview was initially conducted to determine eligibility. The inclusion criteria were age 70–89 years; body weight less than or equal to 285 lb for men and less than or equal to 250 lb for women; body mass index 20–32kg/m2; ability to walk without the assistance of a device or another person; free of basic activities of daily living disability, defined as no difficulty getting in and out of bed or chairs, and no difficulty walking across a small room; no history of hip fracture; no heart attack, angioplasty, or heart surgery within the past 3 months, no cerebral hemorrhage within the past 6 months, stroke within the past 12 months, or chest pain during walking in the past 30 days; no symptomatic cardiovascular or pulmonary disease; no regular pain, aching, or stiffness in the legs, hips, knees, feet, or ankles when walking; no bilateral difficulty bending or straightening fully the knees; not regularly taking Coumadin, Plavix, Aggrenox, Ticlid, or Agrylin/Xagrid. All participants provided written informed consent. The study was approved by the University of Pittsburgh Institutional Review Board.
Testing Schedule
The clinic examination involved three visits. During the first visit potential participants were asked to read and sign an informed consent document. Measurements included height, weight, blood pressure, and resting pulse. A physical examination was also conducted along with a review of clinical information including self-reported physical function, medical history, and medication inventory. A physical activity scale for the elderly questionairre was completed and a final summary score was calculated (24). A short physical performance battery was conducted and an overall score was calculated (25). A 400-m walk test was conducted to determine self-selected walking speed. Participants were also given a 5-minute practice session on the treadmill to become acquainted with treadmill walking prior to the VO2 peak test conducted in a subsequent visit. The second visit involved 31P MRS and imaging and a graded exercise test to determine VO2 peak. The third visit involved muscle tissue collection.
The 400-m Walk Test
The 400-m walk test assessed the participant’s ability to complete a 400-m walking course in 15 minutes or less without sitting down or stopping, without help, or the use of any assistive device. Participants were instructed to complete the distance at their usual pace and without overexerting themselves. Participants were remined to walk at their usual pace every lap. Seated blood pressure and pulse were reviewed for safety before the walk. Prefered walking speed was calculated as total meters walked/total time in seconds.
VO2 Peak Test
Maximal whole-body oxygen consumption (VO2 peak) was determined by a graded treadmill exercise test (26). A resting 12-lead electrocardiogram was conducted prior to the VO2 peak test to screen for cardiac arrhythmias. To ensure participant safety, continuous electrocardiogram monitoring was also performed during the VO2 peak test. During the test, the participant’s self-selected usual walking speed was used and the treadmill grade was increased by 2% every 2 minutes until attainment of peak VO2. The test was terminated as per the criteria outlined in the American College of Sports Medicine guidelines (26).
Muscle Biopsy Procedure and Preparation of Permeabilized Muscle Fiber Bundle
Percutaneous biopsies were obtained at the University of Pittsburgh’s Clinical Translational Research Center on a morning after an overnight fast. Participants were instructed not to perform physical exercise 48 hours prior to the muscle biopsy procedure. Muscle biopsy samples were obtained from the middle region of the musculus vastus lateralis as described previously (27). Following the procedure, the biopsy specimen was immediately blotted dry of blood and interstitial fluid and dissected free of any connective tissue and intermuscular fat. A portion of the biopsy specimen (~10mg) was immediately placed in ice-cold BIOPS solution (10mM Ca–EGTA buffer, 0.1M free calcium, 20mM imidazole, 20mM taurine, 50mM potassium 2-[N-morpholino]-ethanesulfonic acid, 0.5mM dithiothreitol, 6.56mM MgCl2, 5.77mM ATP, and 15mM phosphocreatine [PCr], pH 7.1). The individual muscle fibers in the sample were then gently teased apart in a petri dish containing ice-cold BIOPS solution using fine-nosed forceps and a dissecting microscope (Leica Microsystems, Heerbrugg, Switzerland). The fiber bundles were then permeabilized with saponin (2mL of 50 ug/mL saponin in BIOPS solution) for 20 minutes at 4°C on an orbital shaker, and then washed twice for 10 minutes at 4°C with Mir05 respiration medium (0.5mM EGTA, 3mM MgCl2·6H2O, 60mM K-lactobionate, 20mM taurine, 10mM KH2PO4, 20mM HEPES, 110mM sucrose, and 1g/L BSA, pH 7.1) on an orbital shaker (28). The permeabilized muscle fiber bundles were then placed into the respiration chambers of an Oxygraph 2K (Oroboros Inc., Innsbruck, Austria).
