Abstract
The study of hematopoietic colony-forming units using semisolid culture media has greatly advanced the knowledge of hematopoiesis. Here we report that similar methods can be used to study pancreatic colony-forming units. We have developed two pancreatic colony assays that enable quantitative and functional analyses of progenitor-like cells isolated from dissociated adult (2–4 mo old) murine pancreas. We find that a methylcellulose-based semisolid medium containing Matrigel allows growth of duct-like “Ring/Dense” colonies from a rare (∼1%) population of total pancreatic single cells. With the addition of roof plate-specific spondin 1, a wingless-int agonist, Ring/Dense colony-forming cells can be expanded more than 100,000-fold when serially dissociated and replated in the presence of Matrigel. When cells grown in Matrigel are then transferred to a Matrigel-free semisolid medium with a unique laminin-based hydrogel, some cells grow and differentiate into another type of colony, which we name “Endocrine/Acinar.” These Endocrine/Acinar colonies are comprised mostly of endocrine- and acinar-like cells, as ascertained by RNA expression analysis, immunohistochemistry, and electron microscopy. Most Endocrine/Acinar colonies contain beta-like cells that secrete insulin/C-peptide in response to D-glucose and theophylline. These results demonstrate robust self-renewal and differentiation of adult Ring/Dense colony-forming units in vitro and suggest an approach to producing beta-like cells for cell replacement of type 1 diabetes. The methods described, which include microfluidic expression analysis of single cells and colonies, should also advance study of pancreas development and pancreatic progenitor cells.
Keywords: extracellular matrix proteins, Sry-related HMG box (Sox) 9, Promonin 1 (CD133), neurogenin 3, dickkopf1 (Dkk1)
Adult pancreatic stem/progenitor cells are a potential unlimited source of insulin-secreting beta-like cells, but their existence is controversial. Candidate progenitor cells include adult duct (1–3), centroacinar (4), acinar (5), and insulinlow (6) cells. However, tissue culture strategies so far have been only marginally effective for expanding and differentiating putative progenitor cells into β-like cells. In addition, the starting materials used for culture often contain a mixture of cells, complicating the interpretation of results (1). This lack of progress has been in part due to the absence of analytical tools that can effectively measure self-renewal and differentiation at the single-cell level. Delineation of lineage potential of a progenitor requires single-cell analysis. Otherwise, a population of cells under study may contain several monopotent progenitors that have various lineage potentials, and the cells will appear collectively as multipotent. In addition, if the cells of interest are scarce, the majority may mask the rare cell’s activities. One example of a population study is the in vivo genetic lineage tracing method using the cyclization recombinase (Cre) and locus of crossing over (Lox) system (7). In these studies, both positive (8, 9) and negative (10–15) results have been reported with respect to the question of whether exocrine pancreas in the adult gives rise to beta-like cells in injury models. These controversial data should be interpreted with care because of the possible failure of labeling rare cell populations (16). A different methodology capable of addressing this issue is needed. The single cell “pancreatosphere” assay in liquid culture addresses lineage potential using limiting dilution (4, 6, 17). However, this method is labor-intensive, does not allow incorporation of the extracellular matrix (ECM) proteins important for cell function, and becomes highly cumbersome when attempting to manipulate individual colonies for replating to address self-renewal capacity.
To address these issues, we have investigated the use of the hematopoietic colony assay, which employs semisolid culture media. For this assay, single cells are mixed in viscous media containing methylcellulose, a biologically inert material purified from wood fibers. The methylcellulose medium restricts the movement of single cells, yet is soft enough to allow colony formation. A cell capable of forming a colony is termed a “colony-forming unit” (CFU). To test whether the adult pancreas contains CFUs, we replaced hematopoietic growth factors with factors that we thought would be helpful for growing pancreatic cells, including various growth factors (18–21) and ECM proteins, such as the commercially available Matrigel or an artificial ECM protein (22) containing an α1 laminin sequence (termed “laminin hydrogel”).
