Abstract
Loss or dysfunction of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) leads to impairment of airway mucus transport and to chronic lung diseases resulting in progressive respiratory failure. Nicotinic acetylcholine receptors (nAChRs) bind nicotine and nicotine-derived nitrosamines and thus mediate many of the tobacco-related deleterious effects in the lung. Here we identify α7 nAChR as a key regulator of CFTR in the airways. The airway epithelium in α7 knockout mice is characterized by a higher transepithelial potential difference, an increase of amiloride-sensitive apical Na+ absorption, a defective cAMP-dependent Cl− conductance, higher concentrations of Na+, Cl−, K+, and Ca2+ in secretions, and a decreased mucus transport, all relevant to a deficient CFTR activity. Moreover, prolonged nicotine exposure mimics the absence of α7 nAChR in mice or its inactivation in vitro in human airway epithelial cell cultures. The functional coupling of α7 nAChR to CFTR occurs through Ca2+ entry and activation of adenylyl cyclases, protein kinase A, and PKC. α7 nAChR, CFTR, and adenylyl cyclase-1 are physically and functionally associated in a macromolecular complex within lipid rafts at the apical membrane of surface and glandular airway epithelium. This study establishes the potential role of α7 nAChR in the regulation of CFTR function and in the pathogenesis of smoking-related chronic lung diseases.
Keywords: chloride efflux, ciliated cell, mouse, mucociliary clearance, submucosal gland
Chronic lung diseases are major causes of morbidity and mortality worldwide (1). Chronic obstructive pulmonary diseases (COPDs) are essentially observed in cigarette smokers and share many clinical features with CF (cystic fibrosis) (2), a disease caused by mutations of the cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. In COPD and patients with cystic fibrosis (CF), the lack of functional CFTR in the airways results in altered ion transport at the apical membrane, mucus dehydration and hyperviscosity, reduced mucus transport, the inability to prevent bacterial infections, and the progressive decline of lung function (2–4). In addition, cigarette smoke decreases cAMP-dependent Cl− secretion in vivo (5, 6) and in vitro (7), a process possibly related to smoke oxidants (8). These observations raise the possibility that some of the clinical lung symptoms in cigarette smokers may be explained by an altered CFTR function. However, to date, the potential mechanism by which cigarette smoking induces an altered CFTR function remains unclear.
Acetylcholine (ACh) regulates epithelial ion and water movements (9). ACh, in addition to exogenous nicotine, regulates airway epithelium function via paracrine and autocrine mechanisms through nicotinic acetylcholine receptors (nAChRs) (10). Recently, nAChRs have been shown to participate in the control of the airway ion transport processes in mice (11). CFTR as well as components of the nonneuronal cholinergic system (10, 12), including α7 nAChR (13) and choline acetyltransferase (14), are present at the apical membrane of airway ciliated cells. The α7 nAChR is characterized by a high Ca2+ permeability (15). Interestingly, α7 nAChR regulates cAMP via Ca2+ entry in the neuronal PC-12 cell line and this interaction is restricted to lipid rafts (16). Otherwise, the localization of CFTR in lipid rafts in epithelial cells is required for the CFTR-induced eradication of bacterial infections (17). We addressed the question of whether α7 nAChR may regulate CFTR activity in the airway epithelium and whether chronic nicotine exposure may modulate this interaction.
Results
α7 nAChR Is Present with CFTR at the Apex of the Human Airway Epithelium.
When using the H-302 antibody, validated for the identification of the α7 nAChR in the airways (Fig. S1), or α-bungarotoxin (α-BTX), a α7 nAChR antagonist, we localized the α7 nAChR in the normal human airway epithelium, both at the apex of the epithelium and in basal epithelial cells (Fig. S1 A and B). α7 nAChR was shown to be present at the apical membrane of ciliated cells and partially colocalized with CFTR protein (Fig. S1 E and F).
Absence of α7 nAChR or Its Inactivation by α-BTX Alters CFTR Function and Mucus Transport in the Airway Epithelium.
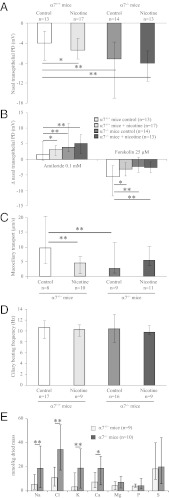
The airway epithelium of α7−/− mice is characterized by a lower nasal transepithelial potential difference (PD) (Fig. 1A), a higher PD increase in the presence of amiloride, an inhibitor of the epithelium sodium channel (Fig. 1B), a lower PD decrease in the presence of amiloride and forskolin, which activates adenyl cyclases (ACs), raises intracellular cAMP levels, and activates CFTR (Fig. 1B). This represents a bioelectric status similar to what is observed in patients with CF (18). Moreover, α7−/− mouse airway is characterized by a lower mucus transport (Fig. 1C), with no modification of the ciliary beating frequency (Fig. 1D), suggesting a defect in α7−/− mice of airway mucus hydration and/or ionic composition. Indeed, we observed higher concentrations of electrolytes such as Na, Cl, K, and Ca in α7−/− mice tracheal airway mucus, whereas concentrations of Mg, P, and S were similar in α7+/+ and α7−/− mice (Fig. 1E). Similarly, elevated NaCl concentrations have been reported in CF airway fluids (19). However, it has then been shown that airway-surface liquid in CFTR-null mice is approximately isotonic (20) and that submucosal gland secretions in airways from patients with CF have normal [Na+], although presenting elevated viscosity (21). Contrary to α7 nAChR, absence of either α5, β2, or β4 nAChR subunit does not impact on airway mucus transport in mice (Fig. S2).
