Abstract
The mechanistic target of rapamycin (mTOR) is a serine/threonine kinase whose activity contributes to leukemia proliferation and survival. Compounds targeting the mTOR active site inhibit rapamycin-resistant functions and have enhanced anti-cancer activity in mouse models. MLN0128 (formerly known as INK128) is a novel, orally active mTOR kinase inhibitor currently in clinical development. Here we evaluated MLN0128 in preclinical models of B-cell acute lymphoblastic leukemia (B-ALL). MLN0128 suppressed proliferation of B-ALL cell lines in vitro and reduced colony formation by primary human leukemia cells from adult and pediatric B-ALL patients. MLN0128 also boosted the efficacy of dasatinib in Philadelphia Chromosome-positive (Ph+) specimens. In a syngeneic mouse model of lymphoid BCR-ABL+ disease, daily oral dosing of MLN0128 rapidly cleared leukemic outgrowth. In primary xenografts of Ph+ B-ALL specimens, MLN0128 significantly enhanced the efficacy of dasatinib. In non-Ph B-ALL xenografts, single agent MLN0128 had a cytostatic effect that was most pronounced in mice with low disease burden. In all in vivo models, MLN0128 was well tolerated and did not suppress endogenous bone marrow proliferation. These findings support the rationale for clinical testing of MLN0128 in both adult and pediatric B-ALL and provide insight towards optimizing therapeutic efficacy of mTOR kinase inhibitors.
Keywords: kinase inhibitor, mTOR, leukemia
Introduction
In B-ALL and other hematological malignancies, cell-intrinsic oncogenic lesions and cell-extrinsic microenvironmental cues converge on a set of intracellular signaling pathways that drive proliferation and survival (1). The development of compounds that inhibit pro-survival signaling proteins has potential to improve patient outcomes and enhance the efficacy of current treatments. The target of rapamycin (mTOR) is a key signaling enzyme whose activity is elevated in most leukemia cells (2–4). mTOR (also known as TOR) is a serine/threonine kinase that exists in two multi-protein complexes, mTORC1 and mTORC2, with different upstream activators and downstream substrates (5). Rapamycin and its analogs (rapalogs) act via an allosteric mechanism and do not fully inhibit the function of mTORC1 or mTORC2 (4–6). Rapalogs have cytostatic activity in many cell contexts but are not strongly cytotoxic, and display limited activity in leukemia models and clinical trials. A novel class of ATP-competitive mTOR inhibitors, here termed mTOR kinase inhibitors (also known as TORKinibs, TORC1/2 inhibitors, asTORi or TOR-KIs), fully inhibit both mTOR complexes and have improved cytotoxic activity and anti-leukemic efficacy in preclinical testing (7–9).
mTOR functions in a complex, non-linear network of kinases that include phosphoinositide 3-kinase (PI3K) and AKT (5). Activation of PI3K and AKT promotes diverse aspects of cell growth, proliferation, survival and metabolism (10). Full AKT activation requires phosphorylation on Thr-308 by phosphoinositide-dependent kinase-1 (PDK-1) and on Ser-473 by mTORC2. Activated AKT can phosphorylate tuberous sclerosis complex-2 (TSC2) and PRAS40 to promote mTORC1 activity, yet AKT activity is not required for mTORC1 function in some cell contexts. Thus, leukemia cells lacking PI3K/AKT activity can survive by maintaining residual mTORC1 activity through other mechanisms (11). Through phosphorylation of S6 kinases and eukaryotic initiation factor 4E (eIF4E) binding proteins (4EBPs), mTORC1 promotes biosynthesis of proteins and lipids required for cell growth and division. However, mTORC1 also initiates negative feedback mechanisms that attenuate the activity of both PI3K and AKT. Rapalogs suppress some of these feedback loops, leading to elevated PI3K/AKT signaling that may promote leukemia cell survival.
The complexity of the PI3K/AKT/mTOR network provides rationale for targeting multiple components of the pathway to achieve maximum anti-cancer efficacy (12, 13). Pharmacological data have supported this concept. Much of the evidence comes from studies of ATP-competitive, pan-selective inhibitors targeting both PI3K and mTOR. These pan-PI3K/mTOR inhibitors (examples include PI-103, NVP-BEZ235, NVP-BGT226, XL765 and GDC-0980) have impressive anti-cancer activity in a wide range of tumor models (14–20). Additional evidence has emerged from studies of mTOR kinase inhibitors, which are selective for the mTOR enzyme compared to PI3K (21–23). Like pan-PI3K/mTOR inhibitors, mTOR kinase inhibitors fully block both mTORC1 and mTORC2 and generally prevent the acute PI3K/AKT rebound effect of rapalogs. mTOR kinase inhibitors are more effective than rapamycin at suppressing proliferation of normal and transformed cell lines. mTOR kinase inhibitors are more cytotoxic than rapamycin in models of Ph+ B-ALL (9) and have some cytotoxic activity in solid tumors, potentially providing an additional advantage in the setting of cancer therapy.
Several mTOR kinase inhibitors have entered clinical trials, and are being tested in patients with solid tumors and hematological malignancies. Optimizing the therapeutic success of these agents in leukemia will be aided by further study in preclinical models. MLN0128 (formerly known as INK128) is a highly potent, orally active mTOR kinase inhibitor (24) currently in phase I clinical trials (Identifiers NCT01058707, NCT01351350, NCT01118689). MLN0128 displays anti-tumor and anti-metastatic activity in prostate cancer models and shows strong synergy with the tyrosine kinase inhibitor (TKI) lapatinib in breast cancer xenografts (24, 25). In this study we evaluated MLN0128 in models of B-ALL, an aggressive malignancy that is the most common leukemia in children (26). Current induction therapies for adult B-ALL rely mainly on variations of conventional chemotherapy followed post remission by allogeneic hematopoetic stem cell transplantation (allo-HSCT), with BCR-ABL-specific TKIs (ie. imatinib, dasatinib, nilotinib) added to the regimen for Ph+ disease. Additional therapies are needed to supplement current pre- and post-remission therapeutic regimens and in cases of relapsed disease. Using both murine BCR-ABL+ transformed cultures and primary patient-derived specimens, we show that MLN0128 suppresses growth and survival of B-ALL cells and enhances the efficacy of dasatinib. We also show for the first time that non-Ph B-ALL specimens are sensitive to mTOR kinase inhibitors in vitro and in vivo. Notably, MLN0128 treatment in vivo has cytostatic effects on Ph+ and non-Ph B-ALL xenografts while sparing normal hematopoietic cell proliferation in the spleen and bone marrow. Overall the results support further exploration of mTOR kinase inhibitors as therapeutic options in combination with existing treatments for B-ALL or as single agents to limit disease progression.
Materials and Methods
Materials
We synthesized MLN0128 and PP242 as previously described (24, 27). We obtained imatinib, dasatinib, and rapamycin from LC Laboratories. PI-103 was synthesized as described in patent # WO 2001083456. Antibodies and other flow cytometry reagents were obtained from Cell Signaling, Invitrogen, eBioscience and Biolegend. We obtained SUP-B15 cells from ATCC. Generation and propagation of p190 cells have been previously described (9, 11). Nalm6 and Blin1 cell lines were kindly provided by Dr. David Rawlings (University of Washington).