Pancreatic development is controlled by sequential activation of key transcription factors (23). Around embryonic day 8.5 (E8.5), commitment to the early pancreas is promoted by factors such as pancreatic and duodenal homeobox 1 (Pdx-1) (24) and NK6 homeobox 1 (Nkx6.1) (25). The early Pdx-1+ cells are tripotent for duct, acinar and endocrine lineages (26, 27), and their subsequent commitment to endocrine cells is dependent on the activation of neurogenin (Ngn) 3 (28–32), a helix–loop–helix transcription factor. The wingless-int (Wnt) proteins are a family of secreted, lipid-modified proteins critical for organ development (33) and for self-renewal and differentiation of several classes of adult stem cells (34). Wnt signaling exerts stage-specific effects during pancreatic development. Although Wnt signaling activation in early (E9) Pdx-1+ cells results in agenesis of the pancreas (35, 36), it enhances the growth and differentiation of more-developed (around E11.5) Pdx-1+ progenitors (36). The roof plate-specific spondin 1 (RSPO1) has recently been identified as a Wnt signaling ligand (37). RSPO1 binds with high affinity to Wnt receptor low-density lipoprotein receptor-related protein (Lrp) 6 (38), which can be blocked by the Lrp6 inhibitor dickkopf1 (Dkk1) (37). Whether RSPO1 may affect activity of adult pancreatic progenitor cells has not been tested.
Here we report that in the adult mouse pancreas there are cells that form colonies in semisolid media. We have named these cells Pancreatic CFUs (PCFUs). These PCFUs make up only ∼1% of the total cells of the adult pancreas, but can give rise to “Ring” or “Dense” ductal-like colonies as well as Endocrine/Acinar colonies in which some cells express endocrine markers and others express acinar markers. Our results demonstrate extensive in vitro progenitor cell activities displayed by adult PCFUs and show that RSPO1 stimulates PCFUs to self-renew and differentiate in vitro.
Results
“Ring” Colonies Can Form from Dissociated Adult Murine Pancreatic Cells.
Dissociated pancreatic cells from adult mice (2–4 mo old) were suspended in colony assay medium containing 5% (vol/vol) Matrigel (referred to as the “Matrigel colony assay”) (Fig. S1). Three weeks later, morphologically distinct pancreatic colonies were observed, which we have named “Ring” (Fig. 1A). Ring colonies are hollow spheres or cysts when viewed under the stereomicroscope. To understand how individual colonies develop, the locations of young colonies on the plate were noted and followed over time. Starting at day 2 postplating, Ring colonies begin as a small cluster of cells that are highly light reflective when viewed under phase-contrast illumination. These young colonies are named “Small Bright” (Fig. 1A). Colony formation is not strain-specific; cells from both CD1 outbred and C57BL/6 (B6) inbred mice form colonies. It will be shown in a later section that a single cell can form a colony. A PCFU that gives rise to a “Ring” colony is termed a PCFU–Ring.
Fig. 1.
“Ring” colonies are formed in Matrigel-containing culture from CD133+Sox9/EGFP+ cells isolated from dissociated adult murine pancreata. (A) A Ring colony starts as a “Small Bright” colony and grows into a Ring colony. (B) Flow cytometry analysis of CD133 and Sox9/EGFP expression of total dissociated adult pancreata. Regions (R) drawn indicate sorting windows. (C) PCFUs–Ring are most enriched in the CD133+Sox9/EGFP+ R3 window. (D) Single-colony microfluidic qRT-PCR analysis demonstrates that Small Bright and Ring colonies express high levels of ductal markers and low but detectable levels of endocrine and acinar cell markers. Each column is from a single colony. (E) Whole-mount and frozen section immunostaining demonstrated protein expression of ductal (Sox9, Mucin1, and Spp1), acinar (Amylase), or endocrine (C-Peptide) markers.
Enrichment for PCFUs–Ring.
PCFUs–Ring comprise ∼1% of plated, dissociated adult pancreatic cells (1.18 ± 0.70%; range, 0.56–2.32%). Therefore, enrichment for these scarce cells is highly desirable. Because cystic cell clusters, similar to Ring colonies, were described in cultures initiated with enriched ducts (1, 39–41), we tested known ductal markers, CD133 (42-46) and Sry-related HMG box (Sox) 9 (15, 47, 48), to see if they could be used as markers for fluorescence-activated cell sorting. CD1 mice transgenic for a Sox9 promoter-driven EGFP reporter (49) were used for subsequent studies. CD133+Sox9/EGFP+ cells (R3 window in Fig. 1B), which accounted for 3.78 ± 1.54% (range, 2.00–6.10%) of total pancreatic cells (Fig. 1B and Fig. S2), had the highest frequency of PCFUs–Ring compared with the other subpopulations (Fig. 1C). CD133−Sox9/EGFP− cells did not give rise to Ring colonies even when plated up to 2.5 × 104 cells/well. Microfluidic quantitative (q) RT-PCR analysis demonstrated that single micromanipulated CD133+Sox9/EGFP+ cells (n = 21) expressed ductal (cytokeratin [CK]19 and CK7) but not acinar (Elastase1) or endocrine (Insulin2) markers (Fig. S3), supporting the ductal identity of CD133+Sox9/EGFP+ cells. Microfluidic qRT-PCR is a relatively new technology that allows reaction volumes to be in the nanoliter range, thus enabling detection of gene expression from as little as a single cell (50).
