Abstract
Embryonic development, lengthening, and repair of most bones proceed by endochondral ossification, namely through formation of a cartilage intermediate. It was previously demonstrated that adult human bone marrow-derived mesenchymal stem/stromal cells (hMSCs) can execute an endochondral program and ectopically generate mature bone. Here we hypothesized that hMSCs pushed through endochondral ossification can engineer a scaled-up ossicle with features of a “bone organ,” including physiologically remodeled bone, mature vasculature, and a fully functional hematopoietic compartment. Engineered hypertrophic cartilage required IL-1β to be efficiently remodeled into bone and bone marrow upon subcutaneous implantation. This model allowed distinguishing, by analogy with bone development and repair, an outer, cortical-like perichondral bone, generated mainly by host cells and laid over a premineralized area, and an inner, trabecular-like, endochondral bone, generated mainly by the human cells and formed over the cartilaginous template. Hypertrophic cartilage remodeling was paralleled by ingrowth of blood vessels, displaying sinusoid-like structures and stabilized by pericytic cells. Marrow cavities of the ossicles contained phenotypically defined hematopoietic stem cells and progenitor cells at similar frequencies as native bones, and marrow from ossicles reconstituted multilineage long-term hematopoiesis in lethally irradiated mice. This study, by invoking a “developmental engineering” paradigm, reports the generation by appropriately instructed hMSC of an ectopic “bone organ” with a size, structure, and functionality comparable to native bones. The work thus provides a model useful for fundamental and translational studies of bone morphogenesis and regeneration, as well as for the controlled manipulation of hematopoietic stem cell niches in physiology and pathology.
Keywords: mesenchymal stem cells, regenerative medicine, stem cell niche, tissue engineering
The term “developmental engineering” has been proposed as an evolution of classic tissue engineering paradigms, consisting of designing regenerative strategies guided by the principles of developmental biology (1). The conceptual transition from the primary target of engineering tissues to that of engineering processes recapitulating the stages of tissue development (e.g., based on self-organization of the cells, activation of specific morphogenetic pathways, and typical spatial and temporal arrangement) has the potential to instruct tissue regeneration to a higher degree of effectiveness and robustness (1). In the context of skeletal tissue biology and repair, this principle has inspired the engineering of hypertrophic cartilage templates as bone substitute materials capable of autonomously progressing through endochondral ossification, the embryonic developmental pathway of long bones and of the axial skeleton (2). The concept was successfully implemented not only using murine embryonic stem cells (ESCs) (3) but also adult human-derived bone marrow (BM) mesenchymal stem/stromal cells (MSCs) (4–6), whose molecular regulation was consistent with the known signaling pathways of fetal bone development (4).
Beyond the potential advantages of adopting a developmental engineering strategy for bone regeneration (4), recapitulation of endochondral ossification could lead to the engineering of a fully functional “bone organ,” defined as an osseous matrix with mature vascularization and including the pivotal component of a hematopoietic BM (7). In mouse models it is well established that osteoblastic/osteoprogenitor cells have a crucial role in what has been defined as the hematopoietic stem cell (HSC) niche (8–10). It was also demonstrated, using human cells, that CD146+ skeletal progenitor cells were able to recreate a hematopoietic microenvironment upon ectopic implantation in mice (11). Interestingly, using mouse embryonic limb-derived MSCs it was reported that the endochondral ossification process is required for the formation of an adult HSC niche (12), consistent with the onset of hematopoiesis at the sites of hypertrophic cartilage remodeling in long bones during skeletal growth (2). To the best of our knowledge, despite phenotypical evidence of the presence of putative HSCs within reconstituted ossicles (12–14), engineering of a human cell-induced bone organ hosting fully functional hematopoiesis has not yet been achieved.
We hypothesized that a functional bone organ can be engineered following a developmental engineering approach, which implies proceeding through a cartilage intermediate. A hypertrophic cartilage template would contain all necessary signals to initiate bone tissue formation, vascularization, remodeling, and establishment of a functional BM. To test this hypothesis, we first developed an upscaled model of endochondral ossification, necessary to study spatial patterns of bone tissue formation and to generate sufficient space for BM cells, in a quantity allowing for further analyses. We then used inflammatory regulators to enhance tissue remodeling and HSC homing and demonstrated the functionality of the cells in the ectopically engineered bone organ by transplantation into lethally irradiated mice.
