Abstract
Ultraviolet (UV) radiation-induced immunosuppression has been implicated in skin carcinogenesis. Grape seed proanthocyanidins (GSPs) have anti-skin carcinogenic effects in mice and GSPs-fed mice exhibit a reduction in UV-induced suppression of allergic contact hypersensitivity (CHS), a prototypic T cell-mediated response. Here, we report that dietary GSPs did not inhibit UVB-induced suppression of CHS in xeroderma pigmentosum complementation group A (XPA)-deficient mice, which lack nucleotide excision repair mechanisms. GSPs enhanced repair of UVB-induced DNA damage (cyclobutane pyrimidine dimers) in wild-type, but not XPA-deficient, dendritic cells (DCs). Co-culture of CD4+ T cells with DCs from UVB-irradiated wild-type mice resulted in suppression of T-cell proliferation and secretion of Th-1 type cytokines that was ameliorated when the DCs were obtained from GSPs-fed mice; whereas, DCs obtained from GSPs-fed XPA-KO mice failed to restore T-cell proliferation. In adoptive transfer experiments, donor DCs were positively selected from the draining lymph nodes of UVB-exposed donor mice that were sensitized to 2,4, dinitrofluorobenzene were transferred into naïve recipient mice and the CHS response assessed. Naïve recipients that received DCs from UVB-exposed wild-type donors that had been fed GSPs exhibited a full CHS response, whereas no significant CHS was observed in mice that received DCs from XPA-KO mice fed GSPs. These results suggest that GSPs prevent UVB-induced immunosuppression through DNA repair-dependent functional activation of dendritic cells in mice.
Keywords: Grape seed proanthocyanidins, DNA repair, contact hypersensitivity, cyclobutane pyrimidine dimer, nucleotide excision repair, ultraviolet radiation
Introduction
Epidemiologic and experimental evidence suggest that ultraviolet (UV) radiation-induced suppression of immune reactions is a risk factor for the development of skin cancer (1, 2). It has been reported that organ transplant recipients and chronically immune-suppressed patients living in regions of intense sun exposure are at a greater risk of skin cancer development (3, 4). These observations are consistent with the concept that immune surveillance plays an important role in prevention of the generation and maintenance of neoplastic cells (5). In mice, exposure of the skin to UV radiation suppresses the development of allergic contact hypersensitivity (CHS), a prototypic T-cell mediated immune response (6, 7). Notably, UV-induced tolerance can be induced in recipient mice by transfer of T cells from UV-treated mice (6-8). Application of a hapten to the UV-treated skin of hapten-sensitized animals fails to elicit a CHS response and transfer of lymphocytes from the spleen and lymph nodes of hapten-sensitized mice that have been UV-irradiated to naïve mice that have not been UV irradiated results in the failure of the recipient mice to develop CHS responses to the same haptens (6, 9). Depletion of UV-induced T-suppressor cells can inhibit UV-induced carcinogenesis (10). These links between immunosuppression and photocarcinogenesis have stimulated considerable interest in the mechanisms by which UVB radiation induces immunosuppression and the development of molecular strategies that may prevent or correct these effects with the goal of developing more effective chemopreventive strategies for skin cancer.
Dietary phytochemicals offer promising options for the development of effective strategies for the prevention of UV radiation-induced immune-suppression and subsequent skin cancer development, and thus can be utilized as complementary and alternative medicine. Grape seed proanthocyanidins (GSPs) are promising phytochemicals that have shown anti-skin carcinogenic effects and exhibit no apparent toxicity in vivo (11-14). GSPs contain primarily proanthocyanidins (89%), including dimers, trimers, tetramers, and oligomers of monomeric catechins and/or (-)-epicatechins (12, 13). It is believed that at least some of the constituents present in this product may act synergistically and thus this product may be more effective than any single constituent. We have demonstrated that dietary GSPs inhibit the immunosuppressive effects of UV radiation by augmenting the levels of interleukin (IL)-12 (11), and stimulating the development of CD8+ effector T cells in mice (15); however, the mechanism(s) by which the GSPs exert these effects have not been elucidated fully. UV-induced DNA damage, predominantly in the form of the generation of cyclobutane pyrimidine dimers (CPDs), is an important molecular trigger for UV-mediated immunosuppression and initiation of photocarcinogenesis (3, 16). UV-induced damage in antigen presenting cells appears to play a key role in UV-induced immunosuppression; for example, UV-irradiated dendritic cells (DCs) can adoptively transfer immune tolerance when they are injected intravenously into mice that are not irradiated with UV. This implies that UV-irradiated DCs are associated with a reduced ability to stimulate T cells, indicating that DNA damage may contribute to the development of UV-induced tolerogenic DCs (17, 18). It also suggests that repair of the UV-induced DNA damage in the DCs may play a central role in the GSPs-mediated amelioration of the UVB-induced immunosuppression.
UV-induced damage of epidermal Langerhans cells (LCs), a subpopulation of DCs in the skin, is considered to be an important mechanism for UV-induced immune suppression (8, 19, 20). There is evidence indicating that DNA repair mechanisms are related directly to the function of DCs in the stimulation of T cells and the induction of immune reactions (17, 18). Here, we report that prevention of UVB-induced immunosuppression by GSPs is mediated, at least in part, through their effects on UVB-irradiated DCs in terms of restoration of their functional activity. We also found that GSPs were unable to inhibit UVB-induced immunosuppression in xeroderma pigmentosum complementation group A (XPA)-deficient mice, which are deficient in repair of UV-induced DNA damage because of absence of nucleotide excision repair (NER) mechanism. Moreover, the GSPs could not rescue the functional activation of UVB-irradiated DCs from these mice, further suggesting that the chemopreventive effects of dietary GSPs are mediated through the enhanced repair of damaged DNA in DCs.