Gene expression analysis by conventional qRT-PCR of sorted subpopulations from B6 pancreas confirmed that markers for acinar (Amylase 2A) and endocrine cells (Glucagon and Insulin genes) were enriched in the CD133− fraction, whereas CD133+ cells were enriched for ductal markers. Only CD133+ but not CD133− cells were able to give rise to Ring colonies (Fig. S4). Thus, we speculate that Ring colonies may not come from acinar dedifferentiation, as suggested by a previous study (51).
Ring colony-forming frequency of dissociated pancreatic cells is the same with or without passing through a sorter, so heterogeneity in colony-forming ability is not due to the physical stress of sorting (Fig. S5). Ring colonies might have been derived from hematopoietic cells present in the pancreas at the time of procurement. However, we found no colony formation from femur-derived bone marrow cells even after plating up to 2.5 × 104 cells/well. This negative result was not due to compromised bone marrow cells, as control experiments showed that the marrow cells formed hematopoietic colonies in the presence of hematopoietic factors (Fig. S6).
Single Cells Can Form Ring Colonies.
To test whether a single cell can form a colony, freshly sorted CD133+Sox9/EGFP+ cells were individually handpicked and plated (1 cell/ well, 96-well plate) to observe colony formation. We found that 23 out of 120 (∼19%) wells developed Ring colonies. This result indicates that (i) a single CD133+Sox9/EGFP+ cell is sufficient to form a colony and (ii) not all CD133+Sox9/EGFP+ cells can form colonies. To test the lineage composition of single handpicked colonies, we examined the expression of a gene panel by microfluidic qRT-PCR analysis (Fig. 1D). We found that all Ring colonies expressed high levels of housekeeping and ductal genes (Fig. 1D, green box) and low levels of endocrine and acinar genes (Fig. 1D, red box). To confirm protein expression, 3-wk-old Ring colonies were handpicked, pooled, fixed, and analyzed by whole-mount or frozen-section immunofluorescent staining and confocal imaging. Consistent with the microfluidic qRT-PCR analysis, ductal protein marker expression was present in all colonies examined, and each colony was positive for ductal proteins, Sox9/EGFP, Mucin1, or Osteopontin1 (Spp1) (52) (Fig. 1E). Three-wk-old Ring colonies were further examined by transmission electron microscopy. Cells in a Ring colony had multilobed nuclei (Fig. S7A), and microvilli and tight junctions were located at the apical surfaces of the cells facing a lumen (Fig. S7B), indicating a ductal cell identity and proper polarity. Cell polarity is important for pancreas development (53). We conclude that most cells in Ring colonies are duct-like.
However, a few cells in many of the colonies express amylase or C-peptide (a surrogate marker for de novo synthesized insulin) (Fig. 1E). About 70% (19/28) of colonies derived from single cells express some endocrine and acinar lineage markers (Fig. 1D, red box). This is evidence that some PCFUs–Ring are tripotent.
“Dense” Colonies Are Induced from PCFUs–Ring by the Wnt Agonist RSPO1 but Not the Wnt Antagonist Dkk1.
To determine if Wnt signaling affects colony formation, adult CD133+Sox9/EGFP+ cells were plated in the Matrigel colony assay with exogenous RSPO1 or Dkk1. A different type of colony (Fig. 2A) was formed after 3 wk only in the presence of RSPO1. We named this type of colony “Dense” due to its high cellularity (3,273 ± 219 cells/Dense vs. 180 ± 14 cells/Ring of ∼200 μm diameter). RSPO1 increased the percentage of Dense colonies in a dose-dependent manner (Fig. 2B), reaching a plateau close to 50% at 750 ng/mL; this dose was used for subsequent studies. The overall colony-forming frequency (number of colonies per input CD133+Sox9/EGFP+ cells) was the same in the RSPO1, vehicle, and Dkk1 groups (Fig. 2C), suggesting that RSPO1 induces a subpopulation of PCFUs–Ring to form Dense colonies in vitro. Because we have not yet identified a marker to separate the PCFUs–Dense from PCFUs–Ring, we will refer to these cells collectively as PCFUs–Ring/Dense.
Fig. 2.
