Abstract
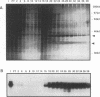
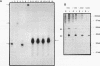
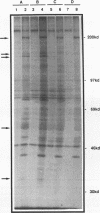
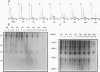
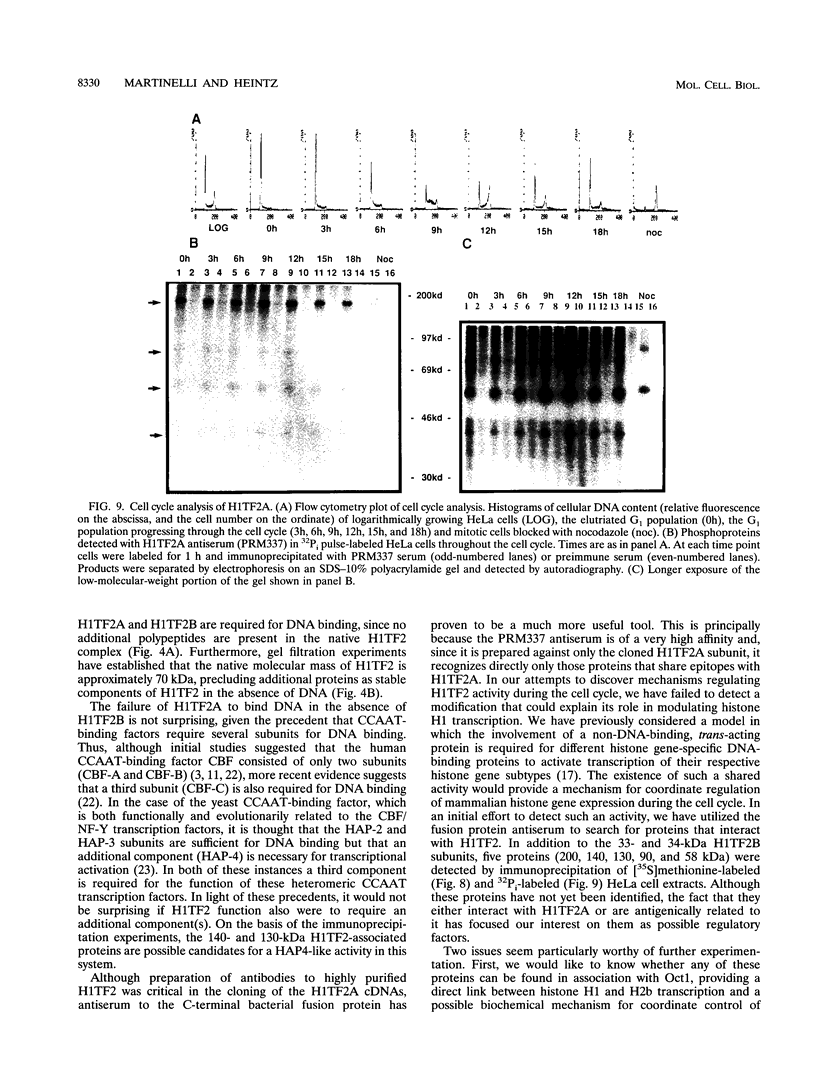
H1TF2 is a CCAAT transcription factor that binds to the histone H1 subtype-specific consensus sequence, which has previously been shown to be necessary for temporal regulation of histone H1 transcription during the cell cycle (F. La Bella, P. Gallinari, J. McKinney, and N. Heintz, Genes Dev. 3:1982-1990, 1989). In this study, we report that H1TF2 is a heteromeric CCAAT-binding protein composed of two polypeptide doublets of 33 and 34 kDa and 43 and 44 kDa that are not antigenically related. The 33- and 34-kDa species were not detected in our previous studies (P. Gallinari, F. La Bella, and N. Heintz, Mol. Cell. Biol. 9:1566-1575, 1989) because of technical problems in detection of these heavily glycosylated subunits. The cloning of H1TF2A, the large subunit of this factor, reveals it to be a glutamine-rich protein with extremely limited similarity to previously cloned CCAAT-binding proteins. A monospecific antiserum produced against bacterially synthesized H1TF2A was used to establish that HeLa cell H1TF2A is phosphorylated in vivo and that, in