Results
Development of an Upscaled Endochondral Ossification Model.
We investigated the possibility of scaling up the size of engineered hypertrophic cartilage tissues while maintaining the previously reported composition and efficiency of ossification (4). Human MSCs were thus seeded into collagen-based scaffolds (8-mm diameter, 2 mm thick), as templates for tissue development. During the last 2 wk, culture medium was further supplemented with IL-1β (50 pg/mL) to accelerate remodeling of a large cartilage mass (15). The resulting constructs resembled most features of the small-scale model, namely a core cartilaginous tissue surrounded by a mineralized ring positive for bone sialoprotein (BSP). Furthermore, the construct contained approximately 10-fold greater amounts of DNA, calcium, and glycosaminoglycans (GAG) and maintaining an approximately fourfold larger diameter, both in vitro and in vivo (Fig. S1 A–C). Likely because of mass transfer limitations in static culture conditions, the construct core included areas devoid of cells and matrix. Successful entering of the endochondral route was mirrored by the marked up-regulation of several key genes compared with postexpanded MSCs, including collagen type 2 (∼107-fold), type 10 (∼106-fold), and BSP (∼105-fold) (Fig. S1D). Upon implantation in vivo for up to 12 wk, samples underwent extensive remodeling, with hypertrophic cartilage areas being progressively and almost entirely replaced by BM and bone at increasing densities of mineralization, as assessed by histological and micro-CT analyses (Fig. 1). Additionally, the central construct core was progressively filled with matrix and ultimately remodeled into trabecular-like bone structures.
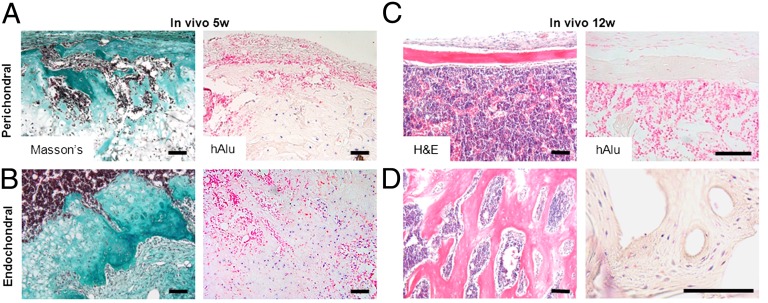
Fig. 1.
Characterization of engineered endochondral bone tissue formation. Engineered hypertrophic cartilage templates underwent extensive remodeling in vivo into bone and BM. (A) Representative sections (Safranin O and Masson’s trichrome) and 3D reconstructed microtomographic images of samples cultured for 5 wk in vitro and implanted ectopically in nude mice for 5 and 12 wk. (Scale bar, 1 mm.) (B) Quantitative histomorphometric data (n = 9) of cartilage, bone, and BM. (C) Quantitative morphometric data (n = 4) of mineral volume and density (P < 0.05).
Contribution of Grafted Cells to the Bone Organ Formation.
The bone matrix derived by remodeling of the hypertrophic cartilage template displayed distinct morphological features in different regions. After 5 wk in vivo, denser extracellular matrix (ECM) was deposited in the outer zone, starting from the external ring, and in the central zone, starting from the inner border of hypertrophic cartilage and progressing toward the core, as shown by Masson’s trichrome staining (Fig. 2 A and B). At the end of the remodeling process, after 12 wk in vivo, the outer zone presented typical characteristics of the compact, cortical bone (e.g., elongated osteocytes, flattened bone-lining cells, and lamellar-like structures), whereas the inner zone resembled the cancellous bone (e.g., bigger cells and trabecular structure with higher surface area) (Fig. 2 C and D). By analogy with embryonic limb development, where cortical bone is formed through direct, intramembranous ossification and cancellous bone is formed through indirect, endochondral ossification, we termed the outer bone “perichondral bone” and the inner bone “endochondral bone.” After 5 wk in vivo, human cells, detected by in situ hybridization (ISH) for human Alu repeats, were interspersed in the cartilaginous template with mouse cells (Fig. 2 A and B). After 12 wk in vivo, human cells were located only in the endochondral bone (Fig. 2D) and colocalized with Osterix-expressing cells with osteoblast and osteocytes appearance (Fig. S2 A and B), whereas the perichondral bone was populated by only mouse cells (Fig. 2C). Consistently with the typical fate of late hypertrophic chondrocytes during endochondral ossification, after 5 wk in vitro many human BM-derived mesenchymal stem/stromal cells (hMSCs) expressed the cleaved form of caspase 3 (Fig. S3A). However, after 5 wk in vivo most of the cells in the cartilaginous template were caspase 3 negative (Fig. S3B) and Osterix positive (Fig. S3C), suggesting survival and osteoblastic differentiation of part of the hMSC embedded in the cartilaginous template.
