Abstract
Objective
To determine the efficacy of an automated, interactive, telephone-based health communication intervention for improving glaucoma treatment adherence among patients in two hospital-based eye clinics.
Method
Randomized controlled trial.
Setting
Two eye clinics located in hospitals in the Southeastern United States.
Participants
312 glaucoma patients aged 18 to 80 years, non-adherent with medication taking, medication refills, and/or appointment keeping
Intervention
The treatment group received an automated, interactive, tailored telephone intervention and tailored printed materials. The control group received usual care.
Main Outcome Measures
Adherence with medication taking, prescription refills, and appointment keeping measured by interviews, medical charts, appointment records, and pharmacy data.
Results
A statistically significant increase was found for all adherence measures in both the intervention and control groups. Interactive phone calls and tailored print materials did not significantly improve adherence measures compared to controls.
Conclusions
During the study period, patient adherence to glaucoma treatment and appointment keeping improved in both study arms. Participation in the study and interviews may have contributed. Strategies that address individuals’ barriers and facilitators may increase the impact of telephone calls, especially for appointment keeping and prescription refills.
INTRODUCTION
Glaucoma affects over 2 million adults over age 40 in the United States.1 Its prevalence is higher among black populations and increases with age.2–3 Substantial personal and economic costs are associated with the progression of glaucoma.4–5 Personal, non-financial costs and consequences of vision loss due to glaucoma include loss of independence (e.g., limitations in driving and reading abilities)6–7 and lower quality of life.8
Medication use can reduce the progressive visual field loss caused by glaucoma, but non-adherence with glaucoma medication remains a primary treatment challenge. Rates of patient-reported adherence with glaucoma medication range from approximately 50% to 55% 9–10 and rates of electronically monitored adherence range from 30%11 to 60%.12
Adherence to regular follow-up medical appointments is also critical to effective management of glaucoma. Only a few studies have reported adherence with follow-up appointments.13–16 An analysis of CDC Behavioral Risk Factor Surveillance System data among adults age 40 or older with glaucoma in 19 states, found that 12% self-reported missing recommended follow-up visits.17
Adherence has been found to be poorer in Blacks and those who do not understand the importance of long-term treatment and follow-up visits.16 Interventions to improve glaucoma treatment adherence and its determinants18–19 are needed. A variety of educational materials, services, resources, tools, and devices are available to support patient adherence to glaucoma treatment,20 but evidence regarding their effectiveness is lacking. The current evidence base regarding any single intervention approach is specifically constrained by the availability of few controlled trials in this area, small sample sizes, and limited duration follow-up.21–22 There is a need for larger and longer-duration studies of interventions to improve glaucoma patients’ treatment adherence with both medication and follow-up appointments.
The Interactive Study to Increase Glaucoma adHerence to Treatment (I-SIGHT) randomized controlled trial was conducted to determine the efficacy of an automated, interactive, telephone-based health communication intervention and accompanying printed materials for improving glaucoma treatment adherence among patients in two hospital-based eye clinics. This article reports the results of the I-SIGHT trial on adherence to glaucoma medications, appointment attendance, and medication refills one year after baseline assessments.
METHODS
Design Overview and Procedures
We evaluated the I-SIGHT intervention in a randomized controlled trial with telephone interviews administered to all participants at baseline, 6, 9, and 12 months. The I-SIGHT intervention is a telephone and print-based intervention that is individually-tailored to a participant’s knowledge, attitudes, and behaviors; psychosocial predictors of adherence; health literacy; race and culture; and prescribed medication regimen. Outcome measures of treatment adherence included self-report data on adherence knowledge, attitudes, and behaviors and administrative data on medication-taking, prescription drug renewals, and appointment adherence. The study protocol was approved by the Emory University and University of Pennsylvania Institutional Review Boards and the research oversight committees of the two participating hospitals, and was fully HIPAA compliant. Recruitment was conducted in 2008 and 2009; follow-up was completed in 2009 and 2010 and the trial ended in 2010.