Fig. 1.
Bioelectric properties and mucociliary transport of murine airway epithelium are altered in α7−/− mice and in nicotine-exposed α7+/+ mice. α7+/+ or α7−/− mice were exposed to saline (control) or nicotine (three 1-mg/kg i.p. injections of nicotine 24 h, 16 h, and 1 h before the measurements) and the following parameters were recorded: nasal transepithelial PD (A), changes in nasal transepithelial PD upon amiloride and amiloride + forskolin exposure (B), mucociliary transport (C), and ciliary beat frequency (D). (E) Ionic composition of tracheal airway mucus in α7+/+ and α7−/− mice. Results are presented as median, with maximal and minimal values, and compared with the Mann–Whitney test (*P < 0.05, **P < 0.01).
Similarly, apical incubation of human airway epithelial cells (HAECs), isolated from patients without CF, with 1–10 µM αBTX dose-dependently induces a higher decrease of short-circuit current (Isc) in the presence of amiloride and a lower Isc increase in the presence of amiloride and forskolin (Fig. 2 A and B). To confirm the effect of αBTX on CFTR functionality, we performed chloride efflux experiments using the halide-sensitive dye 6-methoxy-N-(3-sulfopropyl) quinolinium (SPQ) on HAECs preincubated with 0–10 µM αBTX. αBTX dose-dependently decreased the forskolin-activated chloride efflux, an effect abolished upon CFTR inhibition with CFTRinh-172, a thiazolidinone-specific CFTR inhibitor (Fig. 2D).
Fig. 2.
α7 nAChR inhibition with αBTX or prolonged nicotine exposure alters CFTR function in air–liquid interface HAEC cultures. (A) Representative Isc tracing from air–liquid interface HAEC cultures in baseline condition, in the presence of 0.1 mM amiloride and 0.1 mM amiloride and 25 µM forskolin. (B and C) Changes in Isc upon amiloride and amiloride + forskolin exposure, after a 3 h-incubation with αBTX (0–10 µM) (B) or an overnight incubation with nicotine (0–10 µM) (C), added either apically or basally. (D) SPQ fluorescence variations in air–liquid interface HAEC cultures, induced by 25 µM forskolin in the presence of 0.1 mM amiloride: effect of a preincubation with 10 µM CFTRinh-172 for 1 h and with αBTX (0–10 µM) for 3 h. CFTRinh-172 was added during the last 30 min of the 3-h incubation with αBTX. Results are presented as median, with maximal and minimal values, for six (B and C) or five (D) HAEC cultures derived from different patients and compared with the Mann–Whitney test to the corresponding control in the absence of drug (*P < 0.05, **P < 0.01).
CFTR also controls mucus secretion from airway submucosal glands (22). As observed in the airway surface epithelium, α7 nAChR and CFTR are present at the apical side of the glandular epithelium (Fig. S3A). Fig. S3 shows that αBTX dose-dependently decreased forskolin-activated chloride efflux in MM39, a cell line derived from the normal human airway glandular epithelium and expressing WT-CFTR (23). It had no effect on KM4, a cell line derived from CF human tracheal glands and homozygous for the ΔF508 mutation (24), and decreased forskolin-activated chloride efflux in KM4*, derived from the KM4 cell line after transduction with the lentiviral vector expressing the WT-CFTR cDNA (25). These αBTX effects were abolished after inhibiting CFTR with the CFTRinh-172 (Fig. S3C). These results demonstrate that α7 nAChR controls CFTR function in airway submucosal glands as well as in the surface epithelium.
α7 nAChR Activation Increases Intracellular Calcium and cAMP Concentrations and Chloride Efflux in Airway Epithelial Cells.
CFTR is essentially regulated by cAMP-dependent protein kinase A (PKA) and ATP (26). Adenyl cyclase AC-1 and -8 isoforms are apically expressed in airway epithelial cells in culture (27) and are stimulated by Ca2+ in a calmodulin-dependent manner (28). AC-1 and AC-8 are insensitive to Ca2+ release from intracellular stores, and are rather stimulated by Ca2+ entry (29). α7 nAChR is characterized by an elevated Ca2+ permeability (15). We thus postulated that α7 nAChR-mediated Ca2+ entry, at the apex to the airway epithelium, may positively control AC activity and then CFTR function.