Fig. 2.
Perichondral and endochondral bone is formed over the hypertrophic cartilage templates. (A) After 5 wk, the outer part of the construct displayed invasion by abundant host-derived cells, deposition of dense ECM, and only few human cells left in the cartilaginous template (Left, Masson’s trichrome staining; Right, ISH for human Alu repeats, highlighting human cells in blue and mouse cells in pink). (B) After 5 wk in vivo, in the inner part of the construct denser ECM was deposited over the cartilaginous template where abundant human cells (blue cells) are present. (C) After 12 wk in vivo, the outer part of the construct was completely remodeled into cortical-like bone and BM, as shown by H&E staining. Importantly, ISH demonstrates presence of only host-derived cells (pink cells). (D) After 12 wk in vivo, trabecular-like bone was formed in the inner construct areas, and human cells could still be located within the mature bone tissue. (Scale bars, 100 µm.)
Physiological Response of the Endochondral Ossification Model to IL-1β.
Inflammatory signals are known to play a critical role in bone tissue development and repair. Considering that IL-1β and TNF-α, the two master regulators of the inflammatory process during fracture repair, have a common mode of action (16), we investigated in more detail the response of the system to IL-1β, a well-characterized proinflammatory cytokine. Pretreatment of hypertrophic cartilage with IL-1β before implantation resulted after 5 wk in vivo in more abundant accumulation of matrix metallopeptidase 13 (MMP-13) and of DIPEN (the cryptic epitope of aggrecan, typically exposed upon its degradation) (Fig. 3A). The enhanced remodeling was paralleled by a higher extent of osteoclast recruitment, as assessed by tartrate-resistant acid phosphatase (TRAP) staining (Fig. 3B), a lower amount of Safranin-O–positive residual cartilage, and larger areas of BM (Fig. 3 C and D). Quantification of cytokines in the supernatants after in vitro culture indicated a statistically significant increase (P < 0.05) of stromal cell-derived factor-1 (SDF1), IL-8, macrophage-colony stimulating factor (M-CSF), monocyte chemotactic protein-1 (MCP-1), and MMP-13 in response to IL-1β, substantiating the findings observed in vivo and validating the biological functionality of the system (Fig. S4). Quantification of cytokines retained in the samples after 5 wk of in vitro culture also showed an increase of M-CSF (56-fold), MCP-1 (26-fold), receptor activator of nuclear factor kappa-B ligand (RANKL) (29-fold), MMP-13 (12-fold), and osteoprotegerin (OPG) (sixfold) compared with untreated controls. In particular, the establishment of an environment prone to remodeling was confirmed by a 4.3-fold increase in the RANKL/OPG ratio (17).
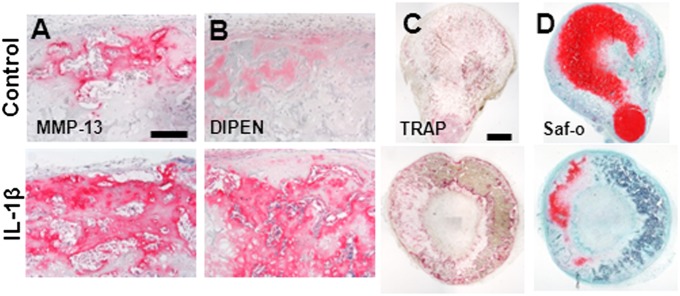
Fig. 3.
Effect of IL-1β on endochondral bone formation. (A) Increased MMP-13 accumulation is evident after 5 wk in vivo in IL-1β–treated samples. (B) This is also paralleled by an enhanced aggrecan remodeling, as demonstrated by increased DIPEN staining. (Scale bar, 200 µm.) (C) Increased host-derived osteoclast recruitment is shown by TRAP staining. (Scale bar, 1 mm.) (D) Notable reduction in cartilaginous ECM and increase in BM content are demonstrated by the Safranin O staining.