Mice
All mice were kept in specific pathogen-free animal facilities at the University of California, Irvine, and procedures were approved by the Institutional Animal Care and Use Committee. We used 8-week-old female BALB/cJ (Jackson Laboratory) mice as recipients of mouse p190 BCR-ABL transformed BM as has been previously described (9, 11). We used 6–12-week-old male and female NSG (JAX mouse stock name NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Jackson Laboratory) as recipients for human leukemic transplants as described below and in reference (9).
In vitro proliferation experiments
Cell growth was determined by the MTS assay (Cell Titer 96 Aqueous One solution cell proliferation assay kit, Promega). Quantitation and normalization of the data were performed as has been previously described (9).
Flow cytometry
Surface phenotyping, intracellular phospho-staining, and EdU incorporation were performed and analyzed with methods that have been previously described (9). Data was acquired using FACSCaliber and LSRII instruments and analyzed using FlowJo software.
Primary leukemia samples, colony formation assays, and stromal co-cultures
Cryopreserved peripheral blood samples were provided by one of the authors (M.B.L.) while treating adult leukemia subjects at Loma Linda Medical Center, under an Institutional Review Board (IRB)-approved specimen bank protocol. Their use for this study was approved by the UC Irvine IRB. We obtained cryopreserved bone marrow of adult leukemia subjects from the University of Texas M.D. Anderson Cancer Center with approval of their IRB. We obtained bone marrow from newly diagnosed pediatric B-ALL patients at CHOC Children’s Hospital under IRB protocols approved by CHOC and by UC Irvine. Leukocytes were isolated from these pediatric specimens by centrifugation over Ficoll and stored frozen in aliquots. Procedures for culturing of leukemic samples in semi-solid methylcellulose and for counting colonies have been previously described (9). For stromal co-culture experiments, hTERT-immortalized human marrow stromal cell (MSC) (104) (provided by D. Campana, St. Jude’s Children’s Research Hospital) were plated in 96 well plates in RPMI1640+10% FBS containing 1 uM hydrocortisone (Sigma). The following day, the media was replaced, and 105 B-ALL cells were plated with hTERT-MSCs in AIM-V media (Life Technologies) with 10% FBS supplemented with human SCF, IL-3, IL-7, and FLT-3L (Peprotech) at 100 ng/ml. Following 24 hr of culture, cells were treated with indicated inhibitors and following 24hr of treatment cells were harvested and stained with human CD19-FITC (Biolegend) and 7-AAD (Life Technologies) and immediately analyzed by flow cytometry.
In vivo transplant with mouse p190 leukemia and xenograft experiments with human leukemia samples
Mouse p190-transformed BM cells were used to initiate leukemia in non-irradiated (immunocompetent) syngeneic (Balbc/J) recipients as described (9, 28). In all in vivo experiments p190 transformed BM was prepared fresh (< 4 week old cultures) to initiate leukemia. Leukemic engraftment was determined in anesthetized animals by retro-orbital bleeds and analyzed by flow cytometry where indicated. For in vivo p190 experiments, mice were injected i.v. with 1×106 cells. Engraftment was assessed 7 days later by enumeration of CD19+hCD4+ cells in peripheral blood. Mice were subsequently randomized into treatment groups and treated as indicated in the figure legends. NSG mice were used as recipients for human samples using methods that have been previously described (9, 28). In brief, non-irradiated NSG mice were injected (i.v.) with leukemic samples (an equivalent amount of 0.3–1 × 106 cells per recipient). Following at least 40 days, engraftment was assessed from peripheral blood bleed, unless otherwise stated. Positive engraftment was considered >1% human CD19, CD34, and/or human CD45+ cells. Mice were subsequently randomized into treatment groups and treated as indicated in the figure legends. In some experiments we used small cohorts of NSG mice for initial engraftment and secondary transplants into larger cohorts for treatment studies. Mice were sacrificed and analyzed for the indicated endpoints 2 hours following the last treatment dose. For EdU experiments, mice were injected with EdU (0.5 mg at 5 mg/ml, i.p.) 1 hour following the last treatment dose and following 1 hour of EdU accumulation mice were sacrificed as has been previously described (9).
In vivo drug preparations
PP242 and MLN0128 were completely dissolved in NMP (1-methyl-2-pyrrolidinone; Sigma-Aldrich) and diluted to 5% in PVP (polyvinylpyrrolidone K 30; Fluka) diluted in water at a 15.8:84.2 wt vol−1 ratio for a final 5% NMP, 15% PVP, 80% water vehicle. Dasatinib was dissolved in a mixture of polypropylene glycol (Sigma-Aldrich) diluted in water (50:50) and administered by oral gavage. Dasatinib/PP242 or MLN0128 combinations were prepared as a 50:50 mixture of completely dissolved dasatinib (polypropylene glycol:water) combined with completely dissolved PP242/ or MLN0128 (NMP/PVP/water vehicle). The combination mixtures had no overt effects on compound solubility. All drug preparations were bath sonicated and stored at RT and used within 5 days at the dosages indicated in the figure legends by oral gavage.
Statistical analysis
Random continuous variables were analyzed using two-sided t tests, one-way ANOVA, and two-way ANOVA. Tukey-Kramer post-hoc analysis was used throughout. We used GraphPad Prism (4.0c) software for all statistical analysis.