Mitochondrial Respiration Protocol
Measurement of oxygen consumption in permeablized fibers was conducted over a period of approximately 1 hour 40 minutes, at 37°C and in the oxygen concentration range 220–150 nmol O2/mL (see Supplementary Method for full protocol). Following the assay, the fiber bundles were recovered and dried. A dry weight was then determined with an analytical balance (Mettler Toledo, XS105). Steady state O2 flux for each respiratory state was determined and normalized to fiber bundle weight using Datlab 4 software (Oroboros Inc.).
Determination of ATPmax by 31P MRS
Maximal mitochondrial ATP production in vivo (ATPmax) following an acute bout of knee extensor exercise was determined using 31P MRS. Recovery of PCr levels after exercise is used to characterize rates of mitochondrial ATP resynthesis (production). The validity of this method is confirmed by animal and human studies showing that ATPmax varies in direct proportion to the oxidative enzyme activity of healthy muscle (29,30) and corresponds with mitochondrial content in human muscle (16). Repeat measurements of muscle ATPmax have been shown to agree to within about 7% (31).
Exercise Protocol
The exercise protocol was designed to deplete PCr of the quadriceps muscles with minimal acidification to achieve a high ADP and thus maximize oxidative phosphorylation (30). Participants lay supine in the scanner’s bore with the knee supported in about 30° of flexion. Sandbags and padding were placed on both sides of the ankle and knee for support and straps placed across the distal leg, thigh, and hips restricted limb movement. Participants performed strong, fast contractions of the quadriceps muscle at the highest rate possible for 24–36 seconds, followed by 6 minutes of rest. Most participants repeated this protocol twice, with two different exercise times, to ensure that in at least one bout PCr was reduced by 33%–66% of basal and that muscle pH did not fall less than 6.80 during recovery. Participants were trained to perform the exercise before entering the magnet.
31P MRS
Phosphorus spectra was collected using a 3T TIM Trio magnetic resonance scanner (Siemen’s Medical System, Erlanger, Germany) (see Supplementary Method). A standard one pulse experiment was used to determine the levels of PCr, ATP, Pi, and pH throughout exercise and recovery.
PCr, Pi, and ATP peak areas in the fully relaxed spectra were measured by integration using Varian VNMR 6.1C software (Varian Medical Systems, Palo Alto, CA). Areas of the PCr and Pi peaks were expressed relative to the ATP peak and quantified using a resting PCr value of 27mM as determined from biopsies of human vastus lateralis muscle (16). Changes in PCr and Pi peak areas during the experiments were analyzed as previously described (32,33).
Determination of Muscle Size
MRI was used to determine quadriceps cross-sectional area and volume according to a previously described method (see Supplementary Method) (35). Using a 3T TIM Trio magnetic resonance scanner (Siemen’s Medical System), images were collected every 3cm from the hip to the thigh (15–25 slices per participant). The patient lay supine for imaging. Standard stereologic techniques were used to determine the largest muscle cross-sectional area for the quadriceps (35). Subcutaneous and intramuscular fat and other noncontractile tissues were excluded from the calculation of muscle contractile cross-sectional area.
Statistical Analysis
All data are presented as mean ± SD unless otherwise stated. Pearson correlation coefficients were used to examine relationships between variables. A multiple linear regression model was used to predict preferred walking speed from VO2 peak and mitochondrial efficiency (ATPmax/St3 respiration).
Results
Participant Characteristics
A total of 179 potential participants were screened by telephone interview. Of those interviewed, 99 individuals were ineligible and 43 were not interested in participating. A total of 37 older adults (21 men and 16 women); who were normal weight to slightly overweight were studied (Table 1). The group had on average a relatively low and widely ranging level of aerobic fitness defined by VO2 peak (Table 1). This group of older participants also had a fairly wide range of preferred walking speed, and the short physical performance battery scores were indicative of low to moderate lower extremity function (Table 1).
Table 1.