Sample/Setting
Study participants were patients recruited from two eye clinics located in hospitals in the Southeastern United States: a Veterans Affairs hospital and a large public hospital. To be eligible for the study, participants had to meet these criteria: receive treatment for their eye condition at one of the two participating eye clinics; be between the ages of 18 and 80; be Caucasian or Black/African American; have a home or cellular telephone; speak and understand English; be diagnosed with glaucoma or ocular hypertension for at least one year; be prescribed daily doses of topical glaucoma treatments for at least the past year; not have had eye surgery within the past 3 months; have better than 20/200 vision in at least one eye; and be able to read or have someone who can help them with reading printed materials. Participants also had to acknowledge non-adherence, in the past one year, with medication taking, obtaining refills, or clinic appointments in a screening interview. Potentially eligible participants were identified through chart reviews and physician referrals, and contacted by mail and then phone for further eligibility screening and informed consent to participate.
Using a two-group design and a planned sample size of 300 patients, we have adequate power (>80%) to detect a 15 to 20 percentage point difference in adherence with glaucoma treatment at 12-month follow-up. We used a software program “Power and Precision” by Cohen 23 to assess statistical power.
Randomization
After completing the baseline interview, each participant was randomized into either the control or intervention group (with a 1:1 ratio). A random number generator was used in Excel and participants were randomized in blocks of 10. The sequence was generated in advance by the research project manager and participants were assigned in the order that they were enrolled. Randomization was stratified by clinical site because of expected differences in gender, race, and educational level between the sites. Research interviewers were not blinded to assignment because it was necessary to determine treatment group participants’ preferences for intervention delivery (e.g., preferred phone number, time of day). Medical providers were blind to assignment as they were not directly involved in the trial.
Description of the Intervention
The treatment group received a tailored telephone intervention and printed materials. The control group received usual care, including the recommendation for medical appointments and prescription refills on each patient’s physician-prescribed schedule. Both groups received birthday cards from the study team.
The telephone intervention consisted of twelve educational phone calls over a 9 month period: a call every two weeks in months one and two; a call every 3 weeks in months three to five; and a call every 4 weeks in months six to nine. The objectives of the calls were to provide individually-tailored messages to encourage adherence with medication taking, appointment keeping, and refills; provide information about glaucoma; and intervene on barriers to adherence. The intervention was administered in 2009 and 2010.
The intervention calls utilized interactive voice recognition technology to facilitate interest, participation, and interaction with call recipients, and to standardize the content and delivery of the calls. Participants had the option to speak their responses or use a telephone key pad. Calls were primarily outbound but participants had the option to call into the system if they missed a call. After 5 days of unsuccessful attempts to deliver a call, a reminder card was sent requesting that the participant call in to receive his or her message. For each missed call (10 days of unsuccessful attempts), contact was made with participants to ensure that accurate contact information was on file.
Each call was structured to include: a salutation (i.e., greeting, participant verification, and introduction); medication regimen review (i.e., confirmation of medication regimen, assessment of adherence); conversation core with tips to address barriers to adherence (e.g. tip on administering drops); general glaucoma information; and a closing (i.e., synopsis of call and reminder to take medication). Each intervention call was recorded for quality assurance and results of each call attempt were reviewed weekly for each participant.
In addition to the intervention calls, participants received printed materials after each phone call. The printed materials were designed to reinforce tips and strategies to improve treatment adherence, to provide additional glaucoma information, and to be easy to review and reference at the participant’s own time and convenience. They were designed as one-page, double-sided flyers written in large print at an 8th grade reading level. Written materials were mailed one week after completion of a call.
Data Sources: Interviews, Chart Reviews, Pharmacy Records
Baseline and follow-up interviews
Upon enrollment in the study, subjects in both the intervention and control groups were administered a baseline interview over the telephone by a trained research assistant. This structured interview included questions about: demographic and background characteristics of the patient; facilitators and barriers to medication taking, proper medication administration, refills, and appointment-keeping; medication regimen complexity; glaucoma knowledge, information-seeking behavior, health literacy; and psychosocial factors such as self-efficacy, outcomes expectancies, quality of life, and social support.