contrast to the H2b transcription factor Oct1 (S. B. Roberts, N. Segil, and N. Heintz, Science 253:1022-1026, 1991; N. Segil, S. B. Roberts, and N. Heintz, Cold Spring Harbor Symp. Quant. Biol. 56:285-292, 1991), no gross change in H1TF2A phosphorylation is evident during the cell cycle. Further immunoprecipitation studies demonstrated that H1TF2 is heterodimeric in the absence of DNA in vivo and identified several H1TF2-interacting proteins that may play a role in H1TF2 function in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D. M., Fikes J. D., Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Kannan P., Imhof A., Bauer R., Yim S. O., Glockshuber R., Van Dyke M. W., Tainsky M. A. An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol Cell Biol. 1993 Jul;13(7):4174–4185. doi: 10.1128/mcb.13.7.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. A gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase. EMBO J. 1988 Jan;7(1):49–56. doi: 10.1002/j.1460-2075.1988.tb02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
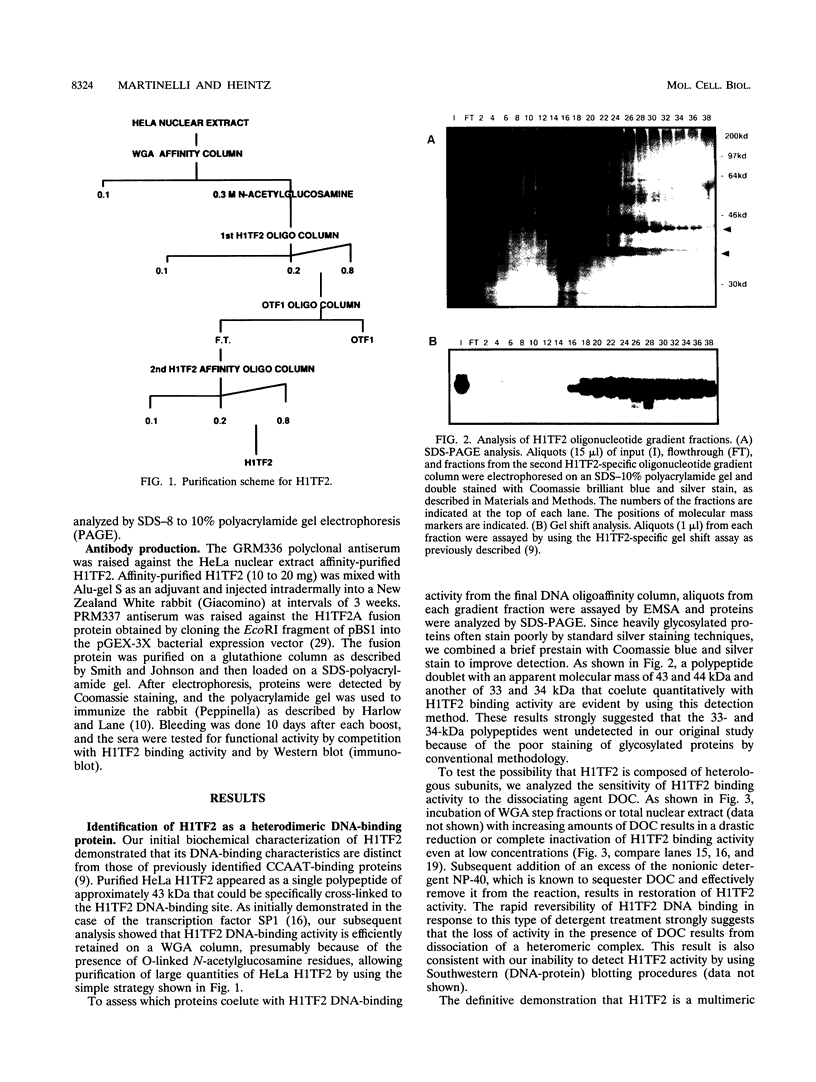
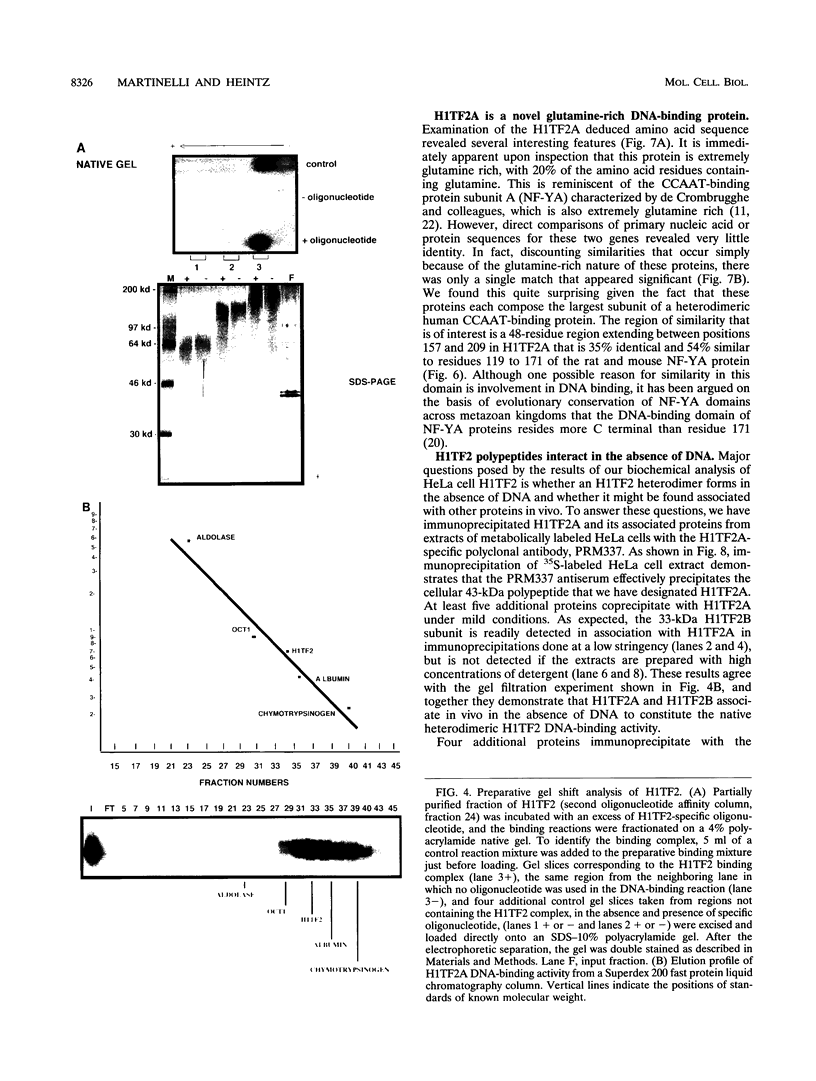
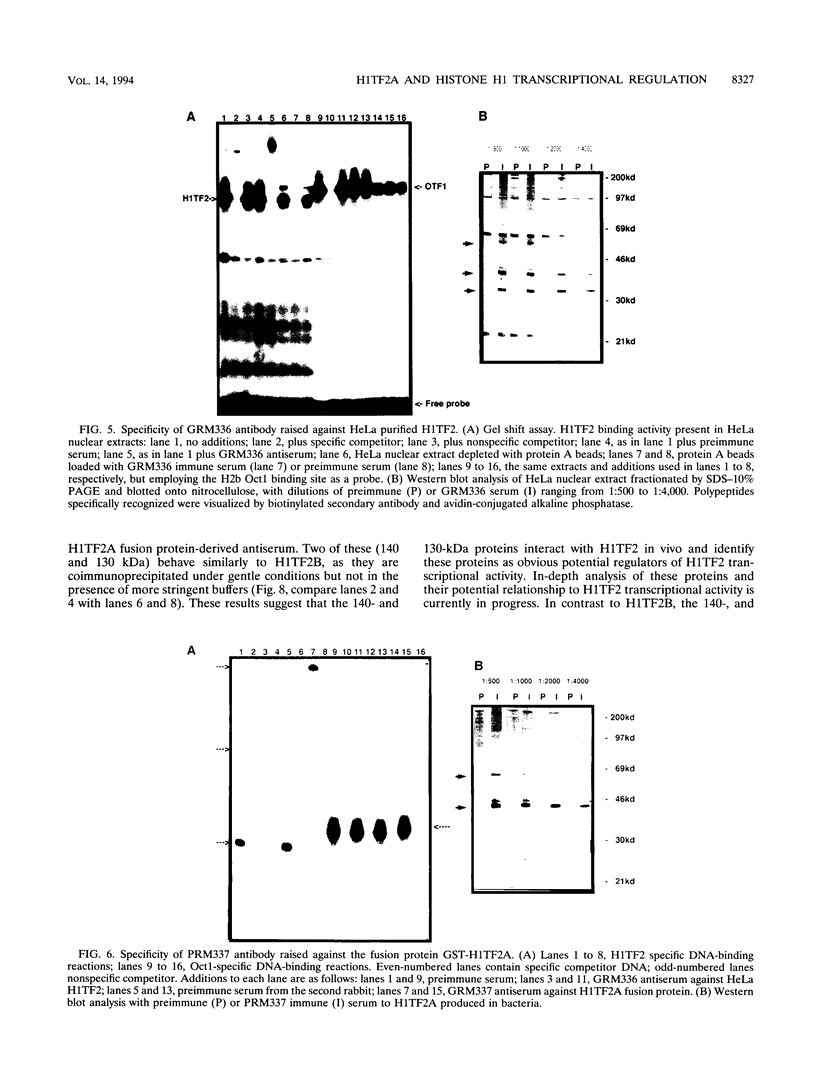
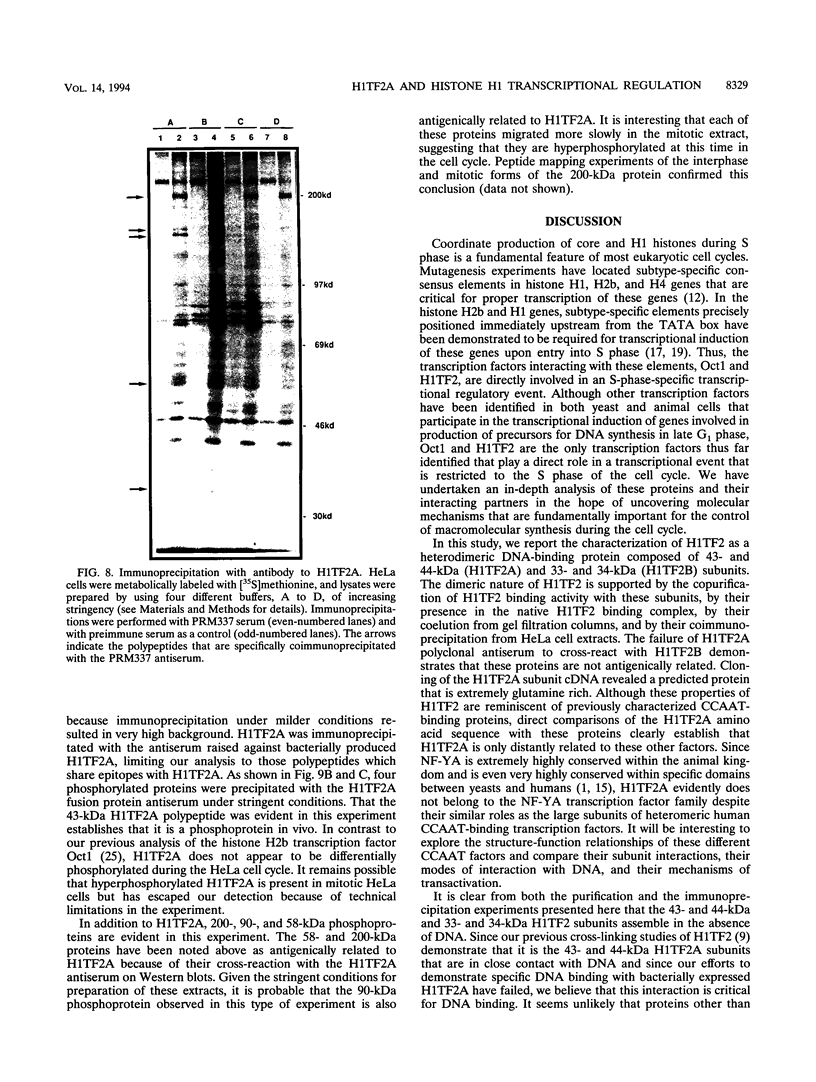