Descriptive, Metabolic and Physiological Data for Study Participants
| n | 37 (M = 21, F = 16) |
| Age (y) | 78.3±4.9 (60–89) |
| Weight (Kg) | 72.0±12 (53–97) |
| BMI (Kg/m2) | 25.7±2.6 (21.4–31.2) |
| Quadriceps contractile volume (mL) | 1159±324 (589–1886), n = 30 |
| ATPmax (mM ATP/s) | 0.52±0.1 (0.32–0.83), n = 30 |
| State 3 respiration (pmol/s*mg DW) | 174±68 (52–303), n = 22 |
| State 4 respiration (pmol/s*mg DW) | 16.4±7.1 (4.0–30.8), n = 22 |
| Respiratory control ratio | 11.8±5.1 (6.1–26), n = 22 |
| Mitochondrial efficiency; ATPmax/State 3 respiration ((mM ATP/s)/(pmol O2/s*mg DW)) | 3.5±1.7 × 103 (1.8 × 103–7.9 × 103), n = 18 |
| Quadriceps oxidative capacity; State 3 respiration × muscle volume ((pmol O2/s*mg DW)*mL muscle) | 209±89.4 × 103 (78 × 103–488 × 103), n = 22 |
| VO2 peak (mL/min) | 1551.5±408 (750–2724) |
| VO2 peak (mL/KgBW/min) | 22.0±5.5 (7.8–33.4) |
| Preferred walking speed over 400 m (m/s) | 1.2±0.2 (0.74–1.58) |
| SPPB score | 10.9±1.3 (7–12) |
| PASE score | 133±55 (15–274) |
Notes: Values are average ± SD (Min-Max). BMI = body mass index; BW = body weight; DW = dry weight of tissue; PASE = physical activity scale for the elderly participants; SPPB = short performance physical battery.
Muscle Magnetic Resonance Measurements
Due to exclusions from MRI, for example, history of metal work or claustrophobia, a subsample of individuals (n = 30; 16 men and 14 women) had ATPmax determined by 31P MRS. The average ATP resynthesis rate (ATPmax) was 0.52mM ATP/s and covered a greater than 2.5-fold range (0.32–0.83) in agreement with reports on older adults (16). The quadriceps contractile muscle size determined from MRI was also consistent with values previously reported for older participants (36). The coefficient of variation for repeat determinations on 8 participants was 7.2% for ATPmax, and 3% for quadriceps volume.
Respirometry Measurements
A subsample of individuals (n = 22; 12 men and 10 women) had muscle biopsies that were studied by high-resolution respirometry. The maximal coupled respiratory capacity (St3 respiration) of vastus lateralis–permeabilized fiber bundles ranged greater than fivefold (Table 1). The nonphosphorylating rate of respiration (State 4 or St4 respiration) displayed a similar wide range as St3. A respiratory control ratio (St3/St4) of 11.8 indicated good preparation of permeabilized muscle fiber bundles (37). These data are in agreement with previous respirometry measurements on permeabilized fibers from older adults (75 years old) with St3 and St4 respiration averaging 199 and 24 pmol/s–1mg DW, respectively, based on Figure 1 from reference (38) and the conversion ratio of wet-to-dry weight from reference (39). The coefficient of variation of the respirometry assay for this study was determined to be 16.9% (St3 respiration, determined from 6 participants). A representitive oxygraph is presented in Supplementary Figure 1.
Associations Among In Vitro and In Vivo Muscle and Whole-Body Oxidative Capacity
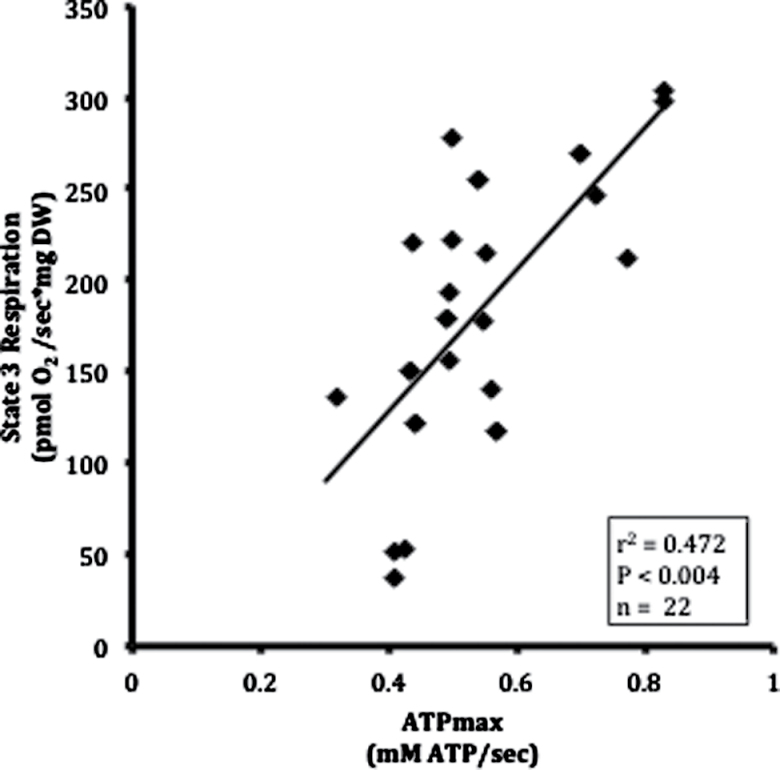
Maximal coupled, St3 respiration (r 2 = .47, p = .004; Figure 2), and maximal uncoupled respiration (r 2 = .47, p = .002; Supplementary Table 1) measured in intact muscle fibers from the biopsy were significantly correlated with whole-muscle ATPmax, indicating a close relationship between the measurements of oxidative capacity of permeabilized fibers and phosphorylation capacity of intact muscle.