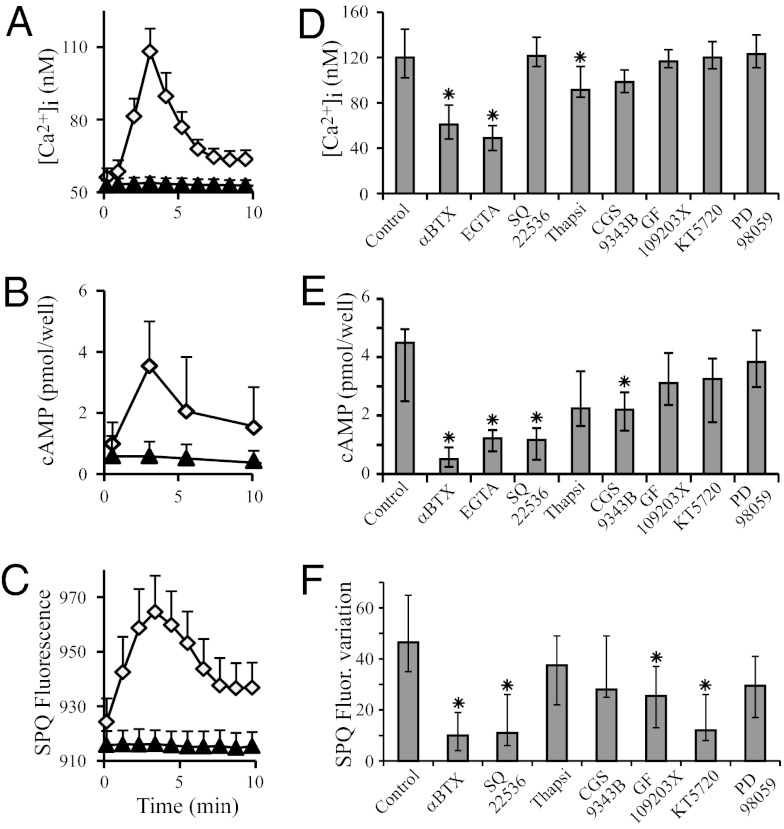
When MM39 cells were exposed to 10 µM PHA 568487 (Tocris Bioscience), a specific agonist for α7 nAChR (30), intracellular Ca2+ and cAMP and chloride efflux followed the same evolution with a maximal increase observed 3 min after PHA addition (Fig. 3 A–C). PHA induced a dose-dependent rise in [cAMP]i, with a maximal effect, observed with a 10-µM concentration, similar to that observed after the direct activation of ACs with forskolin (Fig. S4). We then studied the effect of different inhibiting drugs (αBTX for α7 nAChR, EGTA for extracellular Ca2+ sequestration, SQ22536 for ACs, thapsigargin to deplete [Ca2+]i stores, CGS 9343B for calmodulin, GF 109203× for PKC, KT 5720 for PKA, and PD 98059 for ERK1/2 MAP kinases) on [Ca2+]i, [cAMP]i, and chloride efflux variations after 3-min exposure to PHA 568487. We observed that PHA 568487-induced [Ca2+]i increase depended mainly on [Ca2+]e (Fig. 3D). PHA 568487-induced [cAMP]i increase depended also mainly on [Ca2+]e with a role of calmodulin (Fig. 3E), and PHA 568487-induced increase of chloride secretion mainly depended on the activity of ACs with a subsequent role of PKA and to a smaller extend PKC (Fig. 3F), suggesting that, upon α7 nAChR activation in airway epithelial cells, the increase in [Ca2+]i essentially results from an entry of extracellular calcium, and the subsequent CFTR activation mainly depends upon the activation of ACs, PKA and PKC.
Fig. 3.
α7 nAChR activation induces increases of [Ca2+]i, [cAMP]i, and chloride secretion in airway epithelial cells. MM39 cells were exposed to 10 µM PHA 568487 (white diamonds) and [Ca2+]i (A) and SPQ fluorescence in the presence of 0.1 mM amiloride (C) were monitored for 10 min. [cAMP]i was also discontinuously measured (B). Controls consisted of 0.1% DMSO (black triangles). Results are expressed as mean ± SD for 15 different cells (calcium and SPQ) or five independent experiments (cAMP). (D–F) Effect of different inhibitors on PHA 568487-induced α7 nAChR activation-dependent increases of [Ca2+]i, [cAMP]i, and chloride secretion. MM39 cells were exposed for 60 min to 10 µM αBTX or for 15 min to one of the following drugs: 1 mM EGTA, 50 µM SQ22536, 1 µM thapsigargin, 10 µM CGS9343B, 2 µM GF109203X, 1 µM KT5720, or 50 µM PD98059. A total of 10 µM PHA 568487 was then added and [Ca2+]i and SPQ fluorescence were monitored for 10 min. [Ca2+]i (D), [cAMP]i (E), and SPQ fluorescence variations (F) were measured 3 min after PHA 568487 addition. Results correspond to four different experiments and were compared with the control exposed only to 10 µM PHA 568487.
Chronic Nicotine Exposure Mimics the Absence of α7 nAChR in Mice or Its Inhibition by αBTX in HAEC Cultures.