Results
MLN0128 has more potent anti-leukemic effects than PP242
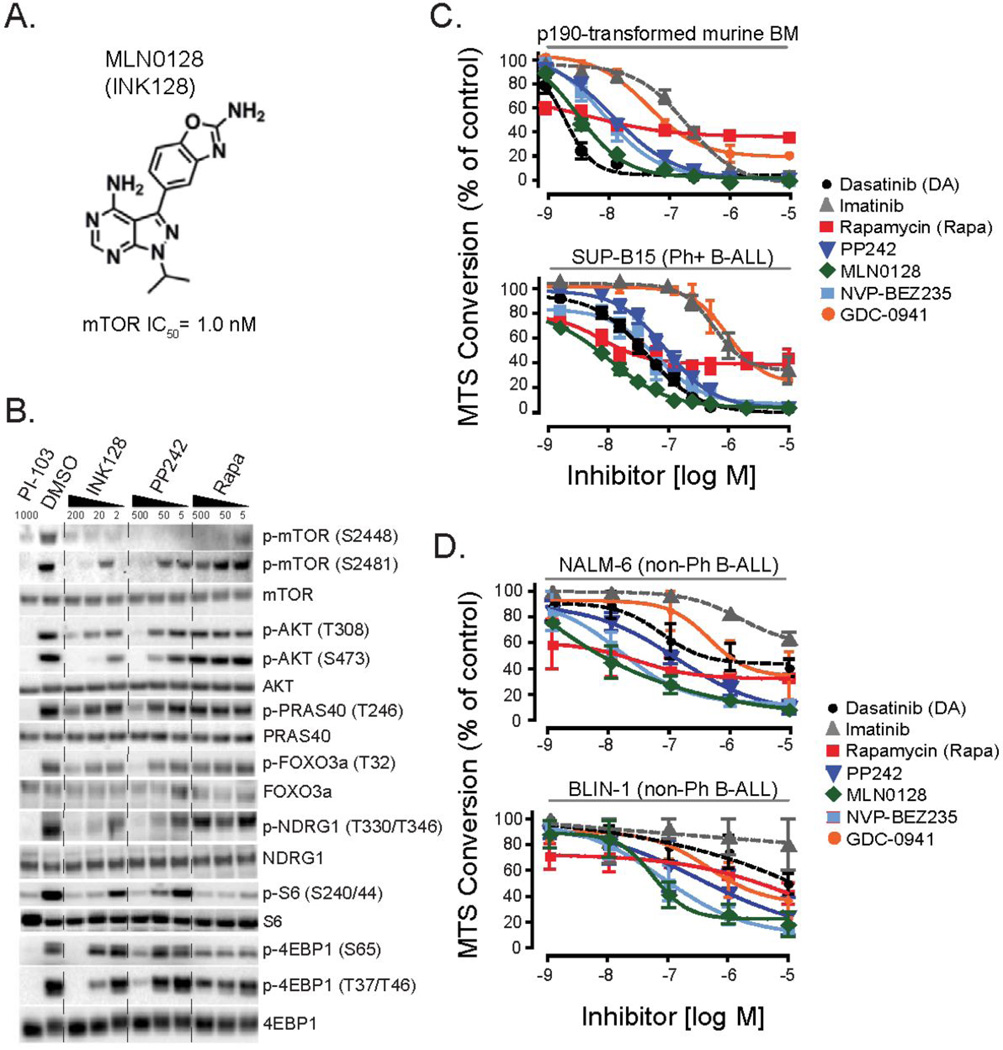
MLN0128 (INK128) is structurally related to PP242 (Fig. 1A) but is approximately 10-fold more potent while retaining high selectivity for mTOR in both biochemical and cellular assays (24). A hallmark of mTOR kinase inhibitors is their inhibition of rapamycin-resistant outputs of mTORC1 and mTORC2 (21, 23). In a previous study, we used two first generation mTOR kinase inhibitors (PP242 and Ku-0063794) and showed that these compounds suppressed proliferation and survival of leukemia cells expressing the BCR-ABL oncoprotein (9). To confirm the biochemical effects of MLN0128, we assessed the inhibition of mTOR signaling in human Ph+ SUP-B15 cells by immunoblot analysis. Similar to PP242, MLN0128 reduced the phosphorylation of mTORC1 and mTORC2 substrates on rapamycin-resistant sites including p4EBP1 (S65) and p4EBP1 (T37/46) (Fig. 1B). MLN0128 inhibited AKT phosphorylation on the mTORC2 site S473, and reduced phosphorylation of the AKT substrates PRAS40 and FOXO3a and the SGK substrate NDRG1. Phosphorylation of mTOR on S2481 (a mTORC2-selective autophosphorylation site) was also reduced by MLN0128 but not rapamycin. MLN0128 exerted these biochemical effects at concentrations at least 5–10 fold lower than PP242. MLN0128 inhibited phosphorylation of S6K substrates (S6, and mTOR on S2448) to a similar extent as rapamycin. Similar results were observed in murine leukemia cells expressing BCR-ABL (Supplementary Figure 1A). MLN0128 did not alter the phosphorylation of STAT5, another signaling output of BCR-ABL (Supplementary Figure 1B). Together, these biochemical experiments establish that MLN0128 shares with PP242 the ability to completely suppress mTOR activity with minimal compensatory effects on parallel survival pathways in BCR-ABL+ leukemia cells.
Figure 1. MLN0128 potently inhibits mTORC1 and mTORC2.
(A) Chemical structure of MLN0128 (previously known as INK128). mTOR activity was assessed by LanthaScreen Kinase assay kit (Invitrogen). (B) Human SUP-B15 Ph+ B-ALL cells were treated for 2 hr with inhibitors at the indicated concentrations (nM). Lysates were prepared and analyzed by Western blotting. (C) p190 BCR-ABL transformed mouse BM (termed p190 cells) or SUP-B15 were cultured for 48hr in the presence of inhibitors and growth was measured by the MTS assay. n = 3–7. (D) Nalm6 or Blin-1 cells were cultured for 48hr in the presence of inhibitors and growth was measured by the MTS assay. n = 3.
To compare the cellular potency of mTOR inhibition, we used primary B lymphoid progenitors transformed by the p190 isoform of BCR-ABL (termed p190 cells). Using the MTS assay as a readout of cell proliferation and survival, we measured a 50% growth-inhibitory concentration (GI50) for MLN0128 (~1 nM) that was approximately 10-fold lower than for PP242 (~10 nM) (Fig. 1C). In the human Ph+ B-ALL cell line SUP-B15, the GI50 for MLN0128 was 10 nM and for PP242 was ~100 nM (Fig. 1C). In both cell lines the response to rapamycin was potent (~5 nM) but showed a plateau in efficacy of around 50–70% inhibition. The pan-class I PI3K inhibitor GDC-0941 also showed a plateau in efficacy, whereas the dual PI3K/mTOR inhibitor NVP-BEZ235 suppressed to a similar extent as the selective mTOR kinase inhibitors. The BCR-ABL tyrosine kinase inhibitors imatinib and dasatinib were both active as expected. In general, SUP-B15 cells were less sensitive than p190 cells to all inhibitors. We also included 2 mixed karyotype B-lineage ALL cell lines, Nalm-6 and Blin-1, that lack the t(9;22) translocation (non-Ph B-ALL). Again we observed greater potency of MLN0128 compared to PP242 and a plateau in efficacy of rapamycin (Fig. 1D).