Figure 2.
Pearson correlation of maximum respiratory capacity with maximum oxidative phosphorylation in muscle. State 3 respiration in permeabilized fiber bundles was determined by high-resolution respirometry. Maximum oxidative phosphorylation (ATPmax) elicited by exercise was determined by phosphorus magnetic resonance spectroscopy (31P MRS). DW = dry weight.
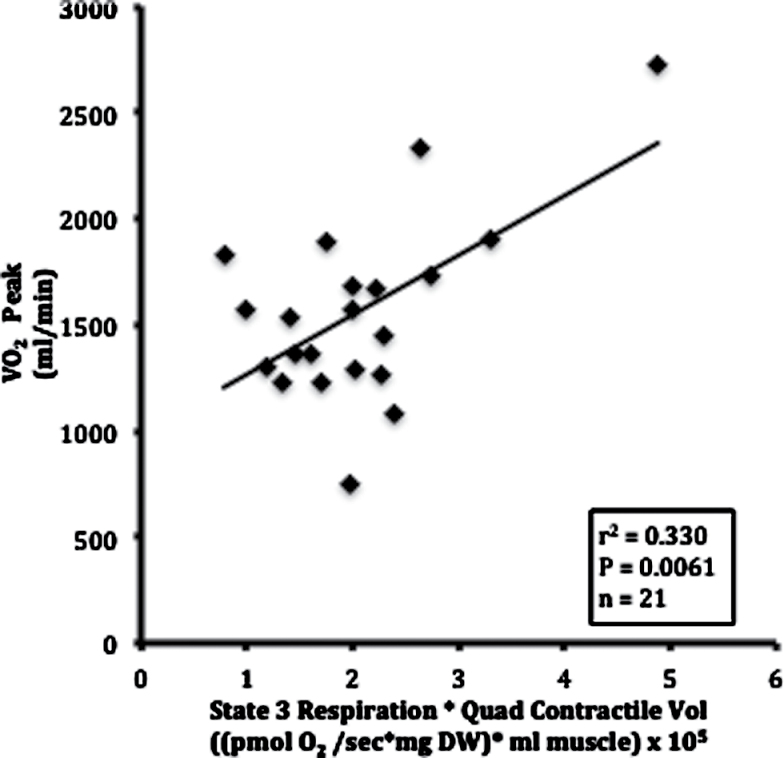
The relationship of muscle respiratory and oxidative capacity to whole-body aerobic capacity was evaluated by combining respirometry data and MRI measurements of quadriceps contractile volume. A measure of muscle oxidative capacity was derived from the product of St3 respiration and the volume of quadriceps muscle (St3·V Q). Figure 3 shows that variation in VO2 peak is significantly correlated with the quadriceps’ oxidative capacity (r 2 = .33, p < .0061). These associations were consistent for both men and women. This finding is also consistent with a prior study in older adults (36).
Figure 3.
Pearson correlation of whole-body aerobic capacity with muscle oxidative capacity. Aerobic capacity (VO2 peak) was determined by a graded exercise test. Muscle oxidative capacity was defined as the product of State 3 respiration and quadriceps contractile volume (State 3 respiration * quadriceps contractile volume). DW = dry weight.