- Gallinari P., La Bella F., Heintz N. Characterization and purification of H1TF2, a novel CCAAT-binding protein that interacts with a histone H1 subtype-specific consensus element. Mol Cell Biol. 1989 Apr;9(4):1566–1575. doi: 10.1128/mcb.9.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamochi A., Golumbek P. T., Van Schaftingen E., de Crombrugghe B. A CCAAT DNA binding factor consisting of two different components that are both required for DNA binding. J Biol Chem. 1988 Apr 25;263(12):5940–5947. [PubMed] [Google Scholar]
- Heintz N. H., Dailey L., Held P., Heintz N. Eukaryotic replication origins as promoters of bidirectional DNA synthesis. Trends Genet. 1992 Nov;8(11):376–381. doi: 10.1016/0168-9525(92)90298-i. [DOI] [PubMed] [Google Scholar]
- Heintz N. The regulation of histone gene expression during the cell cycle. Biochim Biophys Acta. 1991 Mar 26;1088(3):327–339. doi: 10.1016/0167-4781(91)90122-3. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R., Li X. Y., Black D., Matthes H., Benoist C., Mathis D. Co-evolution from yeast to mouse: cDNA cloning of the two NF-Y (CP-1/CBF) subunits. EMBO J. 1990 Oct;9(10):3119–3127. doi: 10.1002/j.1460-2075.1990.tb07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. C., Qi M., DeFranco D. B. Cell cycle regulation of glucocorticoid receptor function. EMBO J. 1992 