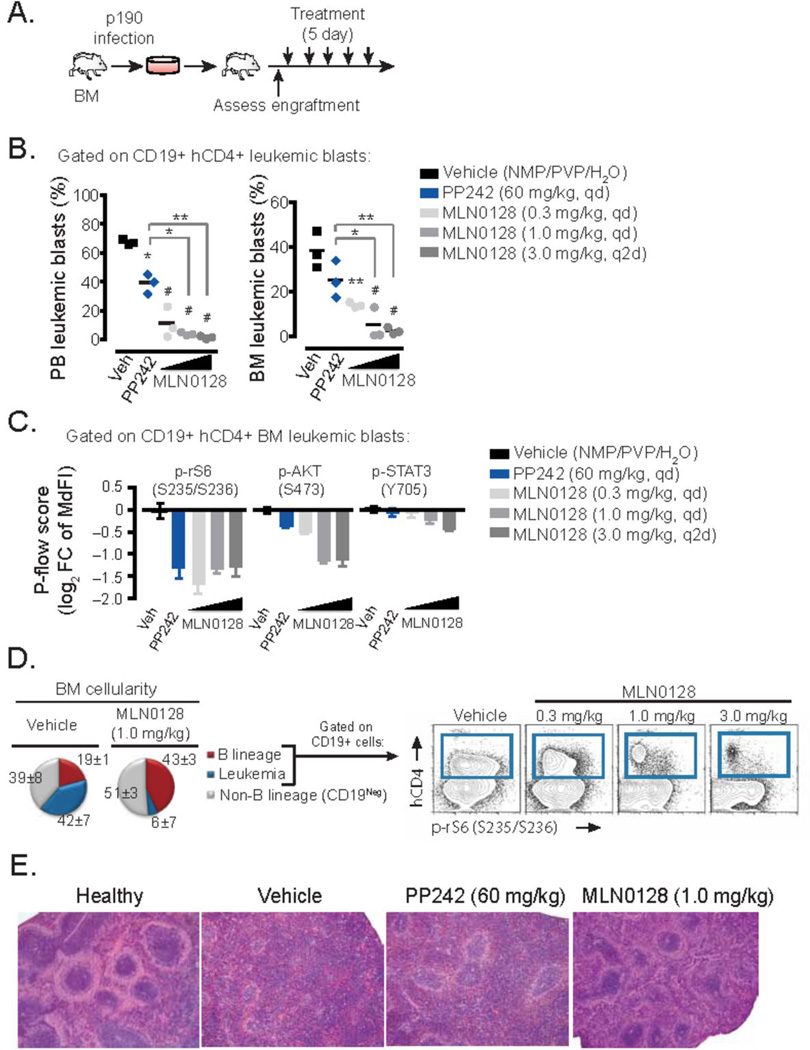
MLN0128 has improved pharmacologic properties compared to PP242 (24, 29). The improved pharmacology of MLN0128 was readily apparent in a mouse leukemia model. p190 cells expressing hCD4 as a marker of blasts containing BCR-ABL were transplanted into syngeneic hosts and seven days later the recipients were treated with daily oral doses of either PP242, MLN0128 or vehicle alone (Fig. 2A). In this model, at the onset of treatment disease burden represents 20–30% of the bone marrow with 30–50% peripheral blood presence. Following a brief 5-day treatment schedule, even at 0.3 mg/kg, MLN0128 suppressed leukemic expansion more effectively than PP242 given at 60 mg/kg (Fig. 2B). Nearly complete eradication of leukemia (CD19+hCD4+ cells) was achieved with MLN0128 at a dose of 1 mg/kg/day or 3 mg/kg every other day. Thus, MLN0128 shows significantly improved efficacy at much lower doses (approximately 100-fold) than PP242 when compared in a syngeneic in vivo transplant assay.
Figure 2. In vivo efficacy of MLN0128 in mouse syngeneic transplant model of Ph+ B-ALL.
(A) Schematic outline of in vivo transplant model of p190 cells into syngeneic Balb/cJ mice, followed by a short treatment period in randomized recipients. (B) Analysis of leukemic burden following 5 day treatment period in vivo with vehicle alone, PP242, or MLN0128 at the dose schedules indicated (n = 3 per group). Mice were euthanized 2 hr after the last dose. Bone marrow (BM) and peripheral blood (PB) were obtained and the percentage of leukemia cells (CD19+ cells marked with human CD4) was quantitated by flow cytometry. * P <0.05, ** P <0.01, # P <0.001, one way ANOVA. (C) Flow cytometry analysis using phospho-specific staining in p190 cells (CD19+hCD4+) from the BM was immediately performed following the treatment period. To assess mTOR inhibition the log2 fold change (FC) of the median fluorescent intensity (MdFI) of phospho-specific staining in CD19+hCD4+ cells from vehicle treated vs. drug treated animals was calculated. (D) Flow cytometry analysis of B lineage (CD19+) and non-B lineage (CD19neg) in the BM immediately following the treatment period. Pie charts represent the percentages of leukemic versus resident endogenous BM populations (left panel). Blue gate represents the abundance and mTORC1 activity of p190 cells (right panel) compared to normal CD19+ lymphoblasts. (E) Representative images of spleen morphology following the treatment period (H&E stained).
To determine whether MLN0128 inhibits mTOR signaling in vivo, we carried out pharmacodynamic analysis of drug action using phospho-specific flow cytometry. Ex vivo analysis of the CD19+hCD4+ leukemic cells from the bone marrow and peripheral blood showed that MLN0128 (0.3 mg/kg/day) suppressed phosphorylation of mTORC1 and mTORC2 readouts (p-S6 and p-AKT) as effectively as PP242 (60mg/kg/day), while having minimal off-target effect on JAK/STAT signaling as measured by STAT3 phosphorylation (Fig. 2C). Interestingly, the phosphorylation of S6 was more uniformly suppressed with MLN0128 in the leukemic subset of CD19+ cells. This loss of mTOR activity correlated with specific clearance of leukemic CD19+hCD4+ cells, which were replaced by normal bone marrow hematopoietic populations (Fig. 2D). The normalization of spleen architecture was also observed with MLN0128 at the doses showing anti-leukemic effects (Fig. 2E).
MLN0128 suppresses colony formation in Ph+ and non-Ph B-ALL specimens
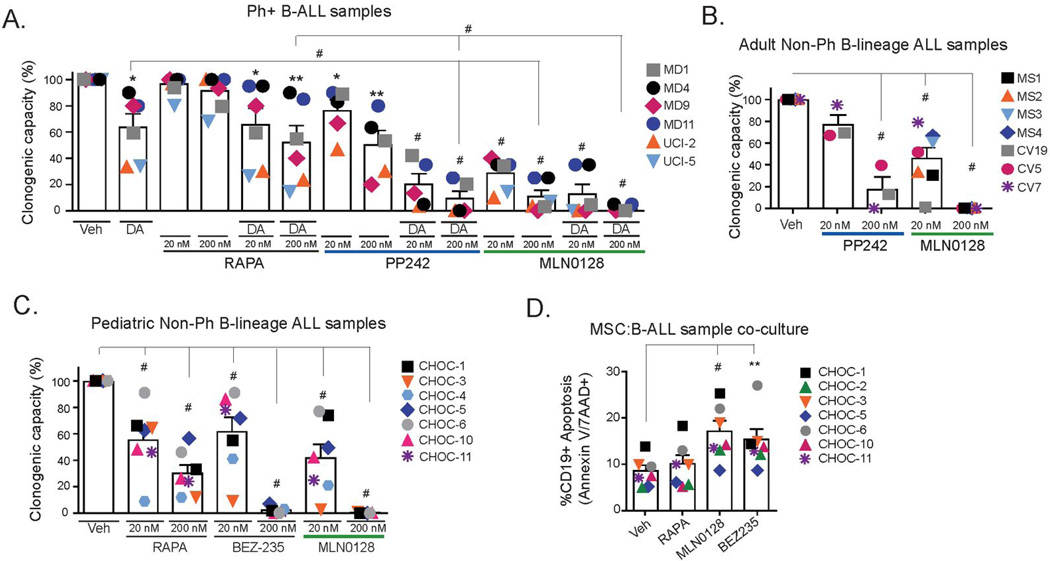
We assessed the effects of MLN0128 on clinical samples representing both Ph+ B-ALL and non-Ph B-ALL (Supplementary Table 1). Treatment of 6 distinct Ph+ B-ALL specimens with MLN0128, but not rapamycin, significantly reduced colony formation in methylcellulose (MethoCult GF+H4435) cultures containing supportive human cytokines (Fig. 3A). MLN0128 was more potent than PP242 in each case when both were compared in the same specimen (Fig. 3A). These trends were also observed when MLN0128 was combined with dasatinib (DA) (Fig. 3A). Although ineffective alone, rapamycin also enhanced the effect of dasatinib to reduce colony formation. In a set of 14 distinct cases of adult and pediatric non-Ph B-ALL (7 each), MLN0128 significantly suppressed colony formation in a concentration-dependent manner (Fig. 3B and C). In the pediatric specimens, rapamycin had a significant but partial effect, and the pan-PI3K/mTOR inhibitor NVP-BEZ235 reduced colony formation to a similar extent as MLN0128.