Follow-up interviews followed the same structure as the baseline interview and were conducted by telephone at 6, 9, and 12 months. Each follow-up interview measured glaucoma treatment adherence (i.e. medication-taking, refills, and appointments). The 9-month interview included questions for participants in the treatment group to evaluate the intervention. The final interview occurred at 12 months and measured glaucoma treatment adherence, facilitators and barriers to glaucoma treatment adherence, and psychosocial factors including self-efficacy and outcome expectancies. Interviews lasted between 20 and 45 minutes, and participants were compensated with a $25 gift card for their time and effort expended on each interview.
The adherence measure, developed and pilot tested by the I-SIGHT study team, assessed adherence with medication taking, refills, and appointment-keeping by self-report. Subjects were considered nonadherent with medication-taking if they reported missing doses of any glaucoma medication within one month of the interview.24 Levels of medication-taking nonadherence were further differentiated by missed doses within 7 days, 2 weeks, or 1 month of the interview. Nonadherence with refills was defined as running out of any glaucoma medication and subsequently missing a dose within a specified time frame (i.e. 1 year prior to the baseline interview; 6 months prior to 6-month interview; and 3 months prior to the 9 and 12 month interview). Appointment-keeping nonadherence was indicated by self-report of missing a glaucoma treatment appointment and not rescheduling during the specified time frame. Self-report of nonadherence in any of these three areas classified the subject as nonadherent with glaucoma treatment.
Chart, administrative, and pharmacy records
Eye clinic medical charts were reviewed at baseline, 6, 9, and 12 months to obtain objective data on nonadherence with each aspect of treatment and to supplement self-reported data. Medication taking nonadherence by chart review was identified through physician notes about missed doses or issues with medication-taking consistency. Refill nonadherence was defined as pharmacy records indicating failure to refill any glaucoma medication prescription within a one month period after it was prescribed, or a physician note on refill nonadherence. Nonadherence with appointment-keeping was assessed as any missed appointment within the specified time frame that was not rescheduled within 3 months, or a physician note on appointment-keeping nonadherence. Three months was chosen as the allowable time to make up an appointment because the participating clinics generally have a wait time of 3 months to schedule an appointment. Adherence data from data abstractions were coded by two raters independently who met in cases of disagreement to resolve discrepancies.
Process Evaluation
We obtained data on patients’ evaluations of the intervention from all treatment group participants who completed the 9-month telephone interview. Closed-ended questions measured the likability/interest, acceptability, and usefulness of the telephone and print content. Sample questions included: How easy to understand were the telephone calls/print materials?; How much did you like the information we sent you in the written materials? Additionally, we sought to evaluate if participants had any logistical problems using the IVR, for example: How much did you have a problem with the system recognizing your voice? The closed-ended question responses used a 1–5 point Likert scale, ranging from 1-Not at all to 5-A lot. To conclude the process evaluation, we asked if they would recommend this study to others.
Statistical analysis
Descriptive statistics were used to describe the study participants, to compare characteristics across treatment groups and study sites, and to summarize participant reactions to the intervention.
The 12 month interview and chart data were used as the main study endpoints. For most patients, an adherence measure at 12 months was available (>90% for all self-report measures, chart refills data, and chart appointment-keeping). However, if the 12 month value on any of the three self-report and three chart indicators of adherence was not available, the 9 month value was used. If the 9 month value was not available, the 6 month value was used. If none of those three were available for a given indicator, the patient was not included in that analysis. Thus, if a patient was nonadherent at 6 months, adherent at 9 months, and adherent at 12 months, then they were considered adherent at their last visit.
The two treatment groups were compared on change in the percent of adherent patients between the baseline visit and the follow-up visit using a longitudinal logistic regression model fit using a generalized linear model.25 The model included a term for treatment, for visit, and for the interaction of treatment and visit. The comparison between the groups is based on the p-value for the interaction term. A separate model was fit for each of the adherence measures. A p-value less than 0.05 was considered statistically significant. No adjustments were made for multiple testing. A stratified analysis by clinical site (VA hospital vs. public hospital) was also conducted. The statistical calculations were done using PROC GENMOD of SAS version 9.2. To examine a possible dose-response effect within the treatment group, a comparison of the proportion of patients adherent at 12 months was performed between those who received all 12 intervention calls and those who did not, using a chi-square test.