“Dense” colonies are induced by RSPO1 but not Dkk1 in Matrigel-containing culture and have enhanced progenitor cell marker expression compared with Ring colonies. (A) Representative photomicrographs of 3-wk-old colonies grown in the presence of designated factors. (B) Proportion of Dense colonies is increased by exogenous RSPO1 in a dose-dependent manner, with optimal dose at 750 ng/mL. (C) Colony-forming efficiency of CD133+Sox9/EGFP+ cells, determined by the total number of colonies formed from 2,500 input cells, was not changed by RSPO1 or Dkk1. (D) Microfluidic qRT-PCR analysis of individually handpicked colonies. Each bar is from a single colony. (E) Whole-mount immunostaining of a Dense colony.
Dense Colonies Have Enhanced Expression of Neurogenin 3 (an Endocrine Progenitor Marker) Compared with Ring Colonies.
Individual 3-wk-old Ring and Dense colonies both expressed the ductal markers Sox9 and Mucin1 at similar levels (Fig. 2 D, 1). Microvilli and multilobed nuclei are also observed in Dense colonies (Fig. S7C). However, Dense colonies expressed higher levels of fetal progenitor cell markers (Pdx-1, Nkx6.1, and Ngn3) (Fig. 2 D, 2). Protein expression of NGN3 was confirmed (Fig. 2E). RSPO1 did not further enhance mRNA levels of Insulin1, Insulin2, nor Elastase1 in individual Dense colonies compared with Ring colonies (Fig. 2 D, 3 and 4). Thus, although RSPO1 enhanced progenitor marker expression, it did not push the cells to further commit toward endocrine lineage cells.
Fig. 3.
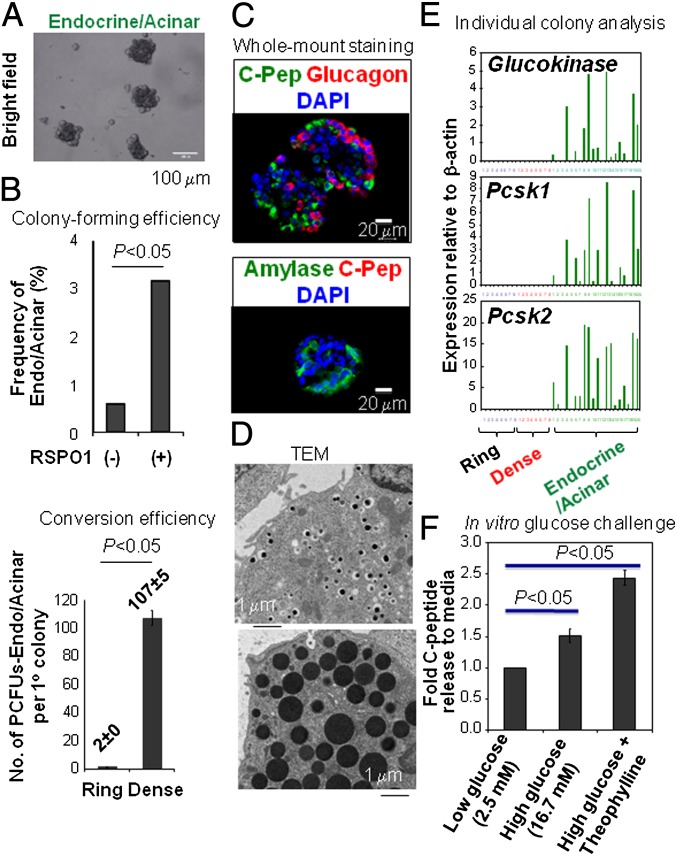
“Endocrine/Acinar” colonies are formed from dissociated and replated Ring and Dense colonies in laminin hydrogel-containing culture. (A) Representative photomicrographs of 2-wk-old Endocrine/Acinar colonies. (B, Upper) Total 3-wk-old colonies grown in Matrigel-containing culture (stimulated without or with 750 ng/mL RSPO1) were dissociated and replated into laminin hydrogel colony assay for 2 wk. Colony-forming efficiency was calculated as the number of Endocrine/Acinar colonies generated divided by total number of input cells. (Lower) A total of 20 3-wk-old Ring (grown in Matrigel) or Dense colonies (grown in Matrigel and RSPO1) were picked, pooled, and dissociated into single-cell suspension. Total cell number was determined and the cells subsequently were replated into laminin hydrogel colony assay in quadruplicated wells for 2 wk. The conversion efficiency was calculated as percentage Endocrine/Acinar colony-forming efficiency times total cell number and then divided by 20. (C) Whole-mount immunostaining of Endocrine/Acinar colonies. (D) Transmission electronmicroscopy of Endocrine/Acinar colonies showing cells with insulin- (Upper) or acinar-like (Lower) granules. (E) Microfluidic qRT-PCR analysis of individually handpicked colonies. Each bar is from a single colony. (F) In vitro glucose change assay on pooled Endocrine/Acinar colonies. Concentrations of theophylline are 10 mM.