Sep;11(9):3457–3468. doi: 10.1002/j.1460-2075.1992.tb05425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- La Bella F., Gallinari P., McKinney J., Heintz N. Histone H1 subtype-specific consensus elements mediate cell cycle-regulated transcription in vitro. Genes Dev. 1989 Dec;3(12A):1982–1990. doi: 10.1101/gad.3.12a.1982. [DOI] [PubMed] [Google Scholar]
- LaBella F., Sive H. L., Roeder R. G., Heintz N. Cell-cycle regulation of a human histone H2b gene is mediated by the H2b subtype-specific consensus element. Genes Dev. 1988 Jan;2(1):32–39. doi: 10.1101/gad.2.1.32. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Mantovani R., Hooft van Huijsduijnen R., Andre I., Benoist C., Mathis D. Evolutionary variation of the CCAAT-binding transcription factor NF-Y. Nucleic Acids Res. 1992 Mar 11;20(5):1087–1091. doi: 10.1093/nar/20.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. Mitosis-specific phosphorylation of the nuclear oncoproteins Myc and Myb. J Cell Biol. 1992 Aug;118(4):775–784. doi: 10.1083/jcb.118.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S. N., Vuorio T., de Crombrugghe B. The B subunit of a rat heteromeric CCAAT-binding transcription factor shows a striking sequence identity with the yeast Hap2 transcription factor. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5378–5382. doi: 10.1073/pnas.87.14.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. T., Guarente L. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 1990 Oct;4(10):1714–1729. doi: 10.1101/gad.4.10.1714. [DOI] [PubMed] [Google Scholar]
- Osley M. A. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Roberts S. B., Segil N., Heintz N. Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science. 1991 Aug 30;253(5023):1022–1026. doi: 10.1126/science.1887216. [DOI] [PubMed] [Google Scholar]
- Segil N., Roberts S. B., Heintz N. Cell-cycle-regulated phosphorylation of the transcription factor Oct-1. Cold Spring Harb Symp Quant Biol. 1991;56:285–292. doi: 10.1101/sqb.1991.056.01.035. [DOI] [PubMed] [Google Scholar]
- Segil N., Roberts S. B., Heintz N. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science. 1991 Dec 20;254(5039):1814–1816. doi: 10.1126/science.1684878. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stoykova A. S., Sterrer S., Erselius J. R., Hatzopoulos A. K., Gruss P. Mini-Oct and Oct-2c: two novel, functionally diverse murine Oct-2 gene products are differentially expressed in the CNS. Neuron. 1992 Mar;8(3):541–558. doi: 10.1016/0896-6273(92)90282-i. [DOI] [PubMed] [Google Scholar]