RESULTS
Participant Characteristics
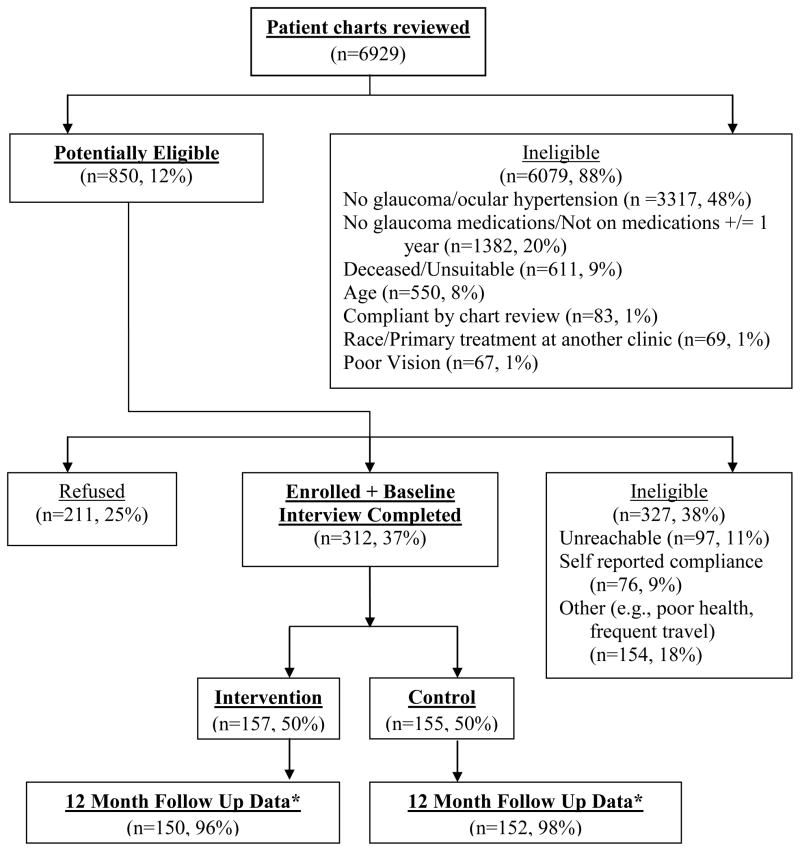
Figure 1 shows the flow chart of potentially eligible, enrolled, randomized patients and those completing the 12-month interview. Of the 850 potentially eligible patients, there were more ineligible participants from the VA Hospital and more refusals from public hospital patients. Participants in the trial (see Table 1) had a mean age of 62.6 years (SD ± 10.2 years) and were mostly in their 50’s and 60’s. There were no significant differences between treatment groups on background characteristics. Just over 60 percent were male, with men comprising over 90 percent of participants from the VA Hospital and only 31 percent from the public hospital (p < .001). More than 90 percent were Black with nearly all the White participants coming from the VA hospital. VA hospital participants were significantly more educated than public hospital subjects (p < .001 for both). VA hospital patients were more likely to be married and were higher-income than public hospital patients.
Figure 1.
Consort Diagram of Eligibility, Participation, Group Assignment, and Study Completion
*12 Month Follow Up Data reflects the >90% of participants who completed the 12 month interview combined with data collected from 6 and 9 month interviews for those without 12 month data
Table 1.