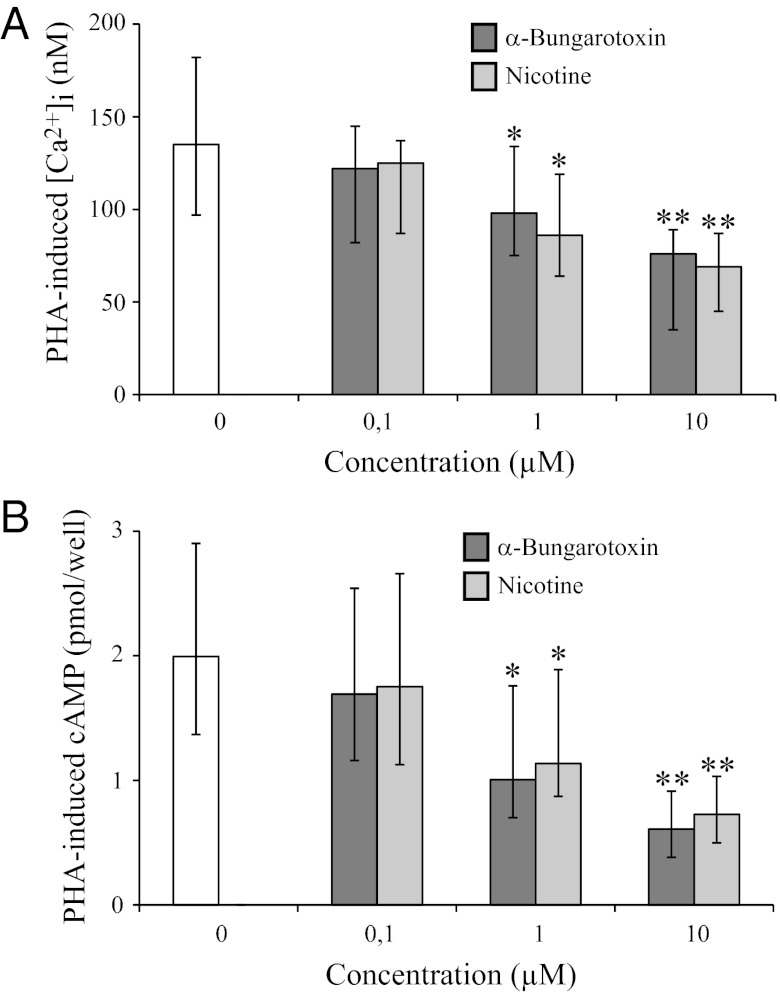
When α7+/+ mice received three 1-mg/kg i.p. injections of nicotine 24 h, 16 h, and 1 h before the measurements, we observed that nicotine exposure decreased nasal transepithelial PD (Fig. 1A) and induced a higher PD increase upon amiloride treatment and a lower PD decrease upon forskolin treatment (Fig. 1B). It decreased mucus transport in the airways (Fig. 1C), with no effect being observed on ciliary beating frequency (Fig. 1D), whereas nicotine exposure did not significantly change these parameters in α7−/− mice. Similarly, apical incubation of HAECs, isolated from patients without CF, with 1–10 µM nicotine dose-dependently induces a higher decrease of Isc in the presence of amiloride and a lower increase of Isc in the presence of amiloride and forskolin (Fig. 2C). Moreover, overnight incubation of HAEC cultures with nicotine (1–10 µM) mimics αBTX in inhibiting PHA 568487-induced α7 nAChR activation-dependent increases of [Ca2+]i and [cAMP]i (Fig. 4 A and B).
Fig. 4.
Chronic nicotine exposure mimics αBTX in inhibiting PHA 568487-induced α7 nAChR activation-dependent increases of [Ca2+]i and [cAMP]i in human airway epithelial cells. Air–liquid interface HAEC cultures were apically incubated for 3 h with αBTX (0–10 µM) or overnight with nicotine (0–10 µM). Three minutes after the addition of PHA 568487 (10 µM), [Ca2+]i (A) and [cAMP]i (B) were measured (Fig. 3 A and B). Results correspond to five HAEC cultures derived from different patients and were compared with the corresponding control exposed to only 10 µM PHA 568487.
α7 nAChR Is Associated with CFTR and AC-1 Within Lipid Rafts at the Apical Plasma Membrane of Ciliated Cells in the Airway Epithelium.
We have confirmed that in vivo in human bronchial tissue samples AC-1 and AC-8, two ACs activated by Ca2+ in a calmodulin-dependent manner (28), are distributed at the apex of the airway epithelium (27), whereas AC-3, for which activation by Ca2+ is not clear (28), is expressed in airway epithelial basal cells (Fig. S5A). By using confocal microscopy, we have observed a partial colocalization of α7 nAChR with CFTR, AC-1, and AC-8 at the apical membrane of airway ciliated cells (Figs. S1E and S5B). In control mice, both CFTR and α7 nAChR were identified at the apex of the airway epithelium (Fig. S5 C and D). Whereas α7 nAChR localization did not change in the absence of CFTR in mice (Fig. S5D), CFTR was rather observed delocalized in the cytoplasm in the upper part of ciliated cells in α7−/− mice (Fig. S5C), suggesting that the absence of α7 nAChR in mice alters CFTR localization at the apical membrane. Immunoprecipitation techniques revealed that α7 nAChR is present along with CFTR and AC-1 in the same macromolecular protein complex in the plasma membrane of airway epithelial cells (Fig. S5 E and F).