Fig. 4.
RSPO1, but not Dkk1, supports expansion of PCFUs–Ring/Dense long term in vitro. As illustrated in A, CD133+Sox9/EGFP+ cells were plated in culture containing Matrigel and RSPO1. Three weeks later, colonies were counted and total colonies from each well (n = 4 for each group) were procured, dissociated, and a fraction of total cells was replated in the presence of Matrigel and designated factors. After 2 wk, the colonies were counted and all cells were procured, dissociated, counted, and replated. Counting, procurement, dissociation, and replating procedure was repeated a total of four times. (B) The number of total resulting colonies (adjusted for fractions of plated cells) was analyzed. Data represent two experiments with similar trends. *P < 0.05 compared with vehicle control. (C) Morphological change of colonies toward smaller Dense colonies with increased passages was noted in cultures continuously receiving exogenous RSPO1.
“Endocrine/Acinar” Colonies Form from Dissociated Dense or Ring Colonies in a Laminin Hydrogel.
ECM proteins, especially laminin, are important for adult murine β-cell function (54). The inefficient endocrine commitment in the Matrigel colony assay (Figs. 1 and 2) prompted us to test whether other ECM proteins affected β-cell survival or differentiation. An artificial ECM protein (22) containing an 18-amino-acid sequence from α1 laminin (designated laminin hydrogel) was produced and tested. The laminin hydrogel-containing colony assay will be referred to as the “laminin colony assay.” Importantly, when total colonies from 3-wk-old culture, grown from adult CD133+Sox9/EGFP+ cells in Matrigel were collected, dissociated, and replated into the secondary laminin colony assay, another type of colony formed after 2 wk. We termed it “Endocrine/Acinar” (Fig. 3A) to reflect its cellular composition. These colonies express endocrine and acinar markers and reduced ductal cell markers (Fig. 2D). A PCFU that gives rise to an Endocrine/Acinar colony is termed a PCFU–Endocrine/Acinar. PCFUs–Endocrine/Acinar are a minor population (∼0.5%) among dissociated pancreatic cells grown in Matrigel; addition of RSPO1 enhanced their frequency by fivefold (Fig. 3B, Upper). Taking into account the total number of cells contained in a colony, an average of 2 or 107 PCFUs–Endocrine/Acinar was produced per Ring or Dense colony, respectively (Fig. 3B, Lower), demonstrating a ∼50-fold enhancement of PCFUs–Endocrine/Acinar by RSPO1. Individually handpicked Endocrine/Acinar colonies contain cells that are C-peptide+Glucagon−, C-peptide−Glucagon+, or Amylase+C-peptide− (by immunostaining) (Fig. 3C) and cells with insulin or acinar zymogen granules (by transmission electronmicroscopy) (Fig. 3D). They also express markers indicative of β-cell differentiation/maturation—e.g., Glucokinase and Prohormone convertase (PCSK) 1 and 2 (Fig. 3E). We therefore tested their ability to secrete C-peptide in vitro. Pooled (∼100) Endocrine/Acinar colonies released 2.4-fold more C-peptide to media when presented with high (16.7 mM) concentrations of D-glucose plus 10 μM theophylline (a cAMP potentiator) compared with low (2.5 mM) concentrations of D-glucose (Fig. 3F). These results indicate that the beta-like cells in Endocrine/Acinar colonies can secrete insulin in vitro.
To confirm lineage potential of single PCFUs–Dense, individual CD133+Sox9/EGFP+ cells (n = 120 in 96-well plate) were implanted in Matrigel and RSPO1, and the resulting 3-wk-old Dense colonies (n = 4) were individually handpicked and dissociated into single-cell suspension. Half of the dissociated cells were analyzed for ductal gene expression, while the other half were replated into the laminin colony assay. The resulting 2-wk-old Endocrine/Acinar colonies were then analyzed for endorine and acinar gene expression. We found that all of the single Dense colonies and their derivatives expressed trilineage markers, suggesting that all PCFUs–Dense are tripotent (Fig. S8). In contrast, half of the control single Ring colonies (6 and 8) did not have endocrine potential.
Wnt Signaling Promotes PCFUs–Ring/Dense Expansion.
Adult CD133+Sox9/EGFP+ cells were plated in the Matrigel and RSPO1 colony assay for 3 wk. The resulting colonies were dissociated and serially replated in the Matrigel colony assay over an additional four generations in the presence of RSPO1, vehicle, or Dkk1. Continuous exposure of PCFUs–Ring to exogenous RSPO1 induced exponential growth of PCFUs–Ring/Dense (Fig. 4) and led to a ∼5 × 105-fold net expansion over 11 wk. Thus, PCFUs–Ring/Dense are expandable in culture.
