Abstract
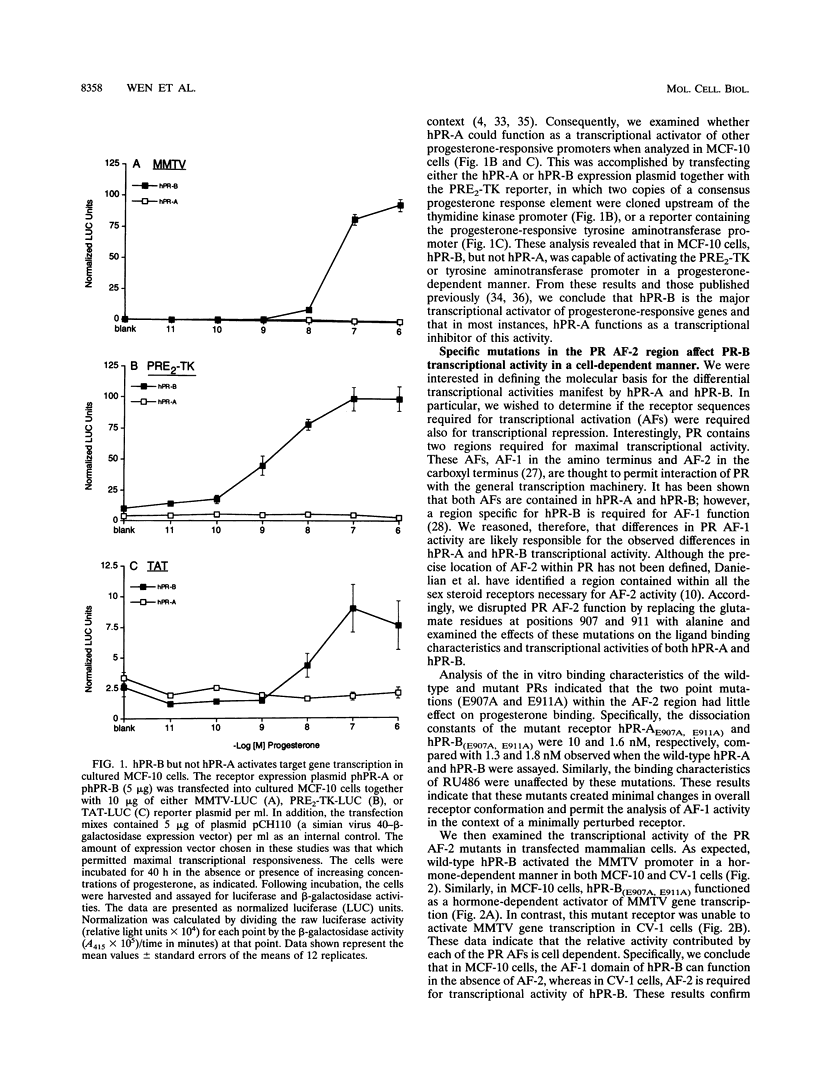
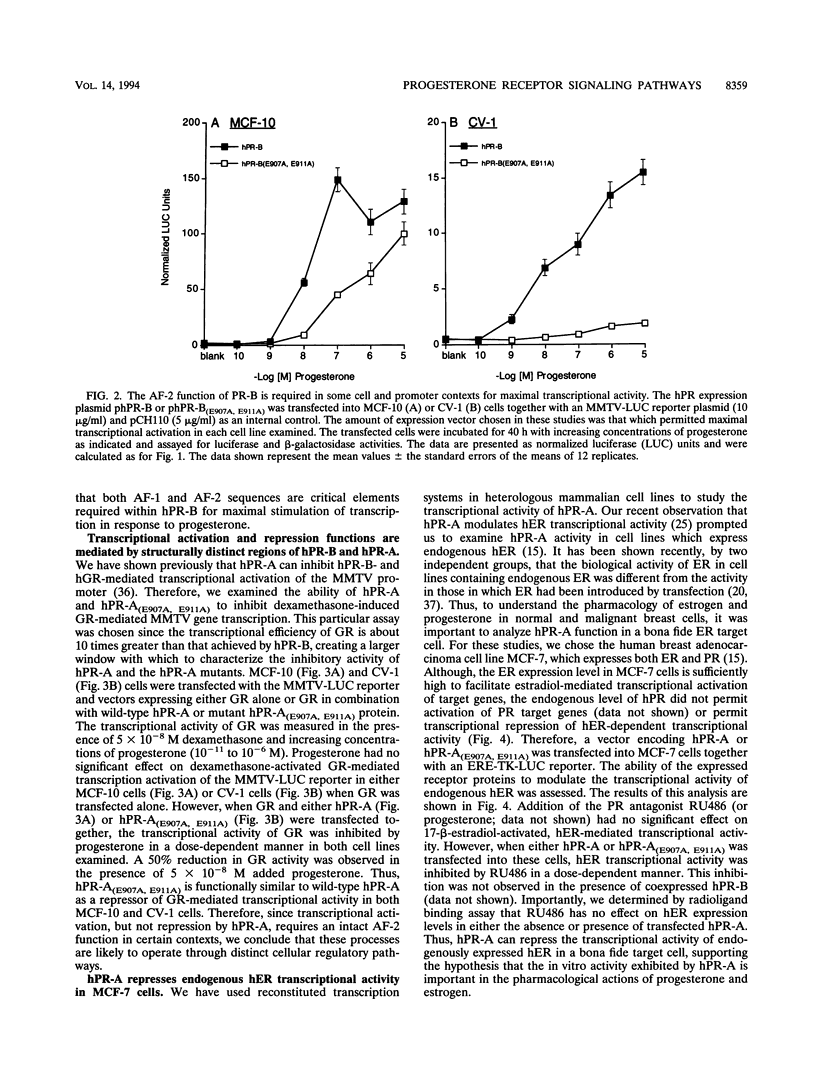
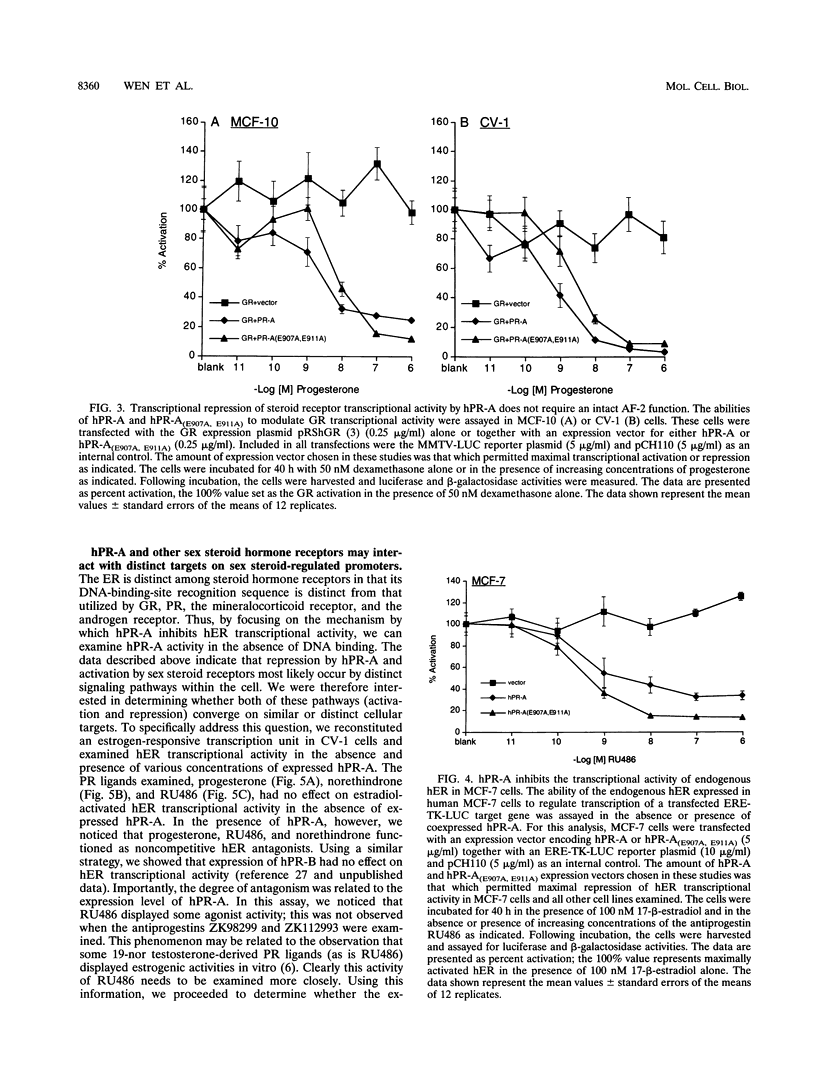
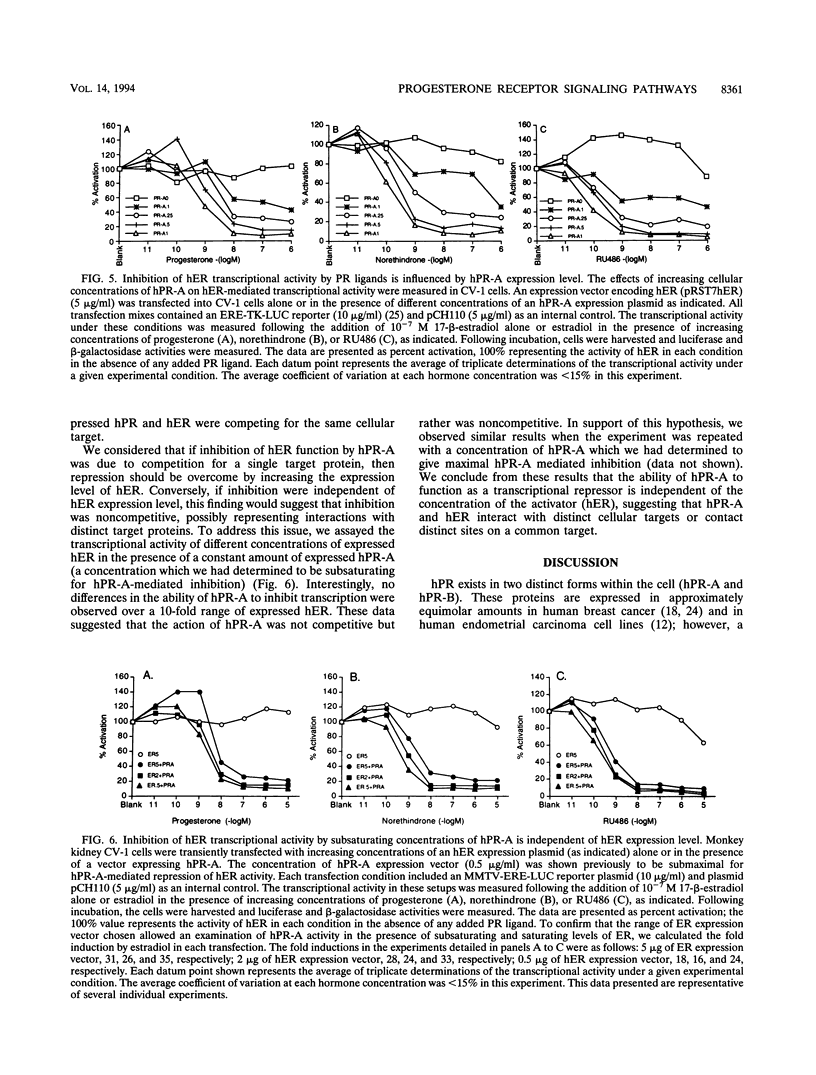
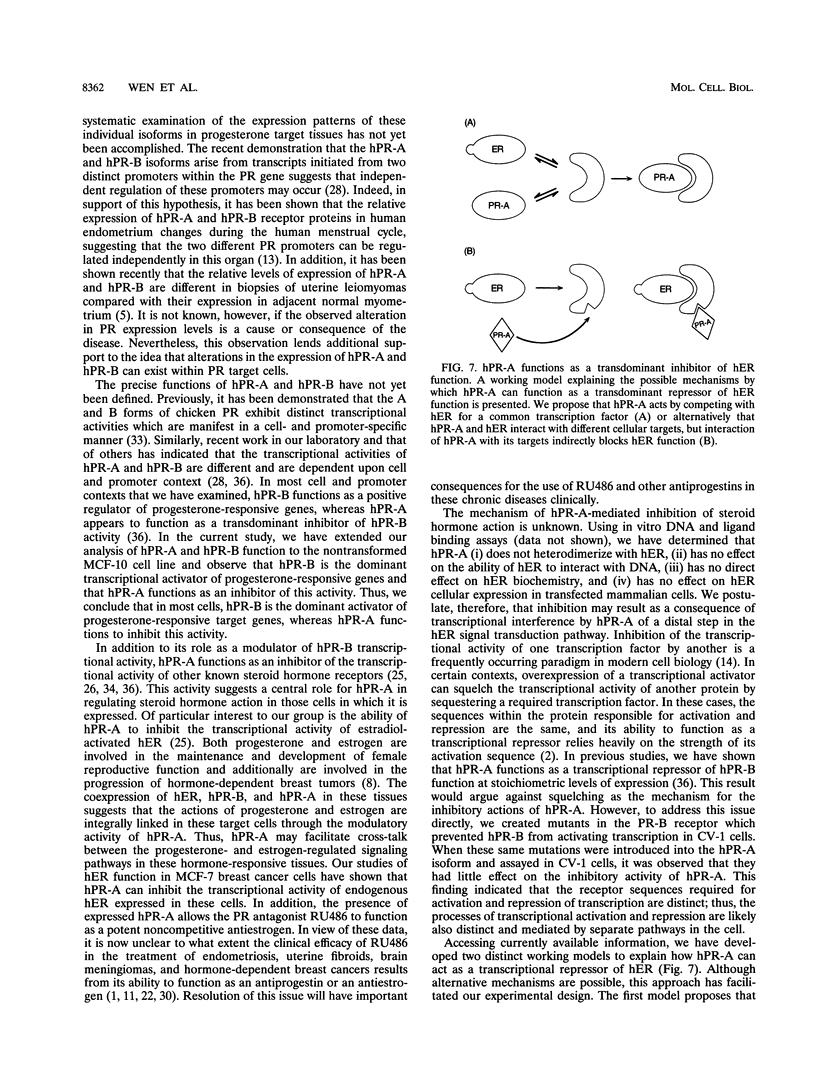
The biological response to progesterone is mediated by two distinct forms of the human progesterone receptor (hPR-A and hPR-B). In most cell contexts, hPR-B functions as a transcriptional activator of progesterone-responsive genes, whereas hPR-A functions as a transcriptional inhibitor of all steroid hormone receptors. We have created mutations within the carboxyl terminus of hPR which differentially effect the transcriptional activity of hPR-B in a cell- and promoter-specific manner. Analogous mutations, when introduced into hPR-A, have no effect on its ability to inhibit the transcriptional activity of other steroid hormone receptors. The observed differences in the structural requirements for hPR-B and hPR-A function suggest that transcriptional activation and repression by PR are mediated by two separate pathways within the cell. In support of this hypothesis, we have shown that hPR-A mediated repression of human estrogen receptor (hER) transcriptional activity is not dependent on hER expression level but depends largely on the absolute expression level of hPR-A. Thus, it appears that hPR-A inhibits