The Impact of Energetics on Walking Speed
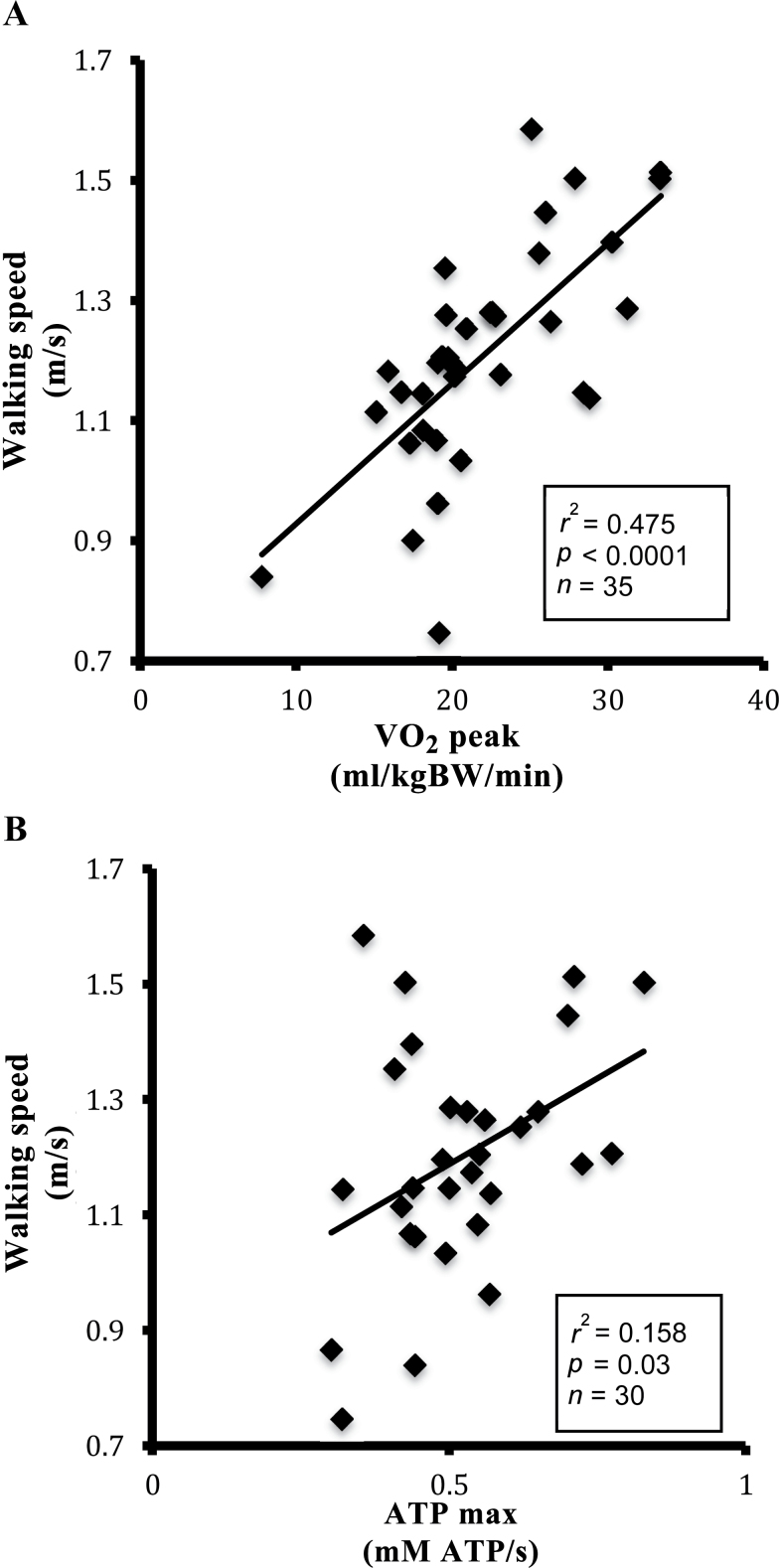
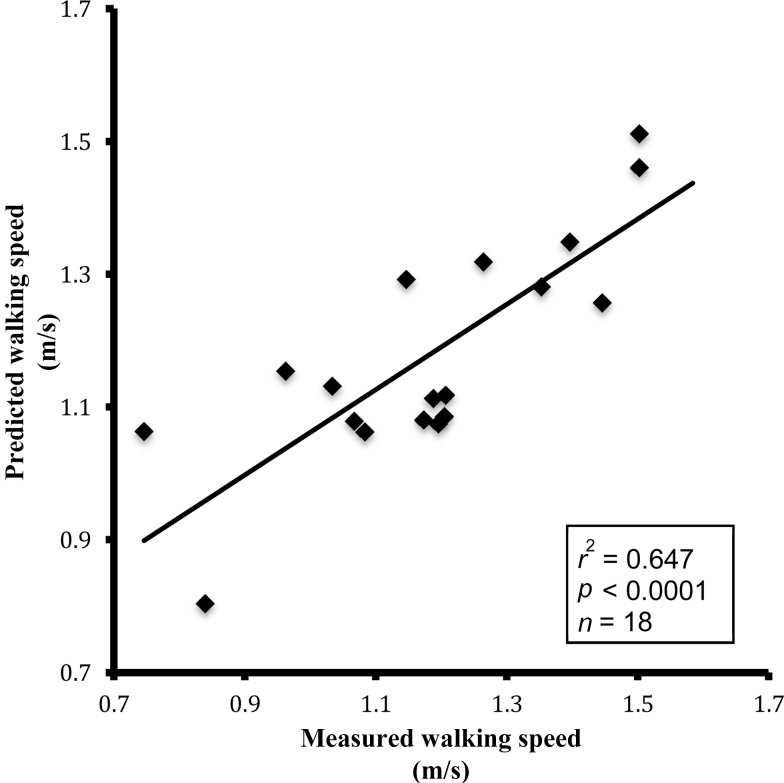
The range of VO2 peak among participants accounted for 48% of the variation in preferred walking speed over 400 m (Figure 4, Panel A: r 2 = .48, p < .0001), in agreement with previous studies of energetics in older adults (12,13). It was also found that ATPmax accounted for 15.8% of the variation in preferred walking speed (Figure 4, Panel B: r 2 = .158, p = .03). The impact of mitochondrial efficiency (ATPmax/St3) on the relationship between aerobic capacity (VO2 peak) and walking performance was tested using a multiple linear regression model. Table 2 shows that independent of VO2 peak, mitochondrial efficiency was borderline associated with preferred walking speed (ATPmax/St3; p = .057). Together, however, VO2 peak and ATPmax/St3 predicted approximately 65% of the variation in preferred walking speed (Figure 5, r 2 = .647, p < .0001) as compared with 47.5% of the variation by VO2 peak alone. Adding gender to the model did not significantly affect these associations. The correlations between all respirometry states, MRS, VO2 peak, and walking speed are presented in Supplementary Table 1.