Discussion
We report here the use of single-cell methylcellulose colony assays to delineate the ex vivo differentiation and expansion properties of adult pancreatic cells. Structures similar to Ring colonies have been described previously using semipurified pancreatic ducts grown in culture containing high [>33% (vol/vol)] concentrations of Matrigel (1, 39–41, 55). However, in our culture system, low [5% (vol/vol)] concentrations of Matrigel, when mixed with methylcellulose, are sufficient to grow adult pancreatic cells. Advantages of semisolid, methylcellulose-based assays are the ability to vary all active components and easily quantitate and manipulate the resulting colonies. Colonies are evenly distributed across the well and can be counted accurately, and a single colony can be easily handpicked for subsequent analysis. The culture system we describe is efficient and easy to maintain. Up to 2.5 × 104 cells can be plated in 500 µL of semisolid media in one well of a 24-well plate. No media change is required during the 3-wk culture period.
Using these semisolid media, 3D assays, we have found the following (Fig. 5): (i) In the presence of Matrigel and exogenous RSPO1, two types of colonies form, ∼50% are “Dense” and the rest are “Ring” colonies. Matrigel is known to contain many growth factors. Whether other factors are required for RSPO1 to induce Dense colony formation remains to be determined. Because PCFUs–Dense constitute a subset of the total PCFUs–Ring (Fig. 2 B and C) and there is no marker to distinguish the two populations, we refer to these progenitor-like cells collectively as PCFUs–Ring/Dense. (ii) PCFUs–Ring/Dense are rare (∼1%) cells in the adult mouse pancreas but are enriched in the CD133+Sox9/EGFP+ cell fraction (Fig. 1C). It should be noted that although CD133+Sox9/EGFP+ cells are enriched for ductal cells as well as PCFUs–Ring/Dense, this does not prove that ductal cells are the originator of the Ring, Dense, or Endocrine/Acinar colonies. Further investigation is required. (iii) When colonies grown in Matrigel are dissociated and replated in the laminin colony assay, “Endocrine/Acinar” colonies result. These colonies express low levels of ductal genes, but higher levels of genes specific to endocrine and acinar cells (Fig. 2D). Included in these Endocrine/Acinar colonies are β-like cells expressing C-peptide that are responsive to D-glucose and theophylline in vitro (Fig. 3 C and F). (iv) PCFUs–Ring/Dense are capable of trilineage differentiation and can be expanded at least 105-fold in vitro (Figs. 1D and 4).
Fig. 5.

Model of PCFU–Ring/Dense expansion and differentiation.
In vivo animal studies using cell-tracing strategies have yielded contradictory results about the existence of progenitor cells (8–15), so the very existence of adult pancreatic progenitor cells in vivo is hotly debated. Note that the current results only demonstrate activities of adult murine PCFUs in vitro, not in vivo, and our studies do not yet prove progenitor cells exist in vivo in the adult pancreas. However, our results do call for further investigation of progenitor cells in the adult pancreas in vivo, a research activity that has largely ceased after a series of recent publications reporting negative findings (12–15). In particular, the issue of heterogeneity among the ductal cell population should be examined.
Experimental Procedures
Mice.
Adult (2–4 mo) Sox9/EGFP transgenic mice (CD1 background) (49) or C57BL/6 (Charles River Laboratory) were used in this study. All mice were maintained under specific pathogen-free conditions, and animal experiments were conducted according to the Institutional Animal Care and Use Committee at the City of Hope.
Dissociation of Adult Pancreas.
Dissected pancreata were cleaned of fatty tissues and minced with a spring scissor for 3 min in a dry Petri dish on ice. The minced tissue was placed in PBS/0.1% (wt/vol) BSA containing collagenase B (2–4 mg/mL per pancreas) (Roche) and DNase І (2,000 U/mL per pancreas) (Calbiochem) and incubated at 37 °C for 20–30 min to yield a predominately single-cell suspension. To hasten the digestion, the tissue was gently pipetted every 5–10 min. The single-cell suspension was filtered through 20-μm cell strainers before use.
Flow Cytometry and Cell Sorting.