hER transcriptional activity as a consequence of a noncompetitive interaction of hPR-A with either distinct cellular targets or different contact sites on the same target. We propose that hPR-A expression facilitates a ligand-dependent cross-talk among sex steroid receptor signaling pathways within the cell. It is likely, therefore, that alterations in the expression level of hPR-A or its cellular target can have profound effects on the physiological or pharmacological responses to sex steroid hormone receptor ligands.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Piña B., Silverman N., Marcus G. A., Agapite J., Regier J. L., Triezenberg S. J., Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992 Jul 24;70(2):251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Berger T. S., Parandoosh Z., Perry B. W., Stein R. B. Interaction of glucocorticoid analogues with the human glucocorticoid receptor. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):733–738. doi: 10.1016/0960-0760(92)90414-e. [DOI] [PubMed] [Google Scholar]
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon D. D., Bethea C. L., Strawn E. Y., Novy M. J., Burry K. A., Harrington M. S., Erickson T. E., Warner C., Keenan E. J., Clinton G. M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993 Jul;169(1):78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- Catherino W. H., Jeng M. H., Jordan V. C. Norgestrel and gestodene stimulate breast cancer cell growth through an oestrogen receptor mediated mechanism. Br J Cancer. 1993 May;67(5):945–952. doi: 10.1038/bjc.1993.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clarke C. L., Sutherland R. L. Progestin regulation of cellular proliferation. Endocr Rev. 1990 May;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Hoare S. A., Fawell S. E., Parker M. G. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993 Feb;7(2):232–240. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil P. D., Clarke C. L., Satyaswaroop P. G. Progesterone receptor structure and protease activity in primary human endometrial carcinoma. Cancer Res. 1988 Mar 1;48(5):1143–1147. [PubMed] [Google Scholar]
- Feil P. D., Clarke C. L., Satyaswaroop P. G. Progestin-mediated changes in progesterone receptor forms in the normal human endometrium. Endocrinology. 1988 Nov;123(5):2506–2513. doi: 10.1210/endo-123-5-2506. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Gottardis M. M., Robinson S. P., Jordan V. C. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30(1-6):311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993 Jan 29;72(2):247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Alexander