Figure 3. Effects of MLN0128 alone or in combination with dasatinib in primary non-Ph and Ph+ B-ALL specimens.
(A) Anti-clonogenic effects of compounds were compared in Ph+ specimens (n=6) treated with rapamycin (Rapa), PP242 or MLN0128 alone or in the presence of 5.0 nM dasatinib (DA). Cytokine supplemented Methocult GF+H4435 cultures were scored for colonies after 12–14 days and normalized to control (vehicle-treated). Some of the data shown here were from specimens included in a broader comparison of rapamycin and PP242 published previously (9), though MLN0128 data were not included at that time. (B–C) Adult and pediatric non-Ph mixed karyotype B-ALL specimens (n=7) were cultured with the indicated compounds at 20 nM or 200 nM and colonies were scored after 14 days. (D) Co-culture of human hTERT-immortalized marrow stromal cells (MSC) with pediatric non-Ph mixed karyotype B-ALL specimens (n=7) for 24 hr, followed by 24 hr treatment of indicated inhibitors. % death was analyzed by 7-AAD dye exclusion by flow cytometry in human CD19+ cells.
To assess the pro-death effects of inhibitors, we cultured pediatric B-ALL specimens on hTERT-immortalized human marrow stromal cell (MSC) layers under conditions that facilitate ex vivo survival (30). In the presence of MSCs and cytokines, B-ALL cells maintained 92% (± 1%) viability over a 48 hr co-culture period. We monitored survival in CD19+ cells by flow cytometry. MLN0128 (100 nM) increased the fraction of dying leukemia cells by approximately 2-fold (84% ± 6 viability), similar to the effect of NVP-BEZ235 (85% ± 6) whereas rapamycin had no significant effect (91% ± 2) (Fig. 3D). These results suggest that MLN0128 can suppress mTOR-dependent supportive survival signals from cytokines and stromal cells. However, the modest effects of MLN0128 on survival compared to colony formation suggests that this compound is more cytostatic than cytotoxic to primary B-ALL cells.
MLN0128 suppresses outgrowth of B-ALL xenografts without inhibiting bone marrow function
To assess in vivo efficacy against B-ALL (both Ph+ and non-Ph), we utilized multiple primary human specimens in xenograft models that we have previously established as a platform for preclinical testing of mTOR selective kinase inhibitors (9, 28). We assessed 4 separate cases of relapsed Ph+ B-ALL (Fig. 4, Suppl. Fig. 2) and 7 cases of non-Ph mixed karyotype pre-B-ALL engrafted into NSG mice (Fig. 5, Suppl. Fig. 3).
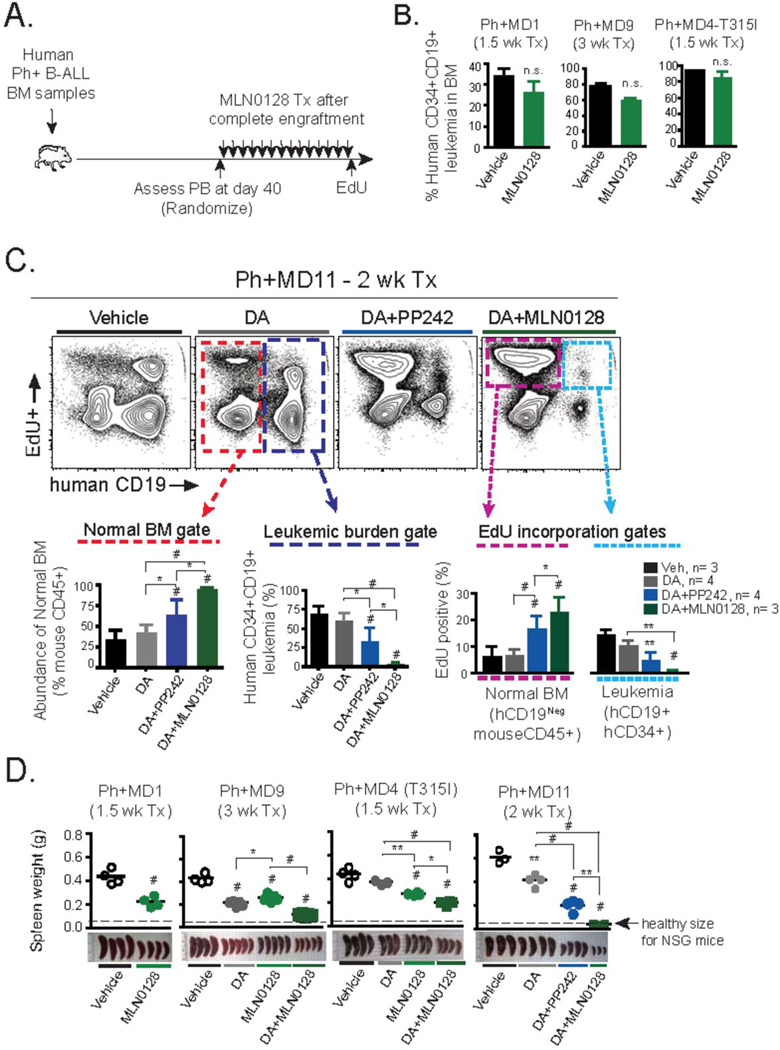
Figure 4. In vivo effects of MLN0128 in primary human Ph+ B-ALL xenografts.