The cell suspension was first incubated with anti-mouse CD16/32 (10 μg/mL; BioLegend) for 5 min on ice to diminish nonspecific binding. Biotin-conjugated anti-mouse CD133 (clone 13A4; 5 μg/mL; eBioscience) or the control biotin-conjugated rat immunoglobin (Ig)G1 isotype (5 μg/mL; eBioscience) antibodies were added, and the cells incubated on ice for 20 min. After washing twice, cells were treated with streptavidin-labeled allophycocyanin (2 μg/mL; BioLegend) on ice for 15 min. Cells were washed twice and resuspended in PBS/BSA/DNase I containing DAPI (0.2 μg/mL). Cell sorting was performed on an Aria-special order research product (SORP) (Becton Dickinson). All analyses included an initial gating of forward and side scatters to exclude cell debris. Sorting further excluded doublets by gating on forward scatter width and side scatter width, and live cells were selected by DAPI negative staining (Fig. S2). The purity of the sorted population was routinely more than 95%.
In Vitro Colony Assays.
Unless specified otherwise, cells were resuspended at a density of 2.5 × 103 cells/0.5 mL in methylcellulose-based colony culture medium as described previously (20, 21). In short, 1 mL culture mixture contained DMEM/F12 media, 1% (wt/vol) methylcellulose (Sinetsu Chemical), 5% (vol/vol) Matrigel or 100 μg/mL laminin hydrogel (see sequence in Fig. S9), 50% (vol/vol) conditioned media from murine embryonic-stem-cell–derived pancreatic-like cells, 5% (vol/vol) FCS, 10 mmol/L nicotinamide (Sigma), 10 ng/mL human recombinant activin-βB, 0.1 nmol/L exendin-4, and 1 ng/mL vascular endothelial growth factor–A. When indicated, RSPO1 or Dkk1 (R&D Systems) was used at 750 ng/mL or 200 ng/mL, respectively. Cells were plated in 24-well ultralow protein-binding plates (Costar) and incubated in a humidified 5% (vol/vol) CO2 atmosphere. Primary colony numbers were scored after 3 wk in culture. For replating experiments, individual colonies were lifted from the methylcellulose medium by using a 10-μl Eppendorf pipette under direct microscopic visualization, collected in microcentrifuge tubes, and dissociated into single-cell suspension by incubation with 0.25% (wt/vol) trypsin-EDTA at 37 °C for 5 min. The single-cell suspension was then mixed in Matrigel or laminin hydrogel colony assay as described above.
Single-Cell Manipulation.
Freshly sorted cells were mixed in 1% methylcellulose and 15% FCS at a density of 3,000 cells/mL and plated into a 35-mm Petri dish. Individual cells were visualized under a microscope and lifted one by one using a fine Pasteur pipet with a diameter of ∼30 μm at the opening. The presence of a manipulated single cell is confirmed by visualization under a microscope.
qRT-PCR.
Total RNA extraction and reverse transcription were as described (56). β-actin was used as an internal control for normalization. Duplicate samples were used in all analyses. Microfluidic qRT-PCR was performed using the BioMark 48.48 Dynamic Array system (Fluidigm). Single handpicked colonies or cells were collected in 10 μL reaction buffer, followed by preamplification (14 or 22 cycles for a colony or cell, respectively) according to the manufacturer’s instructions (Fluidigm). Amplified cDNA was loaded onto a 48.48 Dynamic Array using the NanoFlex integrated fluidic circuit (IFC) controller (Fluidigm). Threshold cycle (Ct), as a measurement of fluorescence intensity, was determined by the BioMark PCR analysis software (Fluidigm) and expressed as a heat map or relative expression (delta Ct) of the gene of interest to the internal control, β-actin. All reactions were performed along with negative (water) and positive (adult pancreatic cells) controls in all experiments. The Taqman probes (Life Technologies) and their catalog numbers used are listed in Table S1.
Expression and Purification of Laminin Hydrogel.
Methods for cloning, expression, and purification of the artificial ECM protein were as described previously (22). The amino acid sequence of laminin hydrogel used in this report, which is comprised of an elastin backbone plus and α1 laminin ECM protein domain, is shown in Fig. S9.
Statistical Analysis.
All values are shown as mean ± SD. P values were calculated using student’s two-tailed t test; P < 0.05 was considered significant.
Other Methods.
Please see SI Experimental Procedures. Antibodies used in immunostaining are listed in Table S2.
Supplementary Material
Acknowledgments
We thank Lucy Brown, Alexander Spalla, and Pavinder Kaur from the Analytical Cytometry Core of City of Hope for assistance in flow sorting and Dr. Vincenzo Cirulli at the University of Washington for evaluating photomicrographs of electron microscopy. This work is supported in part by National Institutes of Health (NIH) Grant R01DK081587 (to H.T.K.), U01DK089533 (to A.D.R.), DK078803 (to M.S.), National Science Foundation NSF-DMR-1206121 (to D.A.T.), Office of Naval Research ONR-N00014-02-1 0958 and NSF-DBI-9970143 (to Electron Microscopy Core), NIH Grant P30 CA33572 (to Analytical Cytometry Core at City of Hope), a Juvenile Diabetes Research Foundation postdoctoral fellowship (to H.P.S.), and a City of Hope Eugene and Ruth Roberts Summer Student Academy fellowship (to A.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301889110/-/DCSupplemental.