P. S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983 Dec;113(6):2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Tsai S. Y., Tsai M. J., O'Malley B. W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J Biol Chem. 1992 Sep 5;267(25):17617–17623. [PubMed] [Google Scholar]
- Jiang S. Y., Jordan V. C. Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst. 1992 Apr 15;84(8):580–591. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettel L. M., Murphy A. A., Mortola J. F., Liu J. H., Ulmann A., Yen S. S. Endocrine responses to long-term administration of the antiprogesterone RU486 in patients with pelvic endometriosis. Fertil Steril. 1991 Sep;56(3):402–407. doi: 10.1016/s0015-0282(16)54531-2. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Lessey B. A., Alexander P. S., Horwitz K. B. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology. 1983 Apr;112(4):1267–1274. doi: 10.1210/endo-112-4-1267. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Goldman M. E. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem. 1994 Apr 22;269(16):11945–11949. [PubMed] [Google Scholar]
- McDonnell D. P., Shahbaz M. M., Vegeto E., Goldman M. E. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994 Apr;48(5-6):425–432. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Quirin-Stricker C., Lerouge T., Bocquel M. T., Gronemeyer H. A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem. 1992 May 25;267(15):10882–10887. [PubMed] [Google Scholar]
- O'Malley B. W. 13th San Antonio Breast Cancer Symposium--Plenary Lecture. Steroid hormone receptors as transactivators of gene expression. Breast Cancer Res Treat. 1991 May;18(2):67–71. doi: 10.1007/BF01980968. [DOI] [PubMed] [Google Scholar]
- Romieu G., Maudelonde T., Ulmann A., Pujol H., Grenier J., Cavalie G., Khalaf S., Rochefort H. The antiprogestin RU486 in advanced breast cancer: preliminary clinical trial. Bull Cancer. 1987;74(4):455–461. [PubMed] [Google Scholar]
- Tjian R., Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994 Apr 8;77(1):5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Tung L., Mohamed M. K., Hoeffler J. P., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993 Oct;7(10):1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Tzukerman M. T., Esty A., Santiso-Mere D., Danielian P., Parker M. G., Stein R. B., Pike J. W., McDonnell D. P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994 Jan;8(1):21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993 Oct;7(10):1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Sager R., Webster L. Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res. 1993 Oct 15;53(20):5004–5011. [PubMed] [Google Scholar]