Development of Mature Vascularization.
Because angiogenesis is a process critically required in the endochondral ossification route, we next assessed the pattern of vascularization of hypertrophic cartilage upon implantation. After 5 wk in vivo, staining for CD31 indicated that blood vessels had penetrated only the outer part of the constructs, corresponding to the areas positive for collagen type 10, whereas cartilaginous regions not overtly hypertrophic were still avascular (Fig. 4A and Fig. S5A). Consistent with the physiology of blood vessels maturation (18), mural NG2 proteoglycan (NG2)+ pericytes surrounded the newly formed vascular structures (Fig. 4B). At 12 wk the constructs, now consisting predominantly of BM and bone/osteoid tissues positive for collagen type 1 (Fig. 4C and Fig. S5B), included vessels with a sinusoid-like structure, consistently stabilized by α-smooth muscle actin (α-SMA) (Fig. 4D). Collectively, these data indicate a coordinated progression of implant remodeling and vascularization, driven by the onset of hypertrophy and thus consistent with patterns observed in the growth plate and during bone repair through callus formation (2, 16).
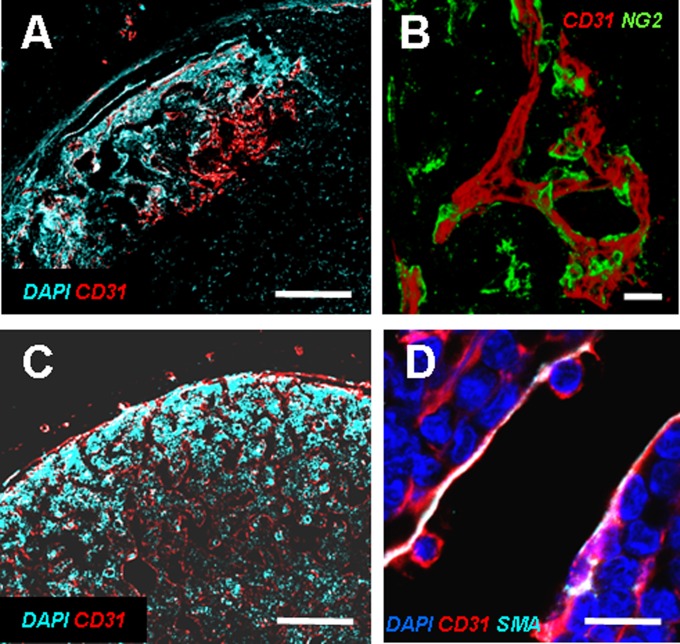
Fig. 4.
In vivo vascularization of the implanted hypertrophic cartilage templates. (A) After 5 wk only the outer region of the engineered tissue was vascularized, as shown by CD31 staining. (B) Vessels were already stabilized by NG2+ pericytes. (Scale bars, 100 µm.) (C) After 12 wk tissues were deeply vascularized, and the BM displayed sinusoid-like vascular structures with a partially SMA-positive wall, indicative of mature stabilization (D). (Scale bars, 20 µm.)
Development of a Functional HSC Niche.
We then investigated whether generated ossicles, previously reported to include regions with morphological features of BM foci (4), could induce homing and maintenance of phenotypically and functionally defined HSC (Fig. 5A). In vitro-manufactured constructs were implanted into B6.Cg-Foxn1nu/J CD45.2+ mice. After 12 wk, ossicles and femurs from transplanted and untreated mice were recovered, crushed, and analyzed by FACS. Interestingly, frequencies of putative HSC (LKS+CD34−CD135−CD150+), megakaryocyte-erythroid progenitors (MEP: LKS−CD34−), and common myeloid /granulocyte-macrophage progenitors (CMP/GMP: LKS−CD34+) (19) in constructs displayed a similar distribution as found in femurs from control mice and mice carrying engineered ossicles (Fig. 5 B and C). The hematopoietic functionality of the recovered ossicle-derived cells was then assessed by transplantation in C57BL/6 CD45.1+/CD45.2+ lethally irradiated mice in a competitive setting using CD45.1+ support cells. As early as 1 mo after transplantation, peripheral blood contained more than 80% ossicle-derived cells (Fig. 5D), consisting predominantly of B cells and myeloid-lineage cells (Fig. 5E). Relative frequencies of donor and competitor-derived blood cells displayed a dose-dependent correlation, as assessed by administering different numbers of ossicle-derived cells (Fig. 5F). Sequential bleeding at 1, 2, and 3.5 mo after transplantation confirmed the long-term self-renewing capacity of the ossicle-derived HSC, with stable engraftment and multilineage reconstitution (Fig. 5E). Relative frequencies of the different lineages were comparable to the control, demonstrating a similar functionality of HSC derived from the native femur or the ectopically engineered ossicles (Fig. 5E). Femoral BM of recipient mice, cytofluorimetrically analyzed 3.5 mo after transplantation, demonstrated a consistent contribution of donor-derived CD45.2+ cells within the compartments of phenotypic HSCs (LKS+CD34−), multipotent progenitors (LKS+CD34+), CMP/GMP (LKS−CD34+), and MEP (LKS−CD34−) (Fig. 5F). Moreover, the efficiency of reconstitution of these pools was similar in the mice transplanted with femur-derived or with different doses of ossicle-derived cells. These data prove the capacity of the engineered hypertrophic cartilage tissues to support and drive the homing and maintenance of functional HSCs.