Figure 4.
Pearson correlation of preferred walking speed with whole-body aerobic capacity and muscle mitochondrial capacity. Panel (A): preferred walking speed versus VO2 peak. Panel (B): preffered walking speed versus ATPmax. Aerobic capacity normalized to body weight (mL/kgBW/min) was determined by a graded exercise test. Preferred walking speed was determined over a 400-m walk test. ATPmax was determiend by 31P magnetic resonance spectroscopy.
Table 2.
Multiple Linear Regression for the Model of Preferred Walking Speed as a Function of Aerobic Capacity Per Body Mass (VO2 peak; mL/kgBW/min) and Mitochondrial Efficiency (ATPmax/State 3)
| Analysis of Variance | ||||
|---|---|---|---|---|
| Variable | Coefficient | SE | F Value | p Value |
| Intercept | 0.4561 | 0.143 | 10.11 | .0062 |
| ATPmax/State 3 | 37.786 | 18.3 | 4.26 | .0567 |
| VO2 peak | 0.0272 | 0.005 | 24.18 | .0002 |
| Summary of Stepwise Selection | ||||
| Step | Variable entered | Partial R-squared | Model R-squared | |
| 1 | VO2 peak | 0.4753 | 0.4753 | |
| 2 | ATPmax/State 3 | 0.1714 | 0.6467 | |
Note: SE = standard error.
Figure 5.
Pearson correlation of predicted preferred walking speed versus measured preferred walking speed. Walking speed was predicted from aerobic capacity (VO2 peak) and muscle mitochondrial efficiency (ATPmax/State 3) by multiple linear regression (Table 2). Measured preferred walking speed was determined over a 400-m walk test.
Discussion
This study provides novel evidence obtained at the cellular, tissue, and whole-body level that skeletal muscle mitochondrial capacity and efficiency are associated with preferred walking speed in older men and women. First, it was found that muscle oxidative and phosphorylation capacities, whole-body aerobic capacity, and walking speed all varied manyfold among older adults in accordance with their fairly broad range in function. Second, muscle mitochondrial capacity and efficiency along with whole-body aerobic capacity were directly associated with walking speed. Aerobic capacity (VO2 peak) varied in proportion to muscle respiratory capacity as measured by St3 respiration (oxidative capacity) of permeabilized fibers combined with quadriceps volume (Figure 3). Aerobic capacity and mitochondrial capacity were also strongly correlated with walking speed (Figure 4). The third key finding was that muscle mitochondrial efficiency (ATPmax/St3) provided independent explanatory power to predict walkng speed, additional to that provided by VO2 peak alone. These data indicate that muscle mitochondrial capacity and efficiency are associated with ambulatory performance of older adults.
Mitochondrial Capacity of Muscle Fibers
Firstly, mitochondrial respiratory capacity determined from permeabilized muscle fibers was compared to phosphorylation capacity of whole muscle. It was found that St3 respiration was directly proportional to ATPmax over the manyfold range of properties found among these participants. Thus, a higher oxidative capacity of the muscle fiber is reflected in a higher oxidative phosphorylation capacity in whole muscle. This finding is in agreement with studies correlating mitochondrial content and enzymatic activity of muscle biopsies with ATPmax (16,29). Thus, for the first time two separate measures of muscle mitochondrial energetics, determined in vitro by respirometry and in vivo by 31P MRS in older adults, show a correspondence between mitochondrial respiratory capacity and whole-muscle phosphorylation capacity.