(A) Schematic outline of Ph+ B-ALL xenograft model and treatment design. Three primary BM samples from dasatinib (DA)-resistant Ph+ B-ALL clinical specimens containing >90% CD45+CD34+CD19+ leukemic blasts were injected intravenously into NOD-SCID-γc−/− (NSG) mice. Following ~40 days, equally engrafted mice (>30% human blasts in peripheral blood) were randomized and treated with the indicated schedule for 1–3 weeks. Animals were injected with EdU (0.5 mg, i.p.) 1 hr before being euthanized, to mark actively cycling cells. (B) Xenografts of Ph+ samples MD1, MD9, and MD4 were treated with MLN0128 (0.75 mg/kg/day, p.o.) for the indicated time period. Percentage of human leukemia in BM was determined by flow cytometry. (C) Xenograft of Ph+ sample MD11 was treated for 2 weeks with DA (5 mg/kg/day, p.o.), or DA in combination with MLN0128 (0.75 mg/kg/day, p.o.) or PP242 (60 mg/kg/day, p.o.). Flow cytometry analysis of the BM is depicted. Dotted gates depict either normal resident endogenous mouse BM cells used to quantify hematopoietic recovery (lower left panel in red), percentage of human CD34+CD19+ leukemic burden (lower middle panel in dark blue), and to quantify EdU incorporation ability of endogenous mouse CD45+ BM cells (magenta) or human CD34+CD19+ leukemic cells (light blue) dotted gates are depicted (lower right panel). (D) Weight and gross pathology of spleens following indicated treatment schedules; note average spleen weight of healthy age-matched NSG mice. Note: data from MD11 xenografts (comparing vehicle, DA and DA + PP242) were shown in a previous publication (9), but the DA + MLN0128 condition from the same experiment was not included at that time. * P <0.05, ** P <0.01, # P <0.001, 2-way ANOVA.
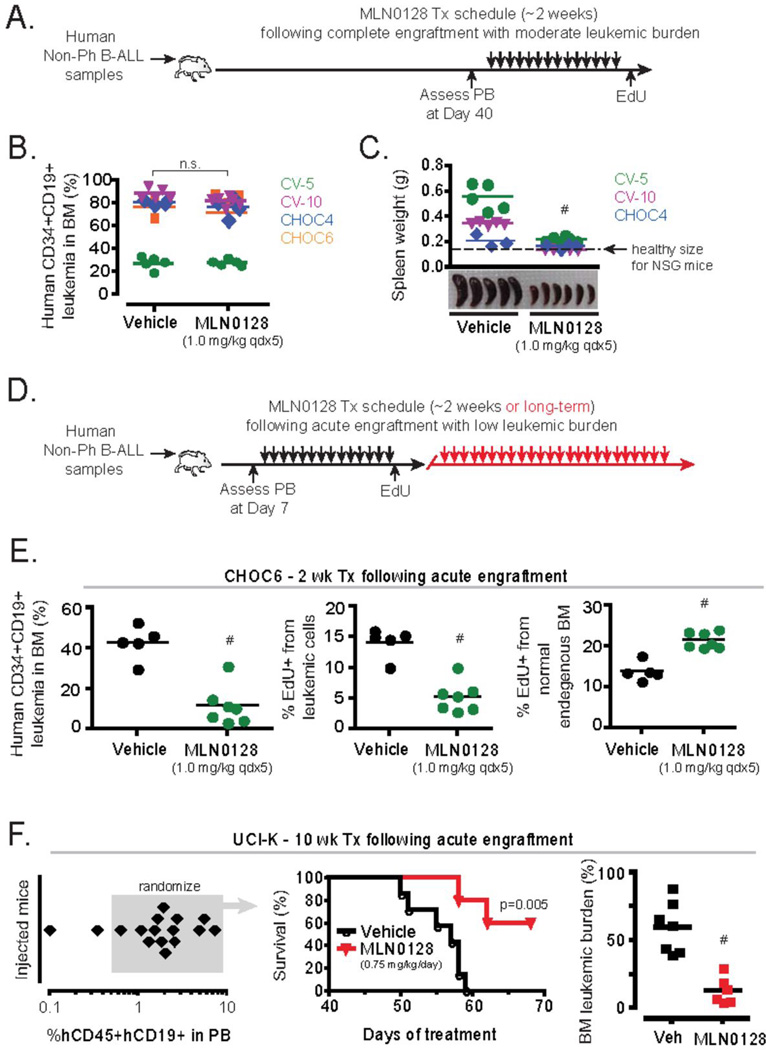
Figure 5. In vivo effects of MLN0128 in primary human non-Ph mixed karyotype B-ALL xenografts.
(A) Schematic outline of B-ALL xenograft model and treatment design. Four primary PB samples from non-Ph B-ALL clinical specimens containing >90% CD45+CD34+CD19+ leukemic blasts were injected intravenously into NOD-SCIDγc−/− (NSG) mice similar as in Figure 4. Secondary transplanted NSG mice were utilized for specimens CHOC4 and 6. (B) The leukemic burden in the BM following 2 weeks of treatment in xenografts of clinical specimens: CV-5, CV-10, CHOC4, and CHOC6. (C) Weight and gross pathology of spleens following treatment with vehicle or MLN0128. Note, CHOC4 displayed preferential localization in the BM and displayed minimal splenic involvement, whereas CV-5 leukemic burden was predominantly localized to the spleen and not the BM. Spleen weights were not recorded for the CHOC6 transplants. (D) Schematic outline of xenograft design with abbreviated engraftment period, where animals were treated approximately 1 week following i.v. injection of clinical specimen. (E–F) Using the design with abbreviated engraftment period, samples CHOC6 and UCI-K were treated for 2 weeks or 10 weeks respectively. (E) Bone marrow leukemic burden (left panel) and % cycling cells (center and right panels) are depicted. (F) Long term survival of NSG mice engrafted with sample UCI-K was assessed (left and middle panel) and the leukemic burden in the BM of mice was assessed when animals were moribund or at the end of 69 days of treatment (right panel).
Daily treatment with MLN0128 alone was unable to significantly reduce the percentage of leukemic cells in the bone marrow in xenografts of three Ph+ B-ALL specimens tested (denoted MD1, MD4, and MD9) (Figure 4B). Therefore, we asked whether MLN0128 could enhance the efficacy of dasatinib in combination, as we showed previously using PP242 (9). In cohorts of mice engrafted with Ph+ cases MD4, MD9, and MD11, we treated with either dasatinib alone (abbreviated DA) or combined with MLN0128. Of the three Ph+ cases, only MD4 contained a BCR-ABL mutation (T315I gatekeeper residue mutation) yet all displayed clinical resistance to imatinib combined with a hyper-CVAD chemotherapy regimen (see Supplementary Table 1 and ref. (9)). Likewise, when transplanted into NSG mice, each specimen exhibited resistance to DA (Fig. 4C and Supplementary Figure 2) at a dose of 5.0 mg/kg/day shown previously to be efficacious in some Ph+ xenografts (9). Remarkably, the combination of dasatinib with MLN0128 (0.75 mg/kg/day) achieved almost complete eradication of MD11 blasts in the marrow, whereas dasatinib + PP242 (60 mg/kg/day) had an intermediate yet significant effect (Fig. 4C). Thus, MLN0128 was significantly more effective than PP242 at a dose approximately 80 times lower given over a 2-week course of treatment. The response to the dasatinib/mTOR combination therapy significantly cleared leukemic burden while sparing the normal marrow precursors. Uptake of 5-ethynyl-2’deoxyuridine (EdU), a method for assessing proliferative capacity by detecting newly synthesized DNA, showed that MD11 blasts were significantly inhibited whereas normal resident mouse CD45 (negative for hCD34 and hCD19) cells recovered (Fig. 4C) to levels approximating healthy age-matched BM proliferative turnover (~20–25% EdU+; data not shown). In xenografts of MD9, DA + MLN0128 significantly reduced leukemic burden compared to single agent treatments (Supplementary Figure 2A). Furthermore, MLN0128 displayed selectivity for malignant cells at the effective dose. The combination of DA + MLN0128 was less effective in the xenografts of MD4 (T315I), despite significant reduction of EdU incorporation in leukemia cells in the bone marrow (Supplementary Figure 2B).
