Abstract
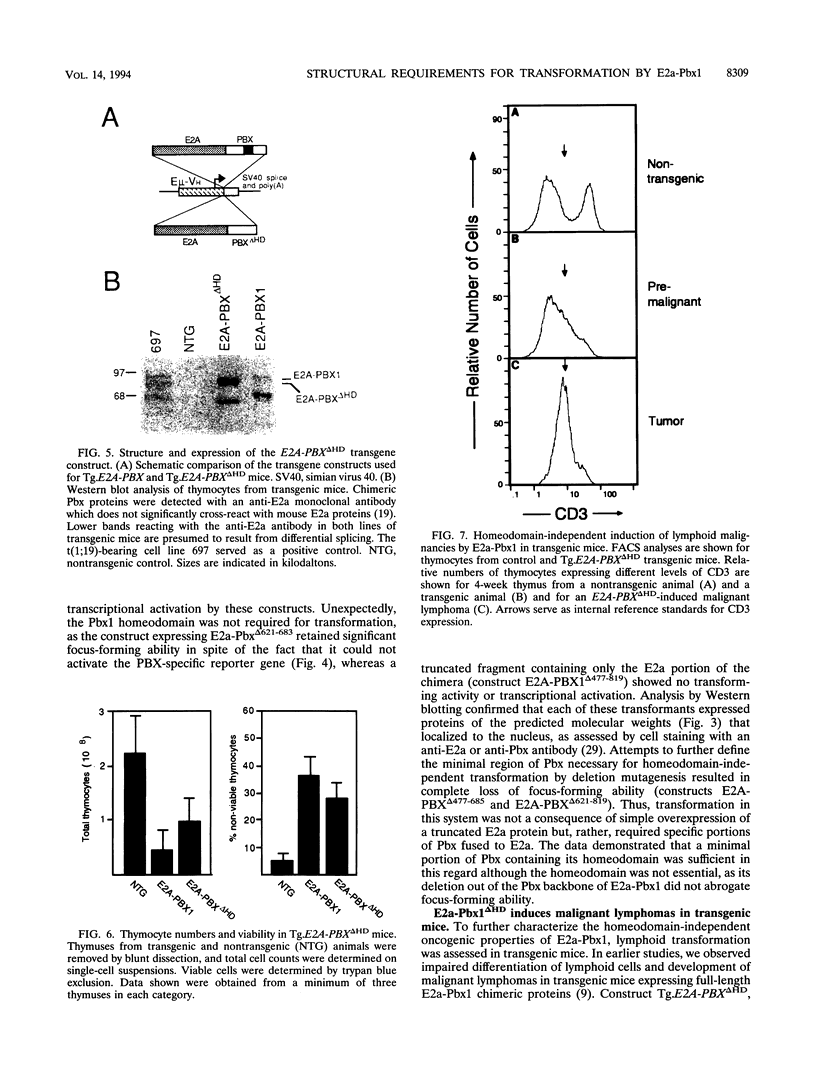
The t(1;19) chromosomal translocation in acute lymphoblastic leukemias creates chimeric E2a-Pbx1 oncoproteins that can act as DNA-binding activators of transcription. A structural analysis of the functional domains of E2a-Pbx1 showed that portions of both E2a and Pbx1 were essential for transformation of NIH 3T3 cells and transcriptional activation of synthetic reporter genes containing PBX1 consensus binding sites. Hyperexpression of wild-type or experimentally truncated Pbx1 proteins was insufficient for transformation, consistent with their inability to activate transcription. When fused with E2a, the Pbx-related proteins Pbx2 and Pbx3 were also transformation competent, demonstrating that all known members of this highly similar subfamily of homeodomain proteins have latent oncogenic potential. The oncogenic contributions of E2a to the chimeras were localized to transactivation motifs AD1 and AD2, as their mutation significantly impaired transformation. Either the homeodomain or Pbx1 amino acids flanking this region could mediate transformation when fused to E2a. However, the homeodomain was not essential for transformation, since a mutant E2a-Pbx1 protein (E2a-Pbx delta HD) lacking the homeodomain efficiently transformed fibroblasts and induced malignant lymphomas in transgenic mice. Thus, transformation mediated by the chimeric oncoprotein E2a-Pbx1 is absolutely dependent on motifs acquired from E2a but the Pbx1 homeodomain is optional. The latter finding suggests that E2a-Pbx1 may interact with cellular proteins that assist or mediate alterations in gene expression responsible for oncogenesis even in the absence of homeodomain-DNA interactions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberdam D., Negreanu V., Sachs L., Blatt C. The oncogenic potential of an activated Hox-2.4 homeobox gene in mouse fibroblasts. Mol Cell Biol. 1991 Jan;11(1):554–557. doi: 10.1128/mcb.11.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan J., Baler R., Morrissey D., Zuo J., Lan Y., Weir M., Voellmy R. Synergistic activation of transcription is mediated by the N-terminal domain of Drosophila fushi tarazu homeoprotein and can occur without DNA binding by the protein. Mol Cell Biol. 1993 Mar;13(3):1599–1609. doi: 10.1128/mcb.13.3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronheim A., Shiran R., Rosen A., Walker M. D. The E2A gene product contains two separable and functionally distinct transcription activation domains. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8063–8067. doi: 10.1073/pnas.90.17.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F. G., Galili N., Holick J., Biegel J. A., Rovera G., Emanuel B. S. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Feb;3(2):113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Winter E., Müller W. A retroviral expression vector containing murine immunoglobulin heavy chain promoter/enhancer. Nucleic Acids Res. 1988 Nov 25;16(22):10939–10939. doi: 10.1093/nar/16.22.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Aberdam D., Schwartz R., Sachs L. DNA rearrangement of a homeobox gene in myeloid leukaemic cells. EMBO J. 1988 Dec 20;7(13):4283–4290. doi: 10.1002/j.1460-2075.1988.tb03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürglin T. R., Ruvkun G. New motif in PBX genes. Nat Genet. 1992 Aug;1(5):319–320. doi: 10.1038/ng0892-319. [DOI] [PubMed] [Google Scholar]