Description of Sample by Treatment Group (n=312)
| Intervention (n=157) | Control (n=155) | p-value a | |
|---|---|---|---|
| Mean (SD) or % (n) | Mean (SD) or % (n) | ||
| Age | 63.13 (9.06) | 62.11 (9.26) | p=0.32 |
| Sex | |||
| Male | 62.4% (98) | 62.6% (97) | |
| Female | 27.6% (59) | 37.4% (58) | p=0.98 |
| Race | |||
| Black/African American | 88.5% (139) | 92.9% (144) | |
| White/Caucasian | 11.5% (18) | 7.1% (11) | p=0.18 |
| Education | |||
| Less than high school | 24.8% (39) | 21.3% (33) | |
| High school/GED | 29.3% (46) | 31.6% (49) | |
| Some college/technical school | 32.5% (51) | 37.5% (58) | |
| College graduate or more | 13.4% (21) | 9.6% (15) | p=0.80 |
| Current Marital Status | |||
| Married | 44.6% (70) | 38.7% (60) | |
| Divorced/separated | 26.1% (41) | 31.0% (48) | |
| Widowed | 19.1% (30) | 18.1% (28) | |
| Never been married | 10.2% (16) | 12.3% (19) | p=0.80 |
| Additional People Living in Household | |||
| No one | 29.9% (47) | 23.9% (37) | |
| One person | 36.9% (58) | 38.7% (60) | |
| Two or more people | 33.2% (52) | 37.4% (58) | p=0.35 |
| Annual Household Income | |||
| $10,000 or less | 32.7% (51) | 33.5% (52) | |
| $10,001 to $20,000 | 24.4% (38) | 26.5% (41) | |
| More than $20,000 | 42.9% (67) | 40.0% (62) | p=0.71 |
Chi-square and t-tests were used
More than half the participants were taking two or more glaucoma medications and more than half had made three or more eye clinic visits during the preceding year. Over 90 percent of participants were nonadherent in at least two of the three areas examined (taking medication, refilling medication, keeping appointments). Based on self-report data, nearly all were nonadherent with medication taking (96.2%), followed by refilling (91.0%) and appointment keeping (65.1%). Chart data showed similarly high nonadherence: medication taking nonadherence at 97.7% was highest, followed by refill nonadherence at 96.1%, and missed appointments at 62.1%. The two treatment groups did not differ in the number of medications, appointments, or adherence rates.
Adherence Outcomes
Table 2 shows the adherence outcomes by treatment group for self-report and chart reports. Adherence increased substantially for all measures and in both groups (all time effects significant at p < 0.01). Self-report medication adherence increased from 10.2% to 30.2% in the treatment group and from 13.5% to 27.0% in the control group (time-by-treatment interaction n.s.). For four of the six outcomes, the treatment group improvements were 4 to 10 percentage points greater than for the control group, but time-by-treatment interactions did not reach statistical significance. A stratified analysis by clinical site revealed similar results, with no treatment group effect among participants at either site. There was no difference in the percent of patients that were adherent at 12 months between those who received all 12 calls and those who did not; however, call completion was very high as 58.7% received all 12 calls and 80.7% received at least 10 calls.
Table 2.
Baseline and last visit adherence by treatment group
| Outcome | Treatment | Baseline | Last visit | Difference baseline to last visit | Interaction p-value† | Visit p-value§ |
|---|---|---|---|---|---|---|
| Self report medication adherence | Intervention | 10.2% | 30.2% | 20% | 0.18 | <0.01 |
| Control | 13.5% | 27.0% | 13.5% | |||
| Self report refill adherence | Intervention | 56.1% | 79.3% | 23.2% | 0.44 | <0.01 |
| Control | 43.2% | 74.3% | 31.1% | |||
| Self report appointment adherence | Intervention | 77.7% | 94.0% | 16.3% | 0.89 | <0.01 |
| Control | 75.5% | 92.8% | 17.3% | |||
| Chart report medication adherence | Intervention | 2.3% | 42.6% | 40.3% | 0.91 | <0.01 |
| Control | 2.4% | 41.5% | 39.1% | |||
| Chart report refill adherence | Intervention | 1.6% | 29.8% | 28.2% | 0.09 | <0.01 |
| Control | 6.2% | 31.0% | 24.8% | |||
| Chart report appointment adherence | Intervention | 38.2% | 69.3% | 31.1% | 0.15 | <0.01 |
| Control | 37.7% | 59.2% | 21.5% |
-Self report measures ranges from 149–157 participants per group
-Chart report measures range from 118–157 participants per group
P-values for treatment*visit interaction (†) and visit (§) in a generalized linear model relating adherence to visit, treatment, and treatment*visit interaction.