The clinical symptoms of B-ALL are caused not only by impaired hematopoiesis but also by dissemination of leukemia cells to peripheral lymphoid organs. Notably, single agent treatment with MLN0128 (0.75 mg/kg/day) significantly reduced leukemic burden in the spleen in all three xenografts tested (MD1, MD4, MD9) and the combination of DA + MLN0128 was even more effective in all cases (Figure 4D). Based on the measurements of leukemic burden in bone marrow and spleen, specimen MD11 showed evidence of nearly complete cure by 2-week treatment with DA + MLN0128.
Adult and pediatric non-Ph B-ALL cases represent a diverse group of leukemias with distinct genetic lesions (26). Unlike Ph+ B-ALL, few cases of non-Ph B-ALL have activating mutations in tyrosine kinases and targeted therapies to activated signaling enzymes have not yet proven effective in the clinic. Targeting mTOR to suppress signals from cytokines and stromal cells could have anti-leukemic effects, as suggested by our in vitro data (Fig. 3B–D). To determine if mTOR kinase inhibition could suppress non-Ph B-ALL expansion in vivo, we tested MLN0128 at different dose schedules in established xenografts of four clinical specimens (2 adult, 2 pediatric cases) using our standardized xenograft protocol used for Ph+ specimens (Fig. 5A). Using a ~2 week treatment schedule with 0.75 mg/kg/day or 1.0 mg/kg qdx5 (Monday–Friday) of MLN0128, we observed no significant effect on bone marrow leukemic burden in any of the xenografts (Fig. 5B). An alternative schedule of 3.0 mg/kg twice per week likewise did not significantly clear disease in the bone marrow (not shown). However, MLN0128 did significantly reduce enlargement of the spleen (Fig. 5C). Overall these data indicate that in established xenografts of non-Ph B-ALL, single agent treatment with MLN0128 lacks the debulking ability observed in Ph+ xenografts treated with MLN0128 + dasatinib.
The data from in vitro studies of colony forming potential and survival on stromal cells suggested that MLN0128 is more cytostatic than cytotoxic to primary non-Ph B-ALL cells (Fig. 3B–D). Hence we considered the possibility that MLN0128 might be more effective at preventing early leukemic expansion than treating advanced disease. Therefore, we altered our standardized xenograft protocol and incorporated an abbreviated engraftment period with treatment schedules starting as little as one week after cell injection—either before human leukemia cells were detectable in the blood (pediatric B-ALL samples CHOC1, CHOC6A and CHOC23), or represented less than 7% of peripheral white blood cells (adult B-ALL sample UCI-K) (Fig. 5D). Using this approach in mice engrafted with the pediatric sample CHOC6, we found that a two-week treatment schedule with MLN0128 (1.0 mg/kg qdx5) significantly reduced disease expansion in the bone marrow (Fig. 5E). Note that the CHOC6 specimen did not respond to MLN0128 when treatment was applied to established xenografts (Fig. 5B). Similar results were observed when xenografts of CHOC1 and CHOC23 were treated at early stages of engraftment (Supplementary Fig. 3). In mice engrafted with an adult B-ALL (sample UCI-K), we found that MLN0128 (0.75 mg/kg/day) could significantly extend survival for greater than 2 months (Fig. 5F). Although the surviving mice did have detectable leukemic involvement in the bone marrow following the end of study, these results suggest that MLN0128 could achieve single agent activity against non-Ph B-ALL cells when disease burden is limited.
Discussion
mTOR kinase inhibitors represent a promising new approach to targeting the PI3K/AKT/mTOR pathway with potentially greater tolerability than dual PI3K/mTOR inhibitors (21–23). Previously we used first generation mTOR kinase inhibitors to demonstrate that this class of compounds has improved efficacy compared to rapamycin in models of Ph+ B-ALL (9). In this study we extend this concept by showing that with a more refined molecule in clinical development, MLN0128, has favorable anti-leukemic activity in non-Ph B-ALL derived from both adult and pediatric subjects. In addition, we show that a low dose of MLN0128 in vivo enhances the efficacy of dasatinib in Ph+ B-ALL while selectively suppressing proliferation of malignant cells. Although MLN0128 has improved pharmacological properties and different off-target effects than PP242, MLN0128 retains the ability to suppress leukemia cell expansion and dissemination while preserving normal bone marrow cell proliferation. This supports the conclusion that selective targeting of leukemia cells is a class effect of mTOR kinase inhibitors and is not unique to PP242.
In non-Ph B-ALL xenografts, MLN0128 showed significant efficacy as a single agent when treatment was initiated at early stages following engraftment. This is consistent with the finding that MLN0128 fully suppresses colony outgrowth of B-ALL cells in vitro, an assay that measures proliferation and survival of isolated leukemic clones. In established xenografts of Ph+ or non-Ph B-ALL with more advanced disease, MLN0128 did not significantly suppress leukemic burden. There are several potential explanations for this observation. First, regression of established disease requires apoptotic effects yet MLN0128 showed only modest cytotoxic activity towards B-ALL cells in vitro. Second, although this compound has a favorable pharmacokinetic profile, it is possible that effective concentrations of the drug are not maintained in protective niches for leukemia cells in the bone marrow. In agreement with this, we found that MLN0128 suppressed proliferation of leukemia cells in the spleen but not the bone marrow of mice bearing established non-Ph xenografts (Suppl. Fig. 4). It is worth noting that syngeneic murine leukemia cells driven by a single oncogene (BCR-ABL) were highly and rapidly sensitive to MLN0128 even in the bone marrow environment. This suggests that the genetic complexity of human leukemia specimens contributes to MLN0128 resistance in vivo.