Impact of Metabolic Capacity and Efficiency on Mobility
A key question of this study was whether—and the extent to which—metabolic capacity and efficiency are related to walking performance. The hypothesis that walking speed is not only affected by whole-body aerobic capacity (VO2 peak) but also by muscle mitochondrial efficiency (ATPmax/St3) was tested. The contribution of both aerobic capacity and mitochondrial efficiency on walking speed is apparent in the multiple linear regression model shown in Table 2. In this model, the positive coefficient for VO2 peak implies that a higher aerobic capacity is associated with a faster preferred walking speed. Similarly, the positive coefficient for ATPmax/St3 implies that greater mitochondrial efficiency has a beneficial effect on walking speed. Together, aerobic capacity and mitochondria efficiency accounted for 64.7% of the variation in walking speed, whereas VO2 peak alone accounted for only 47.5% of the variation. The contribution of mitochondrial efficiency, independent of VO2 peak, can be further highlighted by examining data from individual participants. For example, for 2 participants with similar VO2 peaks, 1 participant had a higher walking speed (1.5 m/s) and high mitochondrial efficiency (7.9 × 103 [mM ATP/s]/[pmol O2/s*mg DW]), whereas the second had a lower walking speed (0.74 m/s) and low mitochondrial efficiency (2.3 × 103 [mM ATP/s]/[pmol O2/s*mg DW]). Alterations in mitochondrial efficiency of ATP production may be caused by reduced inner mitochondrial membrane leak or by reduced electron leak from the electron transport chain. Further studies are warranted to determine the causal factors mediating muscle mitochondrial efficiency in older adults. This is the first study, to the authors’ knowledge, to demonstrate that greater muscle mitochondrial efficiency may play a direct role in gait speed in older adults.
Muscle Impact on Metabolic Capacity
Here, mitochondrial respiratory capacity (St3) was combined with quadriceps contractile volume (V Q) to extend the oxidative capacity of the muscle fibers to that of the whole quadriceps. It was found that the oxidative capacity of the quadriceps accounted for 33% of the variation in VO2 peak (Figure 3), a finding that is in agreement with a prior study of individuals 20–80 years old (36). This agreement suggests that mitochondria play an important role in determining cardiorespiratory fitness in older individuals. In contrast, a study of young and master endurance-trained athletes concluded that cardiac output and O2 delivery to the muscle likely sets the limits to the aerobic capacity (40). These highly active older adults may well have reached the limits to oxygen delivery, as found in younger athletes (average age: 26.1 years old) (41). However, direct measurements demonstrating an O2 delivery limit in athletic individuals (41) fail to find a similar limitation in more sedentary people (42). The lack of O2 delivery limitation in less active older participants is evident in the scaling of maximum O2 uptake in proportion to the muscle’s capacity for O2 consumption in older adults that is apparent in Figure 3 and reported previously (36). These data indicate that in nonathletic older participants with a wide variation in physical function, muscle mitochondrial capacity is an important factor in addition to the cardiovascular system in determining VO2 peak across age. Thus, these data suggest that interventions to enhance muscle mitochondria could have important effects to improve exercise tolerance and function in relatively sedentary older adults.
There are some potential limitations and caveats to this study. Firstly, the strong relationship between VO2 peak and quadriceps oxidative capacity (Figure 3) is dependent on one or two data points. A larger study with more participants would provide a more definitive view of this relationship. Secondly, although the range of functional performance of older adult participants was fairly broad, this study included few very low functioning people. Inclusion of more very low functioning older adults may have further strengthened the observed relationships between muscle mitochondrial capacity/efficiency and gait speed. Nevertheless, it is believed that these findings may be clinically relevant, because walking speed has recently been identified as an important determinant of health and mortality in older men and women (9,10). Thirdly, despite the lack of significant gender effect on these associations, this study was not adequately powered to examine gender-specific associations. Thus larger studies are warranted to determine whether mitochondrial energetics are more or less strongly associated with function in men and women specifically.
In conclusion, muscle mitochondrial capacity and efficiency are related to walking speed in older adults, and that the loss of mitochondrial capacity and efficiency with age may be important contributors to the reduction in mobility and increase in disability. Future prospective longitudinal studies should determine whether mitochondrial energetics predict the decline in walking speed and function as well as incident mobility limitations.
Funding
The Study of Energy and Aging (SEA) Pilot was supported by the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827), the National Institute of Aging, with ARRA funds (1RC2AG036594, 1RC2AG036606), National Institute of Arthritis and Musculoskeletal and Skin (R01 AR 41928), and by the National Center for Research Resources (NCRR) (UL1 RR024153).
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgments
The authors would like to acknowledge the excellent technical assistance of Angela Laslavic and the staff at the University of Pittsburgh Clinical Translational Research Center and the Center for Aging and Population Health.