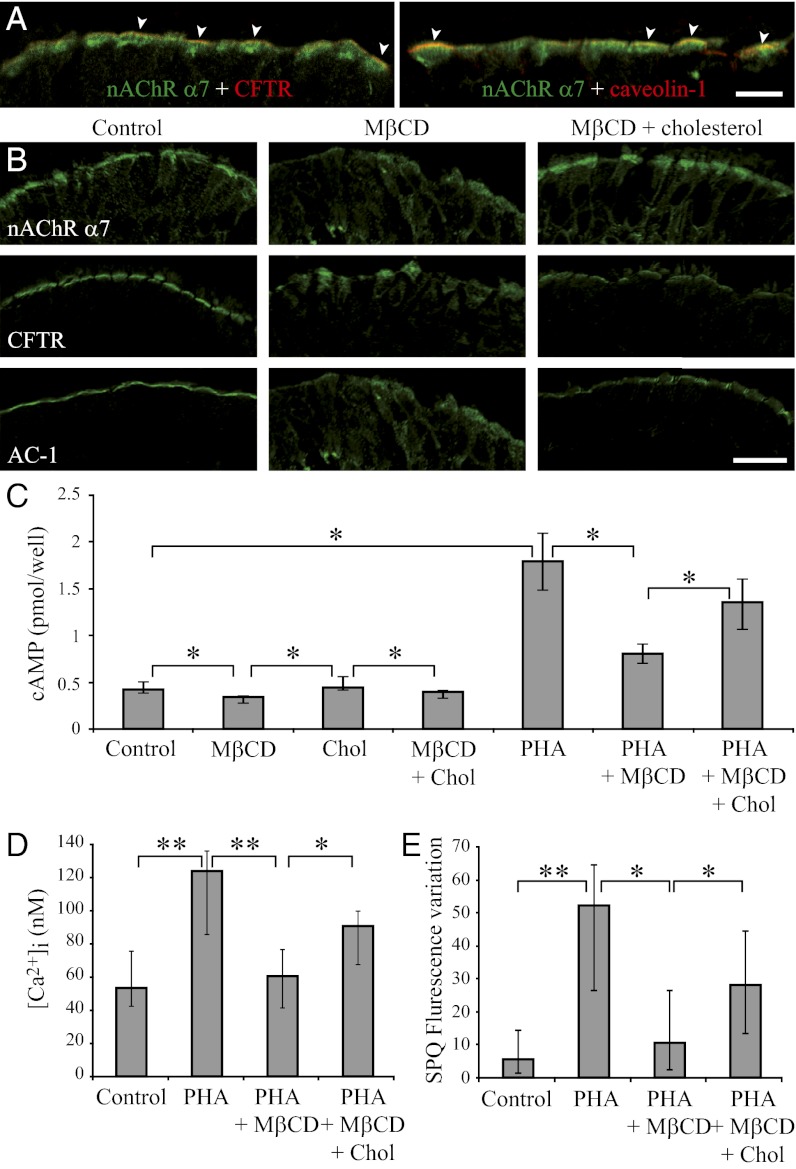
Lipid rafts, including caveolae, are cholesterol and sphingolipid-enriched membrane microdomains, serving as organizing centers for the assembly of signaling molecules. We observed that α7 nAChR partially colocalized both with CFTR and caveolin-1, a marker of caveolae (Fig. 5A). Methyl-β-cyclodextrin (MβCD) depletes plasma membrane cholesterol, which in turn decreases the functionality of molecules that need to be assembled within lipid rafts to interact with each other (31). Recently, ΜβCD has also been shown to strongly reduce α7 nAChR mobility at the cell’s plasma membrane (32). Whereas α7 nAChR, CFTR, and AC-1 are focalized at the apex of the airway epithelium, they are diffusely delocalized in the cytoplasm after incubation of human bronchial tissue samples with MβCD, this effect being partially reversed in the presence of cholesterol (Fig. 5B). Incubating in vitro airway epithelial cells with MβCD decreased both [cAMP]i in control cells and the PHA 568487-induced α7 nAChR activation-dependent increase of [cAMP]i. This effect was reversed in the presence of cholesterol (Fig. 5C). We observed similar effects on PHA 568487-induced α7 nAChR activation-dependent increase of [Ca2+]i and chloride efflux (Fig. 5 D and E). These results suggest that α7 nAChR, CFTR, and AC-1 are coassembled within lipid rafts at the apical plasma membrane of airway ciliated cells and that this association is needed for the functional interaction between these molecules and for the α7 nAChR-mediated control of CFTR functionality.
Fig. 5.
Effect of cholesterol depletion on α7 nAChR, CFTR, and AC-1 distribution and on PHA 568487-induced α7 nAChR activation-dependent increases of [Ca2+]i, [cAMP]i, and chloride secretion in airway epithelial cells. (A) Distribution of α7 nAChR (green) and of CFTR and caveolin-1 (red) at the apical membrane of ciliated cells in human bronchial tissue samples. Arrowheads point to colocalization of α7 nAChR with CFTR or caveolin-1. (B) α7 nAChR, CFTR, and AC-1 were localized in control human bronchial tissue samples (Left column) or after 1-h incubation at 37 °C in the presence of 10 mM ΜβCD either alone (Center column) or with 20 µg/mL cholesterol (Right column). (C–E) MM39 cells were similarly incubated for 1 h in the presence of ΜβCD, cholesterol (Chol), or ΜβCD with cholesterol. Then, 10 µM PHA 568487 (PHA) was added and [cAMP]i was measured 3 min after PHA 568487 addition (C). [Ca2+]i (D) and chloride secretion (SPQ fluorescence variation in the presence of 0.1 mM amiloride) (E) were monitored for 6 min after PHA 568487 addition, and the magnitude of calcium and SPQ fluorescence increases at 3 min (Fig. 3 A and C) was determined. Results correspond to four (C), five (D), and six (E) different experiments. [Scale bars, 8 µm (A) and 20 µm (B).]