It is not unexpected that treatment with MLN0128 alone does not eradicate established B-ALL xenografts in mice. Indeed it is rare for a single anti-cancer drug to provide durable clinical responses. Exceptions are the tyrosine kinase inhibitors (TKIs) targeting BCR-ABL; these agents (imatinib, dasatinib, nilotinib) provide long-term remissions in chronic myeloid leukemia (CML) when treated in chronic phase. However, BCR-ABL TKIs are less effective in the blast-crises CML or in Ph+ B-ALL. It is thought that resistance of blast crises CML and Ph+ B-ALL often arises from additional genetic lesions that bypass cellular addiction to BCR-ABL. Although inhibitors targeting elements of the PI3K/AKT/mTOR pathway are promising approaches for leukemia therapy, there is an increasing consensus that these strategies will also have limited success as single agents even in tumors with activating mutations in the pathway (12). Therefore, a major effort is to identify effective combinations of PI3K/AKT/mTOR inhibitors with other targeted agents or with standard chemotherapy regimens. Our data show that MLN0128 can augment the efficacy of dasatinib in Ph+ B-ALL xenografts that are resistant to either agent alone. Similarly, the combination of MLN0128 with the dual HER2/EGFR inhibitor, lapatinib was significantly more effective than MLN0128 alone in lapatinib-resistant models of HER2-positive breast cancer (25). These findings provide strong rationale for testing mTOR kinase inhibitors such as MLN0128 with BCR-ABL TKIs as front-line regimens in B-ALL patients.
What combinations would potentiate the efficacy of mTOR kinase inhibitors in non-Ph B-ALL? We tested MLN0128 in methylcellulose cultures together with submaximal concentrations of the chemotherapeutic drugs vincristine and doxorubicin, but observed limited and variable additivity of MLN0128 with these agents (data not shown). It is conceivable that mTOR inhibition would actually antagonize the effects of some cytotoxic agents by reducing the frequency of cells undergoing cell division. A more effective approach might be to combine mTOR kinase inhibitors with other targeted agents that suppress survival signaling (i.e. MEK inhibitors, BCL2 family antagonists) or with agents modulating gene expression (i.e. histone deacetylase inhibitors). Ultimately it might be most effective to personalize treatment combinations based on tumor-specific signatures identified by genomic or proteomic approaches.
Other considerations might improve the efficacy of mTOR kinase inhibitors in B-ALL and other leukemias. By using a high dose intermittent schedule, it might be possible to achieve a greater apoptotic effect while maintaining selectivity towards malignant cells. In this study we compared two schedules of MLN0128 in xenografts of pediatric B-ALL and observed that 3.0 mg/kg, given twice weekly (the maximum tolerated frequency at this dose), suppressed leukemic expansion to a similar extent as 1.0 mg/kg dosed five days per week (data not shown). Other variations in dose and schedule are worth testing in mouse models and eventually in clinical trials. An important endpoint to explore is whether mTOR kinase inhibitors would be effective in reducing minimal residual disease in leukemia patients after induction and consolidation regimens. This could be a well-tolerated method to extend remissions or prepare for allo-HSCT. Supporting this concept, starting MLN0128 treatment before leukemia dissemination to advanced stages significantly suppressed expansion of leukemia cells even in the bone marrow.
In conclusion, our data show that an investigational mTOR kinase inhibitor can selectively suppress the growth of B-ALL cells but is likely to be most effective when used in combination or when disease burden is low. As clinical trials of mTOR kinase inhibitors expand, the identification of effective combinations and treatment schedules should be a priority.
Supplementary Material
Acknowledgements
Supported by National Institutes of Health training grant T32-CA009054 (to M.R.J.), a Discovery Grant from the University of California Industry–University Cooperative Research Program (to D.A.F.), a Hope Grant from the Hyundai Hope on Wheels Foundation (to D.A.F.), and a sponsored research agreement from Intellikine (to D.A.F.). We thank Marina Konopleva for access to clinical leukemia samples at M.D. Anderson Cancer Center, Jean-Pierre Bourquin for advice on stromal cell support cultures, Dario Campana for hTERT-immortalized bone marrow stromal cells, and David Rawlings for non-Ph B-ALL cell lines.
Footnotes
Conflict of Interest: M.R.J., L.L., K.A.J. and M.B.M. are employees of Takeda California, a pharmaceutical company involved with the investigation of MLN0128. M.R.J., L.L., K.A.J. and M.B.M., P.R., Y.L. and C.R. were all previous employees of Intellikine, a pharmaceutical company that designed and developed MLN0128 (previously designated INK128). D.A.F. was a scientific advisor to Intellikine in the development and utilization of MLN0128.
References
- 1.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 2.Chapuis N, Tamburini J, Green AS, Willems L, Bardet V, Park S, et al. Perspectives on inhibiting mTOR as a future treatment strategy for hematological malignancies. Leukemia. 2010;24:1686–1699. doi: 10.1038/leu.2010.170. [DOI] [PubMed] [Google Scholar]
- 3.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 4.Vu C, Fruman DA. Target of rapamycin signaling in leukemia and lymphoma. Clin Cancer Res. 2010;16:5374–5380. doi: 10.1158/1078-0432.CCR-10-0480. [DOI] [PubMed] [Google Scholar]
- 5.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 7.Altman JK, Sassano A, Kaur S, Glaser H, Kroczynska B, Redig AJ, et al. Dual mTORC2/mTORC1 Targeting Results in Potent Suppressive Effects on Acute Myeloid Leukemia (AML) Progenitors. Clin Cancer Res. 2011;17:4378–4388. doi: 10.1158/1078-0432.CCR-10-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 11.Kharas MG, Janes MR, Scarfone VM, Lilly MB, Knight ZA, Shokat KM, et al. Ablation of PI3K blocks BCR-ABL leukemogenesis in mice, and a dual PI3K/mTOR inhibitor prevents expansion of human BCR-ABL+ leukemia cells. J Clin Invest. 2008;118:3038–3050. doi: 10.1172/JCI33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer? Oncotarget. 2011;2:1314–1321. doi: 10.18632/oncotarget.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan QW. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 16.Markman B, Tabernero J, Krop I, Shapiro GI, Siu L, Chen LC, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012 doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 17.Prasad G, Sottero T, Yang X, Mueller S, James CD, Weiss WA, et al. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro-oncology. 2011;13:384–392. doi: 10.1093/neuonc/noq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, et al. Pharmacologic Characterization of a Potent Inhibitor of Class I Phosphatidylinositide 3-Kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 19.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 20.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 21.Janes MR, Fruman DA. Targeting TOR dependence in cancer. Oncotarget. 2010;1:69–76. doi: 10.18632/oncotarget.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilar E, Perez-Garcia J, Tabernero J. Pushing the envelope in the mTOR pathway. The second generation of inhibitors. Mol Cancer Ther. 2011 doi: 10.1158/1535-7163.MCT-10-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012 doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Garcia C, Ibrahim YH, Serra V, Calvo MT, Guzman M, Grueso J, et al. Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 26.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 27.Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janes MR, Fruman DA. The in vivo evaluation of active-site TOR inhibitors in models of BCR-ABL+ leukemia. Methods Mol Biol. 2012;821:251–265. doi: 10.1007/978-1-61779-430-8_15. [DOI] [PubMed] [Google Scholar]
- 29.Jessen K, Wang S, Kessler L, Guo X, Kucharski J, Staunton J, et al. INK128 is a potent and selective TORC1/2 inhibitor with broad oral antitumor activity. Mol Cancer Ther. 2009;8:B148. [Google Scholar]
- 30.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.