- Cleary M. L. Oncogenic conversion of transcription factors by chromosomal translocations. Cell. 1991 Aug 23;66(4):619–622. doi: 10.1016/0092-8674(91)90105-8. [DOI] [PubMed] [Google Scholar]
- Dedera D. A., Waller E. K., LeBrun D. P., Sen-Majumdar A., Stevens M. E., Barsh G. S., Cleary M. L. Chimeric homeobox gene E2A-PBX1 induces proliferation, apoptosis, and malignant lymphomas in transgenic mice. Cell. 1993 Sep 10;74(5):833–843. doi: 10.1016/0092-8674(93)90463-z. [DOI] [PubMed] [Google Scholar]
- Dubé I. D., Kamel-Reid S., Yuan C. C., Lu M., Wu X., Corpus G., Raimondi S. C., Crist W. M., Carroll A. J., Minowada J. A novel human homeobox gene lies at the chromosome 10 breakpoint in lymphoid neoplasias with chromosomal translocation t(10;14). Blood. 1991 Dec 1;78(11):2996–3003. [PubMed] [Google Scholar]
- Fitzpatrick V. D., Percival-Smith A., Ingles C. J., Krause H. M. Homeodomain-independent activity of the fushi tarazu polypeptide in Drosophila embryos. Nature. 1992 Apr 16;356(6370):610–612. doi: 10.1038/356610a0. [DOI] [PubMed] [Google Scholar]
- Flegel W. A., Singson A. W., Margolis J. S., Bang A. G., Posakony J. W., Murre C. Dpbx, a new homeobox gene closely related to the human proto-oncogene pbx1 molecular structure and developmental expression. Mech Dev. 1993 May;41(2-3):155–161. doi: 10.1016/0925-4773(93)90045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss G. H., Lieber M. R. DEAE-dextran enhances electroporation of mammalian cells. Nucleic Acids Res. 1992 Dec 25;20(24):6739–6740. doi: 10.1093/nar/20.24.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Hatano M., Roberts C. W., Minden M., Crist W. M., Korsmeyer S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991 Jul 5;253(5015):79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Jacobs Y., Vierra C., Nelson C. E2A expression, nuclear localization, and in vivo formation of DNA- and non-DNA-binding species during B-cell development. Mol Cell Biol. 1993 Dec;13(12):7321–7333. doi: 10.1128/mcb.13.12.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Mol Cell Biol. 1993 Jan;13(1):351–357. doi: 10.1128/mcb.13.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Look A. T., Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991 Mar;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kennedy M. A., Gonzalez-Sarmiento R., Kees U. R., Lampert F., Dear N., Boehm T., Rabbitts T. H. HOX11, a homeobox-containing T-cell oncogene on human chromosome 10q24. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8900–8904. doi: 10.1073/pnas.88.20.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K., Allen J., Adams J. M. Expression of Hox-2.4 homeobox gene directed by proviral insertion in a myeloid leukemia. Nucleic Acids Res. 1989 Mar 11;17(5):1881–1892. doi: 10.1093/nar/17.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence H. J., Largman C. Homeobox genes in normal hematopoiesis and leukemia. Blood. 1992 Nov 15;80(10):2445–2453. [PubMed] [Google Scholar]