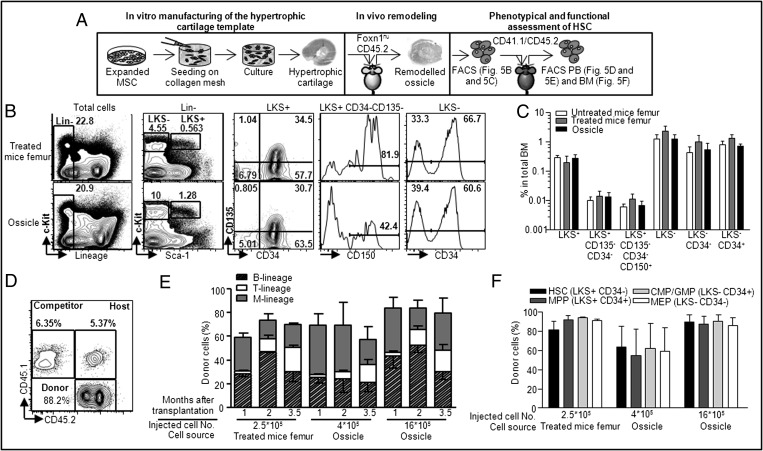
Fig. 5.
Homing and maintenance of functional mouse HSCs in the implanted templates. (A) In vitro manufacturing and in vivo remodeling. Three months after implantation, cells were harvested from untreated, treated femur, or in vivo-remodeled templates and transplanted into lethally irradiated congenic animals. (B) Representative profile of HSCs and progenitors in treated femur or in vivo-remodeled ossicles 3 mo after in vivo implantation (C) Summary of cytofluorimetric analysis of BM HSCs and progenitors (n = 3–4). (D) Representative profile of peripheral blood analysis. (E) Time-course analysis of donor engraftment and lineage distribution in peripheral blood of repopulated recipients (n = 3–5). (F) Summary of cytofluorimetric analysis of BM HSCs and progenitors in recipient mice 3.5 mo after transplantation (n = 3–5). Error bars represent SDs of n = 3–4 measurements.
Discussion
Adherent MSCs isolated from human postnatal BM have long been identified as having the capacity to ectopically generate “miniature ossicles,” including bone matrix and hematopoietic tissue architectures and reproducing physiological and pathological processes of skeletal elements (20). The present study reports that adult hMSCs can be further manipulated to ectopically engineer a full-fledged “bone organ,” at a size, structure, and degree of biological functionality comparable to that of native bones. The claim regarding the functionality of the implant is based on (i) the recapitulation of typical processes of bone tissue development and remodeling, coupled with the formation of a mature vascular network, (ii) the physiological response to inflammatory signals, known to regulate resorption, and of the cartilage intermediate, and (iii) the establishment of large BM spaces, capable of hosting and sustaining fully functional HSCs.
The bone organ model was engineered by activating hMSCs toward an endochondral ossification route and thus by invoking a “developmental engineering” paradigm. The autonomous, self-organized progression of the processes, recapitulating bone development (4), was critically required (i) to promote osteogenesis in the absence of priming by a ceramic-based scaffold, and (ii) to induce efficient vascularization in the absence of cocultured endothelial cells. At 12 wk after implantation, these features allowed the formation of bone matrix by active donor cells down to the core of the grafts, namely at approximately 4 mm from the outer edges. The endochondral ossification route thus seems to address the issues of vascularization of the engineered tissue and cell survival, which are considered as key bottlenecks for a coherent clinical exploitation of cell-based bone regeneration strategies (21).