Participant Reactions to the Intervention
Participant reactions to the tailored, automated phone calls and written materials were very positive. More than 85% of respondents rated the calls as easy to understand, and 78 to 85% said the calls were interesting, personally relevant, and helpful. The written materials were rated as easy to read by 84.6% of recipients, personally relevant by 78.3%, and attractive by 72.7%. Most liked the interactive features, did not have difficulty with the system recognizing their voice, and preferred to speak their responses rather than using the keypad. All respondents said they would recommend the program to other people with glaucoma.
DISCUSSION
A statistically significant increase for all adherence measures was noted in both the treatment group and the control group in the I-SIGHT trial. The treatment group had greater improvements in adherence in 4 of 6 categories, but this did not reach statistical significance. A previous study of an intervention program to improve glaucoma adherence to medication noted an intervention effect similar in size to those noted in both of our study groups.22 Although it is possible that the adherence measures chosen and piloted for this study were too insensitive to capture a modest treatment effect, it is difficult to explain the statistically significant effect in the control group without questioning whether other study-related factors accounted for the changes. There may have been a selection bias that contributed to a placebo effect in the control group. The “control” patients may have already been highly motivated to seek further knowledge or involvement in managing their glaucoma. Furthermore, all subjects in the I-SIGHT trial completed a baseline interview prior to randomization, and were re-interviewed at 6, 9, and 12 months. The trial retention rate was very high, due in part to study interviewers who were very successful at establishing rapport with the subjects. The fact that interviewers were not blinded to treatment group status is a limitation, although the use of closed-ended questions likely limited any potential for bias. Another possible explanation for the statistically significant increase in adherence in both groups is that adherence is dynamic and varies more throughout the course of a year than previously believed.
Treatment adherence in glaucoma is complex and is influenced by many determinants.22 Nonadherence with glaucoma medication is common, ranging from 5–80%.26–28 Patients with poor health literacy and Black/African American patients have been noted to have poor adherence with glaucoma therapy and greater disease progression.29,30 Interventions to improve adherence require multi-faceted approaches.31
A recent Cochrane Review of the efficacy of glaucoma medication adherence interventions identified only 7 randomized, controlled trials and a total of 8 intervention studies that met the review’s inclusion criteria.21 Gray and colleagues concluded that small sample sizes (ranging from 13 to 202 across the studies), missing data, and short term follow-up durations (ranging from 4–12 weeks) greatly limit the extant evidence in support of any one intervention approach.21 Further, most published studies have investigated glaucoma adherence with medication taking22 with few studies also measuring clinic visit adherence. The I-SIGHT trial addressed these limitations by: 1) developing and testing a multi-component intervention designed to address a variety of adherence determinants, 2) measuring medication-taking, refills, and appointment keeping adherence outcomes, and 3) evaluating efficacy at 12 months with an adequately large sample to detect small to moderate effect sizes.
I-SIGHT evaluated an innovative approach utilizing an automated, patient-centered, and interactive telephone-based intervention strategy on glaucoma patient medication adherence, appointment-keeping, and refills in nonadherent patients. Nonadherence determinations were based on self report, and on chart abstraction of medication taking, pharmacy refill data, and clinic appointment keeping. Interestingly, there were some differences between self-report and chart data, with nonadherence rates lower based on administrative data. This is most likely due to the fact that chart notes may not have indicated medication nonadherence, especially if the patient also missed his or her appointment. It would be a greater concern if self-reported rates of nonadherence were found to be lower than chart data, suggesting that patients may have been reluctant to report their nonadherence to research interviewers. This underscores the importance of careful pre-testing and the use of nonjudgmental questions in the adherence measure interview, though there is no perfect way to measure adherence. In other studies, physician chart notes have been shown to correlate with pharmacy refill records and monitored adherence.26, 32
Interactive voice recognition (IVR) has been used successfully for improving adherence in other chronic conditions such as asthma.33 The voice recognition system allows participants to react to questions and prompts using their own voice, allowing for interactivity and active engagement. Telephones have been found to be an effective channel for delivering tailored interventions.34–36
Using a two-arm, randomized trial, I-SIGHT enrolled 312 participants from two clinical sites. There were no significant differences between the treatment and control groups in background characteristics. The majority of participants in this study were Black/African American and low in socio-economic and educational status. I-SIGHT confirmed poor medication and appointment adherence rates in these patients, highlighting the need for effective strategies to improve glaucoma health literacy and adherence to treatment. However, the study findings may not be generalizable to private practice or other non-clinic settings.