References
- 1.Bonner-Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48(11):2296–2304. doi: 10.1007/s00125-005-1935-8. [DOI] [PubMed] [Google Scholar]
- 3.Yatoh S, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56(7):1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 4.Rovira M, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107(1):75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minami K, et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA. 2005;102(42):15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smukler SR, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8(3):281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SJ, Dor Y. Pancreatic cells and their progenitors. Methods Enzymol. 2006;419:322–337. doi: 10.1016/S0076-6879(06)19013-8. [DOI] [PubMed] [Google Scholar]
- 8.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 11.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 16.Ku HT. Minireview: Pancreatic progenitor cells—recent studies. Endocrinology. 2008;149(9):4312–4316. doi: 10.1210/en.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaberg RM, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22(9):1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 18.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92(3):1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movassat J, Beattie GM, Lopez AD, Hayek A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002;87(10):4775–4781. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- 20.Ku HT, et al. Insulin-expressing colonies developed from murine embryonic stem cell-derived progenitors. Diabetes. 2007;56(4):921–929. doi: 10.2337/db06-0468. [DOI] [PubMed] [Google Scholar]
- 21.Winkler M, et al. A quantitative assay for insulin-expressing colony-forming progenitors. J Vis Exp. 2011;(57):e3148. doi: 10.3791/3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5(2):497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 23.Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev Dyn. 2011;240(3):530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev Cell. 2010;18(6):1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129(10):2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13(1):103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Apelqvist A, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400(6747):877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 29.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136(21):3567–3574. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson KA, et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12(3):457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Miyatsuka T, Kosaka Y, Kim H, German MS. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci USA. 2011;108(1):185–190. doi: 10.1073/pnas.1004842108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, et al. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol. 2010;339(1):26–37. doi: 10.1016/j.ydbio.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JP, 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10(10):683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staal FJ, Luis TC. Wnt signaling in hematopoiesis: Crucial factors for self-renewal, proliferation, and cell fate decisions. J Cell Biochem. 2010;109(5):844–849. doi: 10.1002/jcb.22467. [DOI] [PubMed] [Google Scholar]
- 35.Heller RS, et al. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225(3):260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- 36.Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133(10):2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- 37.Kim KA, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309(5738):1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 38.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281(19):13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 39.Githens S. The pancreatic duct cell: Proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr. 1988;7(4):486–506. [PubMed] [Google Scholar]
- 40.Gao R, et al. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52(8):2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 41.Wescott MP, et al. Pancreatic ductal morphogenesis and the Pdx1 homeodomain transcription factor. Mol Biol Cell. 2009;20(22):4838–4844. doi: 10.1091/mbc.E09-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci USA. 2007;104(1):175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshima Y, et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132(2):720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 2008;36(1):e1–e6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 45.Hori Y, Fukumoto M, Kuroda Y. Enrichment of putative pancreatic progenitor cells from mice by sorting for prominin1 (CD133) and platelet-derived growth factor receptor beta. Stem Cells. 2008;26(11):2912–2920. doi: 10.1634/stemcells.2008-0192. [DOI] [PubMed] [Google Scholar]
- 46.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piper K, et al. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116(1-2):223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 48.Seymour PA, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104(6):1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 50.Narsinh KH, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaine SA, et al. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development. 2010;137(14):2289–2296. doi: 10.1242/dev.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilic G, Wang J, Sosa-Pineda B. Osteopontin is a novel marker of pancreatic ductal tissues and of undifferentiated pancreatic precursors in mice. Dev Dyn. 2006;235(6):1659–1667. doi: 10.1002/dvdy.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortijo C, Gouzi M, Tissir F, Grapin-Botton A. Planar Cell Polarity Controls Pancreatic Beta Cell Differentiation and Glucose Homeostasis. Cell Rep. 2012;2(6):1593–1606. doi: 10.1016/j.celrep.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikolova G, et al. The vascular basement membrane: A niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber FS, et al. Successful growth and characterization of mouse pancreatic ductal cells: Functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127(1):250–260. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, et al. Characterization of an in vitro differentiation assay for pancreatic-like cell development from murine embryonic stem cells: Detailed gene expression analysis. Assay Drug Dev Technol. 2011;9(4):403–419. doi: 10.1089/adt.2010.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.