The host origin of the perichondral bone vs. the contribution of human cells in the central endochondral bone could be related to the spatial patterns of composition and vascularization of the cartilaginous template. Formation of the endochondral bone would be driven by the implanted cells, capable of surviving the hypoxic condition of an avascular environment and of switching on the expression of the master osteoblastic transcription factor Osterix (22). Because Osterix expression in the central region was associated with hypertrophic chondrocytes, our findings would argue for the direct contribution of terminally differentiated cartilage cells to endochondral bone formation. Instead, formation of the perichondral bone would be driven by host osteoprogenitor cells, colonizing the construct from the outer surface through the network of ingrowing blood vessels, and primed to intramembranous bone formation by the external mineralized layer, containing BSP. The formation of bone tissue by two distinct processes, namely endochondral and intramembranous, resembles the distinct responses taking place during fracture healing (16). This feature, recognized thanks to the use of a model upscaled in size, suggests the possible use of engineered hypertrophic cartilage as a surrogate of fracture callus, in pathologies where this does not form efficiently (e.g., atrophic nonunions). Moreover, the recruitment of resident cells for bone formation at the implant periphery warrants further investigations on the nature of the delivered instructive signals, which could develop the perspective of engineering decellularized ECMs based on hypertrophic cartilage to induce endogenous regeneration of bone tissue (23).
The use of IL-1β during the last phase of in vitro culture was introduced with the double intent of demonstrating a physiological response of the system to inflammatory signals and accelerating resorption of the large mass of hypertrophic cartilage. In line with the known effect on growth plate and fracture callus cartilage (16), in our model IL-1β induced (i) enhanced MMP-13–mediated endogenous ECM preprocessing; (ii) enhanced host osteoclast-mediated ECM remodeling by increased M-CSF levels and RANKL/OPG ratios; (iii) faster vascularization, despite a minimal reduction in VEGF content (15); and (iv) larger regions of BM, possibly because of an increased synthesis of SDF1, IL-8, M-CSF, and MCP-1. Instead, IL-1β did not induce a reduction of bone mass by increased bone resorption (24), because its administration was temporally confined to the phase preceding bone matrix deposition. Further studies are required to validate the functionality of the engineered bone organ in the context of an immunocompetent model at an orthotopic site, where loading and inflammation would further regulate bone development and homeostasis.
The marrow component of the engineered bone organ, occupying the majority of its volume, was demonstrated to include phenotypically and functionally defined HSCs at a comparable frequency to normal bones of the same mice. These unprecedented findings validate the physiological nature of the in vivo-established ossicle and reinforce the evidence of self-organization ability of hMSC-based hypertrophic cartilage templates into functional hematopoietic niches. Interestingly, the increased amount of host-derived BM in response to IL-1β seemed to mirror the enhanced osteoclast recruitment, as recently outlined in the context of HSC niches in a murine model (25). The developed system, although requiring further investigations to specify the mode of establishment (e.g., a direct vs. indirect niche effect of the human cells in triggering homing of the mouse HSC), offers the opportunity to address a variety of critical questions at the interface between HSC biology and regenerative medicine. The model can be exploited to dissect cues that regulate HSC engraftment, by engineering marrow environments with defined and genetically controlled properties (e.g., by knockin/out of specific factors, possibly defined by high-throughput screening systems) (13, 26). In fact, the capacity of the ectopic marrow to attract and retain rare circulating HSCs advocates its function as a “stem cell trap,” ultimately providing a tool to investigate the physiological factors and mechanisms underlying the still debated concept of the HSC niche. Furthermore, on the basis of the concept of mutual influences between the BM microenvironment and leukemia cells (27, 28), the possibility of establishing a human origin BM microenvironment in humanized hematopoietic murine models (29–31) has a large relevance to investigate the development and progression of blood cell malignancies. Last but not least, the generation of an extramedullary bone organ, accessible to advanced imaging techniques (e.g., intravital confocal microscopy) (14), opens the perspective to investigate and exploit processes underlying the efficient expansion of HSCs for therapeutic purposes (13).