The findings of the I-SIGHT trial suggest that motivated patients participating in an ongoing clinical trial may improve their adherence, even without tailored messages, but because there was not an untailored “attention intervention” condition, this needs to be tested in future research. New technologies, such as IVR and electronic reminder devices, may play a supportive role in the effort to improve adherence in glaucoma patients, but further study is warranted.
Acknowledgments
FUNDING: NIH Grant R01 EY016997
This work was supported in part by an NEI Core Grant for Vision Research (P30 EY 006360) (Beck, Primo, Lynn, Cleveland) and an unrestricted departmental grant from Research to Prevent Blindness (RPB) (Beck, Primo). Karen Glanz’s effort was supported in part by a Georgia Cancer Coalition Distinguished Scholar award, and through the Leonard Davis Institute for Health Economics and the Center for Public Health Initiatives at the University of Pennsylvania.
Footnotes
Karen Glanz led the conception and design of the study. She led the drafting of, reviewed, and revised the publication for intellectual content. She approved the final version to be submitted.
Allen Beck contributed to the conception and design for this study. He participated in the drafting of, reviewed, and revised the publication for content. He approved the final version.
Lucja Bundy contributed to the conception and design for this study. She participated in the drafting of, reviewed, and revised the publication for content. She approved the final version.
Susan Primo contributed to the conception and design for this study. She participated in the drafting of, reviewed, and revised the publication. She provided approval of the final version.
Michael Lynn contributed to the analysis and interpretation of data. He participated in the drafting of, reviewed, and revised the publication content. He approved the final version.
Julia Cleveland contributed to the analysis and interpretation of data. She participated in the drafting of, reviewed, and revised the publication content. She approved the final version.
Katharina Echt contributed to the conception and design for this study. She participated in the drafting of, reviewed, and revised the publication for content. She approved the final version.
ClinicalTrials.gov Registration: NCT00794170
References
- 1.Prevent Blindness America. 2011 www.preventblindness.org.
- 2.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 3.McGwin G, Khoury R, Cross J, Owsley C. Vision impairment and eye care utilization among Americans 50 and older. Curr Eye Res. 2010 Jun;35(6):451–458. doi: 10.3109/02713681003664931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006 Jan;124(1):12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 5.Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011 Oct;152(4):515–522. doi: 10.1016/j.ajo.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009 Oct;116(10):1846–1853. doi: 10.1016/j.ophtha.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophthalmol. 2009 Jan;127(1):82–87. doi: 10.1001/archophthalmol.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez P, Wilson MR, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997 Jun;115(6):777–784. doi: 10.1001/archopht.1997.01100150779014. [DOI] [PubMed] [Google Scholar]
- 9.Rees G, Leong O, Crowston JG, Lamoureux EL. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmology. 2010 May;117(5):903–908. doi: 10.1016/j.ophtha.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Sleath B, Ballinger R, Covert D, Robin AL, Byrd JE, Tudor G. Self-reported prevalence and factors associated with nonadherence with glaucoma medications in veteran outpatients. Am J Geriatr Pharmacother. 2009 Apr;7(2):67–73. doi: 10.1016/j.amjopharm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Rossi GC, Pasinetti GM, Scudeller L, Tinelli C, Milano G, Bianchi PE. Monitoring adherence rates in glaucoma patients using the Travatan Dosing Aid. A 6-month study comparing patients on travoprost 0.004% and patients on travoprost 0.004%/timolol 0.5% fixed combination. Expert Opin Pharmacother. 2010 Mar;11(4):499–504. doi: 10.1517/14656561003601994. [DOI] [PubMed] [Google Scholar]