References
- 1. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005; 102: 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conley KE, Jubrias SA, Esselman PE. Oxidative capacity and ageing in human muscle [published erratum appears in J Physiol. 2001 Jun 15;533(Pt 3):921]. J Physiol. 2000; 526(Pt 1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006; 61: 1059–1064 [DOI] [PubMed] [Google Scholar]
- 4. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006; 61: 72–77 [DOI] [PubMed] [Google Scholar]
- 5. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005; 60: 324–333 [DOI] [PubMed] [Google Scholar]
- 6. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332: 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J Rehabil Res Dev. 1993; 30: 210–223 [PubMed] [Google Scholar]
- 8. Samson MM, Crowe A, de Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging (Milano). 2001; 13: 16–21 [DOI] [PubMed] [Google Scholar]
- 9. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003; 51: 314–322 [DOI] [PubMed] [Google Scholar]
- 10. Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol. 2006; 21: 113–122 [DOI] [PubMed] [Google Scholar]
- 11. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010; 58(suppl 2):S329–S336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982; 37: 560–564 [DOI] [PubMed] [Google Scholar]
- 13. Conley KE, Cress ME, Jubrias SA, Esselman PC, Odderson IR. From muscle properties to human performance, using magnetic resonance. J Gerontol A Biol Sci Med Sci. 1995; 50: 35–40 [DOI] [PubMed] [Google Scholar]
- 14. Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf). 2006; 186: 127–139 [DOI] [PubMed] [Google Scholar]
- 15. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003; 300: 1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000; 526(Pt 1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, Attardi G. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J. 2003; 17: 1706–1708 [DOI] [PubMed] [Google Scholar]
- 18. Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007; 104: 1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005; 569: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf). 2007; 191: 59–66 [DOI] [PubMed] [Google Scholar]
- 21. Larsen FJ, Schiffer TA, Borniquel S, et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011; 13: 149–159 [DOI] [PubMed] [Google Scholar]
- 22. Schrauwen P, Hesselink M. Uncoupling protein 3 and physical activity: the role of uncoupling protein 3 in energy metabolism revisited. Proc Nutr Soc. 2003; 62: 635–643 [DOI] [PubMed] [Google Scholar]
- 23. Hou XY, Green S, Askew CD, Barker G, Green A, Walker PJ. Skeletal muscle mitochondrial ATP production rate and walking performance in peripheral arterial disease. Clin Physiol Funct Imaging. 2002; 22: 226–232 [DOI] [PubMed] [Google Scholar]
- 24. Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993; 46: 153–162 [DOI] [PubMed] [Google Scholar]
- 25. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49: M85–M94 [DOI] [PubMed] [Google Scholar]
- 26. American College of Sports Medicine. Thompson WR, Gordon NF, Pescatello LS. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 27. Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004; 287: E857–E862 [DOI] [PubMed] [Google Scholar]
- 28. Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012; 810: 25–58 [DOI] [PubMed] [Google Scholar]
- 29. McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993; 75: 813–819 [DOI] [PubMed] [Google Scholar]
- 30. Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997; 272: C501–C510 [DOI] [PubMed] [Google Scholar]
- 31. Bajpeyi S, Pasarica M, Moro C, et al. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blei ML, Conley KE, Odderson IB, Esselman PC, Kushmerick MJ. Individual variation in contractile cost and recovery in a human skeletal muscle. Proc Natl Acad Sci USA. 1993; 90: 7396–7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heineman FW, Eng J, Berkowitz BA, Balaban RS. NMR spectral analysis of kinetic data using natural lineshapes. Magn Reson Med. 1990; 13: 490–497 [DOI] [PubMed] [Google Scholar]
- 34. Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 2008; 46: 312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch. 1997; 434: 246–253 [DOI] [PubMed] [Google Scholar]
- 36. Conley KE, Esselman PC, Jubrias SA, et al. Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol. 2000; 526(Pt 1):211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008; 3: 965–976 [DOI] [PubMed] [Google Scholar]
- 38. Hutter E, Skovbro M, Lener B, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007; 6: 245–256 [DOI] [PubMed] [Google Scholar]
- 39. Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004; 286: H1742–H1749 [DOI] [PubMed] [Google Scholar]
- 40. Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol. 1997; 82: 1411–1415 [DOI] [PubMed] [Google Scholar]
- 41. Richardson RS, Grassi B, Gavin TP, et al. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999; 86: 1048–1053 [DOI] [PubMed] [Google Scholar]
- 42. Cardus J, Marrades RM, Roca J, et al. Effects of FIO2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc. 1998; 30: 697–703 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.