In conclusion, we have reported an upscaled model based on adult human cells that displays morphological, phenotypic, and functional features of a “bone organ.” The system can be used in fundamental investigations of the biology of bone development and of HSC niches, as well as in translational studies related to restoration of bone tissue and of normal hematopoiesis.
Materials and Methods
All human samples were collected with informed consent of the involved individuals, and all mouse experiments were performed in accordance with Swiss law. All studies were approved by the responsible ethics authorities and by the Swiss Federal Veterinary Office. MSCs were expanded for two passages (accounting for an average of 13–15 doubling), to minimize the loss of chondrogenic potential, and characterized by flow cytometry for putative MSC markers, with results consistent with our previous report (4). Cells were then seeded onto type I collagen meshes at a density of 70 × 106 cells/cm3 and cultured in chondrogenic conditions for 3 wk in a serum-free chondrogenic medium, followed by 2 wk in a serum-free hypertrophic medium (4). Samples were implanted in s.c. pouches of nude mice (four samples per mouse) and retrieved after 5 or 12 wk. The resulting in vitro and in vivo tissues were analyzed histologically, immunohistochemically, biochemically (glycosaminoglycans, DNA, protein content), by real-time RT-PCR, and by microtomography. Tissue development in vivo was evaluated histologically, immunohistochemically, and by microtomography. Total BM cells, obtained from long bones of control or treated mice (CD45.2+) or from the engineered ossicles by crashing/collagenase digestion, were treated with red blood cell lysis buffer and transplanted into lethally (9.5 cGy)-irradiated congenic animals (CD45.1/2+). Blood samples were collected from the transplants and analyzed by flow cytometer after staining with antibodies against CD45.1/2 allotype, B (CD19), T (CD3ɛ), and myeloid lineage markers (CD11b/Gr-1). The survival and contribution to bone formation by MSC was evaluated with ISH for human Alu sequences. A more complete and detailed description of the methods is included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Allison Hoch for proofreading the manuscript. The work was partially funded by Swiss National Science Foundation Grant NMS1725 (to I.M.), by AO Foundation Grant S-11-13P (to A.P.), and by the Promedica Foundation (Chur, Switzerland) and the clinical research focus program “Human Hemato-Lymphatic Diseases” of the University of Zürich (both to M.G.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220108110/-/DCSupplemental.
References
- 1.Lenas P, Moos M, Luyten FP. Developmental engineering: A new paradigm for the design and manufacturing of cell-based products. Part I: From three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev. 2009;15(4):381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 3.Jukes JM, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105(19):6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotti C, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107(16):7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janicki P, Kasten P, Kleinschmidt K, Luginbuehl R, Richter W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: Enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 2010;6(8):3292–3301. doi: 10.1016/j.actbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Farrell E, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 8.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 11.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Human extramedullary bone marrow in mice: A novel in vivo model of genetically controlled hematopoietic microenvironment. Blood. 2012;119(21):4971–4980. doi: 10.1182/blood-2011-11-389957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, et al. Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc Natl Acad Sci USA. 2012;109(48):19638–19643. doi: 10.1073/pnas.1208384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumme M, et al. Interleukin-1β modulates endochondral ossification by human adult bone marrow stromal cells. Eur Cell Mater. 2012;24:224–236. doi: 10.22203/ecm.v024a16. [DOI] [PubMed] [Google Scholar]
- 16.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 17.Jurado S, et al. Effect of IL-1beta, PGE(2), and TGF-beta1 on the expression of OPG and RANKL in normal and osteoporotic primary human osteoblasts. J Cell Biochem. 2010;110(2):304–310. doi: 10.1002/jcb.22538. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 20.Bianco P, et al. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101(8):1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4(2):e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 23.Sadr N, et al. Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials. 2012;33(20):5085–5093. doi: 10.1016/j.biomaterials.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 24.Polzer K, et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann Rheum Dis. 2010;69(1):284–290. doi: 10.1136/ard.2008.104786. [DOI] [PubMed] [Google Scholar]
- 25.Mansour A, et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209(3):537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gobaa S, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8(11):949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 27.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12(1):49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rongvaux A, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA. 2011;108(6):2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strowig T, et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci USA. 2011;108(32):13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32(7):321–327. doi: 10.1016/j.it.2011.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.