Discussion
The present study highlights a previously unknown macromolecular signaling complex in which α7 nAChR appears as a key regulator of CFTR functional activity in the airway epithelial cells both in the surface epithelium and in submucosal glands. We establish that α7 nAChR and CFTR must be assembled in lipid rafts in a physically and functionally interacting macromolecular complex to ensure an efficient functional coupling between α7 nAChR and CFTR, including key signaling elements such as ACs, PKA and PKC. The absence of α7 nAChR results in decreased mucus transport in the mouse airway, and chronic nicotine exposure mimics the absence of α7 nAChR in mice or its pharmacological inactivation in vitro in HAEC cultures. The biological significance of these findings is particularly relevant to chronic respiratory disorders related either to acquired CFTR dysfunctions or tobacco smoking.
Impairment of airway mucus transport results from dysfunction of CFTR. The nonneuronal cholinergic system also control mucus transport (33) and is deregulated in the airways of patients with CF (34). Recently, Hollenhorst et al. observed that acute nicotine exposure modulated ion transport processes in the murine tracheal epithelium and this effect was mediated by nAChRs (11). Although the nicotine effect was partly mediated by α7 nAChR, CFTR was not likely involved in this process. In this study, the use of nicotine as a general agonist of nAChRs may have underestimated the specific involvement of α7 nAChR. Indeed, Hollenhorst et al. (11) suggested that heteropentameric nAChRs, in relation to Ca2+-activated chloride channels and potassium channels, mostly contribute to ion transport processes in the mouse airway. We specifically explored, in both mouse and human, α7 nAChR function, by using α-BTX and PHA 568487, and CFTR function (forskolin-induced cAMP-dependent CFTR activation). This approach has emphasized the α7 nAChR–CFTR interaction in controlling airway ion and mucus transports. On the contrary, the absence of either α5, β2, or β4 nAChR subunit does not impact on airway mucus transport in mice, suggesting that α5/β2/β4-containing heteropentameric nAChRs are not involved in this process.
Most patients with COPD have a history of chronic smoking and are characterized by an impaired mucus transport, which results in chronic airway infections, but how smoking perturbs this process is still incompletely understood. Cigarette smoke exposure inhibits airway Cl− secretion in vivo and in vitro (5, 7), whereas smokers with no CFTR mutation exhibit nasal transepithelial PD values similar to that of patients with CF (6). We report here that prolonged exposure to nicotine alone of α7+/+ mice or of HAEC cultures, produced the same effects resulting from the absence, in α7−/− mice, or inactivation by α-BTX of the α7 nAChR. This includes decreases of nasal transepithelial PD, of forskolin-mediated CFTR activation, of mucus transport, and of α7 nAChR activation-dependent increases of [Ca2+]i and [cAMP]i and an increase of amiloride-sensitive apical Na+ absorption. These findings suggest that chronic nicotine exposure, through its specific action on the α7 nAChR, has the same inhibitory effect on the airway mucus transport as cigarette smoking.
In patients with COPD who smoke, chronic exposure to nicotine may result in α7 nAChR desensitization. A specific property of nAChRs is their susceptibility to desensitization (35, 36), whereby a decrease or loss of functional response occurs upon chronic exposure to nicotine (37). Given the high affinity of desensitized nAChRs for ligands, regular cigarette smoking may permanently maintain nAChR desensitization (38). Whatever the regulation of α7nAChR expression by nicotine (39–41), neuronal α7 nAChR is particularly sensitive to desensitization after a prolonged exposure to nicotine (37, 42). Moreover, overexposure of bronchial epithelium cells to nicotine in vitro produced an antagonist-like effect (41). We thus hypothesize that changes in airway bioelectric properties, mucus transport, and α7 nAChR activation-induced modulations of [Ca2+]i and [cAMP]i, which we observed upon chronic nicotine exposure, may result from α7 nAChR desensitization. It follows that maintained airway α7 nAChR desensitization contributes to CFTR-related lung diseases in heavy smokers.
We have previously reported that α7 nAChR regulates airway epithelium differentiation by controlling basal cell proliferation. The lack of functional α7 nAChR in the airways leads to squamous metaplasia and loss of ciliary function, alterations also observed in patients with COPD (13). The α7 nAChR thus emerges as a key element of airway epithelium homeostasis. The decrease of α7 nAChR function, as a consequence of either down-regulated expression or desensitization by prolonged nicotine exposure in smokers, may directly alter CFTR activity and consequently mucus transport and antibacterial protection. It may also compromise the ability of the airway epithelium to regenerate upon chronic inflammation and thus contributes to COPD development in smokers. Moreover, several studies have shown that α7 nAChR plays a critical role in the inflammatory response and the consecutive lung injury, by negatively regulating the synthesis and release of proinflammatory cytokines, such as TNFα (43). Whether the dysfunction of α7 nAChR associates an impairment of ion and water airway epithelial transport with airway epithelial inflammation remains to be elucidated. α7−/− mice share with CFTR−/− mice changes in the airway epithelium that are strikingly similar to those observed in patients with CF or smoking-related lung diseases. Surprisingly, α7−/− mice like mouse CF mutants fail to exhibit CF-like lung disease. The lack of lung disease may be explained by the fact that our α7−/− mice were maintained in a pathogen-free environment, thus preventing any chronic pulmonary infections similar to human airway pathologies. Another possible explanation is that the reduced CFTR activity in α7 KO mice is compensated by non-CFTR Cl− channels protecting the lung from disease, as already postulated (44). Otherwise, we have observed that α7−/− mice have a reduction in body weight and are less fertile compared with control mice, phenotypes reminiscent of most mouse CF mutants (45).