- Lawrence H. J., Stage K. M., Mathews C. H., Detmer K., Scibienski R., MacKenzie M., Migliaccio E., Boncinelli E., Largman C. Expression of HOX C homeobox genes in lymphoid cells. Cell Growth Differ. 1993 Aug;4(8):665–669. [PubMed] [Google Scholar]
- LeBrun D. P., Cleary M. L. Fusion with E2A alters the transcriptional properties of the homeodomain protein PBX1 in t(1;19) leukemias. Oncogene. 1994 Jun;9(6):1641–1647. [PubMed] [Google Scholar]
- Lu M., Gong Z. Y., Shen W. F., Ho A. D. The tcl-3 proto-oncogene altered by chromosomal translocation in T-cell leukemia codes for a homeobox protein. EMBO J. 1991 Oct;10(10):2905–2910. doi: 10.1002/j.1460-2075.1991.tb07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulbecker C. C., Gruss P. The oncogenic potential of Pax genes. EMBO J. 1993 Jun;12(6):2361–2367. doi: 10.1002/j.1460-2075.1993.tb05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulbecker C. C., Gruss P. The oncogenic potential of deregulated homeobox genes. Cell Growth Differ. 1993 May;4(5):431–441. [PubMed] [Google Scholar]
- Monica K., Galili N., Nourse J., Saltman D., Cleary M. L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1991 Dec;11(12):6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Cleary M. L. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994 Apr 15;83(8):2261–2267. [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Perkins A., Kongsuwan K., Visvader J., Adams J. M., Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukemia. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quong M. W., Massari M. E., Zwart R., Murre C. A new transcriptional-activation motif restricted to a class of helix-loop-helix proteins is functionally conserved in both yeast and mammalian cells. Mol Cell Biol. 1993 Feb;13(2):792–800. doi: 10.1128/mcb.13.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C., Peifer M., Wieschaus E. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993 Sep 24;74(6):1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- Schier A. F., Gehring W. J. Functional specificity of the homeodomain protein fushi tarazu: the role of DNA-binding specificity in vivo. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1450–1454. doi: 10.1073/pnas.90.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Shapiro D. N., Sublett J. E., Li B., Downing J. R., Naeve C. W. Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 1993 Nov 1;53(21):5108–5112. [PubMed] [Google Scholar]
- Van Dijk M. A., Voorhoeve P. M., Murre C. Pbx1 is converted into a transcriptional activator upon acquiring the N-terminal region of E2A in pre-B-cell acute lymphoblastoid leukemia. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6061–6065. doi: 10.1073/pnas.90.13.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]