- 12.Nordmann JP, Baudouin C, Renard JP, et al. Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a survey. Clin Ophthalmol. 2010;4:731–739. doi: 10.2147/opth.s11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaya FT. Compliance with medicine. Ophthalmol Clin North Am. 2005 Dec;18(4):611–617. doi: 10.1016/j.ohc.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Gwira JA, Vistamehr S, Shelsta H, et al. Factors associated with failure to follow up after glaucoma screening: a study in an African American population. Ophthalmology. 2006 Aug;113(8):1315–1319. doi: 10.1016/j.ophtha.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Kosoko O, Quigley HA, Vitale S, Enger C, Kerrigan L, Tielsch JM. Risk factors for noncompliance with glaucoma follow-up visits in a residents’ eye clinic. Ophthalmology. 1998 Nov;105(11):2105–2111. doi: 10.1016/S0161-6420(98)91134-4. [DOI] [PubMed] [Google Scholar]
- 16.Murakami Y, Lee BW, Duncan M, et al. Racial and ethnic disparities in adherence to glaucoma follow-up visits in a county hospital population. Arch Ophthalmol. 2011 Jul;129(7):872–878. doi: 10.1001/archophthalmol.2011.163. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Eye-care utilization among women aged >40 years with eye diseases - 19 states, 2006–2008. Morbidity & Mortality Weekly Report. 2010;59(19):588–591. [PubMed] [Google Scholar]
- 18.Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003 Oct;12(5):393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Stryker JE, Beck AD, Primo SA, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010 Jan;19(1):66–72. doi: 10.1097/IJG.0b013e31819c4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowing D, Messer D, Slagle S, Wasik A. Programs to optimize adherence in glaucoma. Optometry. 2010 Jul;81(7):339–350. doi: 10.1016/j.optm.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Gray TA, Orton LC, Henson D, Harper R, Waterman H. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev. 2009;(2):CD006132. doi: 10.1002/14651858.CD006132.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009 Dec;116(12):2286–2293. doi: 10.1016/j.ophtha.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J, Borenstein M, Rothstein H, et al. Power and precision. Englewood NJ: Biostat Inc; 2001. [Google Scholar]
- 24.Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006 Mar;113(3):431–436. doi: 10.1016/j.ophtha.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–32. [Google Scholar]
- 26.Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986 May 15;101(5):515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 27.Kass MA, Gordon M, Morley RE, Jr, Meltzer DW, Goldberg JJ. Compliance with topical timolol treatment. Am J Ophthalmol. 1987 Feb 15;103(2):188–193. doi: 10.1016/s0002-9394(14)74225-4. [DOI] [PubMed] [Google Scholar]
- 28.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005 Jun;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Juzych MS, Randhawa S, Shukairy A, Kaushal P, Gupta A, Shalauta N. Functional health literacy in patients with glaucoma in urban settings. Arch Ophthalmol. 2008 May;126(5):718–724. doi: 10.1001/archopht.126.5.718. [DOI] [PubMed] [Google Scholar]
- 30.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009 Jun;116(6):1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol. 2006 Apr;17(2):190–195. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 32.Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology. 2007 Sep;114(9):1599–1606. doi: 10.1016/j.ophtha.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Adams WG, Fuhlbrigge AL, Miller CW, et al. TLC-Asthma: an integrated information system for patient-centered monitoring, case management, and point-of-care decision support. AMIA Annu Symp Proc. 2003:1–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong AW, Watson AJ, Makredes M, Frangos JE, Kimball AB, Kvedar JC. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009 Nov;145(11):1230–1236. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 35.Rimer BK, Halabi S, Sugg Skinner C, et al. Effects of a mammography decision-making intervention at 12 and 24 months. Am J Prev Med. 2002 May;22(4):247–257. doi: 10.1016/s0749-3797(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 36.Lauver DR, Settersten L, Kane JH, Henriques JB. Tailored messages, external barriers, and women’s utilization of professional breast cancer screening over time. Cancer. 2003 Jun 1;97(11):2724–2735. doi: 10.1002/cncr.11397. [DOI] [PubMed] [Google Scholar]