In conclusion, we describe the coupling of α7 nAChR signaling to CFTR Cl− channel function in the human airway epithelium and submucosal glands: α7 nAChR activation leads to calcium entry, AC-1 activation, and cAMP generation. This activates a cascade of signaling pathways, including PKA and PKC and finally results in CFTR-mediated Cl− secretion (Fig. S6). Our report also suggests that alterations in α7 nAChR lead to CFTR dysfunctions that may cause airway CF-like disorders further leading to chronic airway disease, especially in smokers.
Materials and Methods
Detailed protocols are provided in SI Materials and Methods.
Cell Culture and Media.
HAECs were isolated from polyps and bronchial tissues, and cultured as described (13). MM39, a cell line derived the normal human airway glandular epithelium and expressing wt-CFTR, KM4, a cell line derived from CF human tracheal glands and homozygous for the ΔF508 mutation and KM4*, derived from the KM4 cell line and expressing the wt-CFTR cDNA, were cultured as described with modifications (46).
Immunocyto/histochemistry.
An indirect immunofluorescence labeling technique was performed on frozen sections of bronchial tissues or cell cultures as described (47).
Immunoprecipitation and Western Blotting.
Immunoblot techniques were used to demonstrate the association between CFTR, α7 nAChR, and AC-1.
Treatment of Mice with Nicotine.
α7+/+ or α7−/− mice received three i.p. injections of 1 mg/kg nicotine (nicotine tartrate salt) in normal saline 24 h, 16 h, and 1 h before measurements (nasal transepithelial PD, mucus transport, and ciliary beating frequency.
Transepithelial Potential Difference Measurements.
Nasal transepithelial PD measurements were performed in mice as described with modifications (48).
Studies of Chloride Efflux.
Studies of chloride efflux were performed, using the halide-sensitive dye 6-methoxy-N-(3-sulfopropyl) quinolinium (SPQ), as described (46).
Electrophysiology.
A Ussing chamber technique with HAECs was used to record Isc resulting from CFTR-mediated chloride efflux as described (46).
Measurement of Ionic Composition of Tracheal Surface Liquid.
Native airway surface liquid was collected by a cryotechnique and ionic composition was analyzed by X-ray microanalysis as described (49).
Measurement of Mucus Transport Velocity and Mucociliary Frequency of Murine Tracheal Epithelium.
Mucus transport velocity of mouse tracheal epithelium was evaluated by tracking polystyrene fluorescent microspheres added on the epithelial surface. The mucociliary frequency measurement consisted of recording the frequency of the mucus waves propagated by the underlying cilia.
Measurement of α7 nAChR Activation-Dependent [Ca2+]i Variation.
The variations of [Ca2+]i upon α7 nAChR activation were followed with the calcium-sensitive Fura-2 acetoxymethyl ester by a fluorescence ratiometric method as described (47).
Statistical Analyses.
Except for curves illustrating the variations of [Ca2+]i, [cAMP]i, and chloride secretion, where data were presented as mean ± SD, all data were expressed as median with maximal and minimal values and compared with the nonparametric Mann–Whitney test (*P < 0.05, **P < 0.01).
Supplementary Material
Acknowledgments
We thank Edith Puchelle, Béatrice Nawrocki-Raby, Myriam Polette, Mathilde Viprey, and Thierry Chinet for their insights; Robert L. Dormer for the gift of the MPCT-1 anti-CFTR antibody and Marc Merten for the gift of MM39 and KM4 cell lines; all of the surgeons and ear, nose, and throat doctors who provided us with human airway tissues (Profs. Christian Debry and Gaétan Deslee and Drs. Salima Bellefqih, Maryline Dauphin, Anne Durlach, Karine Joseph, Talal Nasser, Christophe Ruaux, and Dominique Zachar); and Dr. Denis Lamiable for the nicotine and cotinine measurements in mice. This work was supported by grants from Vaincre la Mucoviscidose (to C.C. and F.D.), the Lions Club of Soissons and 1 Euro contre le cancer (to P.B.), and by the Région Champagne-Ardenne (K. Maouche and K. Medjber).
Footnotes
The authors declare no conflict of interest.
†This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216939110/-/DCSupplemental.
References
- 1.Barnes PJ. New therapies for chronic obstructive pulmonary disease. Med Princ Pract. 2010;19(5):330–338. doi: 10.1159/000316368. [DOI] [PubMed] [Google Scholar]
- 2.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23(1):146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 3.Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat. 2002;201(4):313–318. doi: 10.1046/j.1469-7580.2002.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Welsh MJ. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J Clin Invest. 1983;71(6):1614–1623. doi: 10.1172/JCI110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin AM, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173(10):1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 7.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 8.Cantin AM, et al. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med. 2007;42(1):15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acevedo M. Effect of acetyl choline on ion transport in sheep tracheal epithelium. Pflugers Arch. 1994;427(5-6):543–546. doi: 10.1007/BF00374272. [DOI] [PubMed] [Google Scholar]
- 10.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollenhorst MI, et al. Evidence for functional atypical nicotinic receptors that activate K+-dependent Cl- secretion in mouse tracheal epithelium. Am J Respir Cell Mol Biol. 2012;46(1):106–114. doi: 10.1165/rcmb.2011-0171OC. [DOI] [PubMed] [Google Scholar]
- 12.Pfeil U, et al. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am J Respir Cell Mol Biol. 2003;28(4):473–477. doi: 10.1165/rcmb.2002-0190OC. [DOI] [PubMed] [Google Scholar]
- 13.Maouche K, et al. alpha7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol. 2009;175(5):1868–1882. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessler I, Kirkpatrick CJ, Racké K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: Expression and function in humans. Pharmacol Ther. 1998;77(1):59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 15.Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshikawa J, et al. Nicotinic acetylcholine receptor alpha 7 regulates cAMP signal within lipid rafts. Am J Physiol Cell Physiol. 2003;285(3):C567–C574. doi: 10.1152/ajpcell.00422.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol. 2004;172(1):418–425. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 18.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 19.Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148(6 Pt 1):1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107(3):317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na(+)] and pH but elevated viscosity. Proc Natl Acad Sci USA. 2001;98(14):8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JV, Krouse ME, Wine JJ. Acinar origin of CFTR-dependent airway submucosal gland fluid secretion. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L304–L311. doi: 10.1152/ajplung.00286.2006. [DOI] [PubMed] [Google Scholar]
- 23.Merten MD, et al. A transformed human tracheal gland cell line, MM-39, that retains serous secretory functions. Am J Respir Cell Mol Biol. 1996;15(4):520–528. doi: 10.1165/ajrcmb.15.4.8879186. [DOI] [PubMed] [Google Scholar]
- 24.Kammouni W, et al. A cystic fibrosis tracheal gland cell line, CF-KM4. Correction by adenovirus-mediated CFTR gene transfer. Am J Respir Cell Mol Biol. 1999;20(4):684–691. doi: 10.1165/ajrcmb.20.4.3341. [DOI] [PubMed] [Google Scholar]
- 25.Baconnais S, et al. Abnormal ion content, hydration and granule expansion of the secretory granules from cystic fibrosis airway glandular cells. Exp Cell Res. 2005;309(2):296–304. doi: 10.1016/j.yexcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Guggino WB, Stanton BA. New insights into cystic fibrosis: Molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7(6):426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 27.Nlend MC, et al. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett. 2007;581(17):3241–3246. doi: 10.1016/j.febslet.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 29.Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca(2+)-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271(21):12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- 30.Walker DP, et al. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorg Med Chem. 2006;14(24):8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Fagan KA, Smith KE, Cooper DM. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem. 2000;275(34):26530–26537. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- 32.Baier CJ, Gallegos CE, Levi V, Barrantes FJ. Cholesterol modulation of nicotinic acetylcholine receptor surface mobility. Eur Biophys J. 2010;39(2):213–227. doi: 10.1007/s00249-009-0521-2. [DOI] [PubMed] [Google Scholar]
- 33.Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130(2):219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wessler I, et al. Dysfunction of the non-neuronal cholinergic system in the airways and blood cells of patients with cystic fibrosis. Life Sci. 2007;80(24-25):2253–2258. doi: 10.1016/j.lfs.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 35.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308(5727):1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 36.Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: Shaping cholinergic signaling. Trends Neurosci. 2005;28(7):371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Corringer PJ, et al. Critical elements determining diversity in agonist binding and desensitization of neuronal nicotinic acetylcholine receptors. J Neurosci. 1998;18(2):648–657. doi: 10.1523/JNEUROSCI.18-02-00648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brody AL, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63(8):907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39(13):2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 40.Carlisle DL, et al. Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respir Res. 2004;5:27. doi: 10.1186/1465-9921-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol. 1997;97(3):243–262. [PubMed] [Google Scholar]
- 42.Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283(2):675–683. [PubMed] [Google Scholar]
- 43.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 44.Clarke LL, et al. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci USA. 1994;91(2):479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilke M, et al. Mouse models of cystic fibrosis: Phenotypic analysis and research applications. J Cyst Fibros. 2011;10(Suppl 2):S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 46.Delavoie F, et al. Salmeterol restores secretory functions in cystic fibrosis airway submucosal gland serous cells. Am J Respir Cell Mol Biol. 2009;40(4):388–397. doi: 10.1165/rcmb.2008-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tournier JM, et al. alpha3alpha5beta2-Nicotinic acetylcholine receptor contributes to the wound repair of the respiratory epithelium by modulating intracellular calcium in migrating cells. Am J Pathol. 2006;168(1):55–68. doi: 10.2353/ajpath.2006.050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahm JM, et al. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol. 1997;272(3 Pt 1):C853–C859. doi: 10.1152/ajpcell.1997.272.3.C853. [DOI] [PubMed] [Google Scholar]
- 49.Zahm JM, et al. X-ray microanalysis of airway surface liquid collected in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L309–L313. doi: 10.1152/ajplung.2001.281.2.L309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.