Materials and Methods
Animals
Female C3H/HeN mice of 4 to 6 weeks of age were purchased from Charles River Laboratories. The XPA-deficient or XPA-knockout (XPA-KO) mice on a C3H/HeN background were bred in our Animal Resource Facility, as described previously (21, 22). All mice were maintained under standard conditions of a 12-hour dark/12-hour light cycle, a temperature of 24±2°C, and relative humidity of 50±10%. The mice were provided a control AIN76A diet with or without supplementation with GSPs and drinking water ad libitum throughout the experiment. Mice in GSPs-fed group were given GSPs-containing diet 7 days before the start of UV irradiation and continued till the end of the experiment. The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Chemicals, antibodies and GSPs
Microbeads conjugated to monoclonal anti-mouse CD8/CD4 or anti-mouse CD11c antibodies and the MACS system used for the purification of immune cells were purchased from Miltenyi Biotec (Auburn, CA). Anti-mouse Langerin/CD207 antibody was purchased from Dendritics (Dardilly France). IL-4, lipopolysaccharide (LPS), and carboxyfluorescein succinimidyl ester (CFSE) were purchased from Sigma Chemical Co. (St. Louis, MO). Anti-mouse CD3e and GM-CSF were purchased from BD Bioscience (San Diego, CA). ELISA kits for mouse IFNγ, IL-12, IL-4 and IL-10 were purchased from eBioscience (San Diego, CA), while antibody specific to cyclobutane pyrimidine dimers was obtained from Kamiya Biomedical (Seattle, WA).
The GSPs were obtained from the Kikkoman Corporation (Tokyo, Japan) and the chemical composition has been described earlier (12, 13). Experimental diets containing GSPs (0.2 or 0.5%, w/w) were commercially prepared in pellet form in the AIN76A powdered control diet by TestDiet (Richmond, IN) using the GSPs that we provide for this purpose.
UVB irradiation
The clipper shaved backs of the mice were UVB irradiated using a band of 4 FS20 UVB lamps (Daavlin; UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage, as described earlier (11). The UV lamps emit UVB (280–320 nm; ≈80% of total energy) and UVA (320-375 nm; ≈20% of total energy), with UVC emission being insignificant. We used two different doses of UVB irradiation depending on the nucleotide excision repair capability of mice used in this study. XPA-KO mice lack DNA repair genes and are sensitive to UVB radiation-induced DNA damage. For this reason, 20mJ/cm2 dose of UVB was used for irradiation of XPA-KO mice. In the case of C3H/HeN mice (wild-types of XPA-KO mice), a dose of 150 mJ/cm2 UVB irradiation was used.
Assessment of contact hypersensitivity
The UV-induced suppression of CHS was determined as described previously (11). The clipper shaved backs of the mice were UVB irradiated for 4 consecutive days. Twenty-four hours after the last UV exposure, the mice were sensitized by painting 25 μL of 0.5% 2, 4-dinitrofluorobenzene (DNFB) in acetone: olive oil (4:1, v/v) on the UVB-irradiated skin site. The CHS response was elicited 5 days later by challenging ear of each mouse with 20 μL of 0.2% DNFB. The ear thickness was measured 24 hours and 48 hours after the challenge using an engineers’ micrometer (Mitutoyo, Tokyo, Japan) and was compared with the ear thickness just before challenge. Non-UVB irradiated mice that received the same dose of DNFB served as a positive control, whereas the non-UVB-irradiated mice that received only ear challenge served as a negative control. Each experiment was repeated at least twice with n=5 per group.
Preparation of bone marrow-derived dendritic cells (BM-DCs)
To assess whether GSPs enhance repair of UVB-induced DNA damage in DCs, DCs were prepared from bone marrow of naïve wild-type and XPA-KO mice, as described previously (15, 23). Briefly, mice were sacrificed and the femurs collected, cleaned and sterilized by dipping in 70% ethanol for 5 min. Under a sterile hood, the bone marrow cells were collected in RPMI media. After lysis of red blood cells, the B cells and T cells were depleted using Dynal beads. The remaining cells were washed with HBSS buffer and suspended in dendritic cell medium [RPMI supplemented with 10% FBS, GM-CSF (10 ng/mL) and IL-4 (10 ng/mL)] in 6-well tissue culture plates. On the 5th day of incubation, LPS (5 μg/mL) was added to the culture media to induce maturation of dendritic cells. Twenty-four hours later, the cells were harvested. Approximately 95% of these cells (BM-DC) are CD11c+ cells.
Photoprotective effect of GSPs on UVB-induced DNA damage in BM-DC: Detection of CPD+ BM-DCs using cytostaining
The BM-DCs (CD11c+ cells) were treated with various concentrations of GSPs (0, 5, 10 and 20 μg/mL) for 30 min, washed with PBS, and then exposed to the UVB radiation (5 mJ/cm2) in PBS. Following UVB exposure, BM-DCs were re-suspended in BM-DCs culture medium and incubated in the dark inside an incubator for another 30 min or 24 hours. BM-DCs were then harvested and were subjected to immunohistochemical detection of CPD-positive cells using cytostaining, as described previously (24, 25). CPD+ cells were detected and counted in 5-6 different fields using an Olympus BX41 microscope. Data are presented as the mean of the percentage of CPD+ cells ± SD from two separate experiments.
Immunohistochemical detection of langerin-positive dendritic cells and CPD-positive cells in vivo mouse skin
Mice were exposed to UV (WT, 150 mJ/cm2; XPA-KO, 20 mJ/cm2) with or without treatment with GSPs in diet. Twenty four hours after exposure, mice were euthanized, and skin samples were collected and frozen in OCT medium. For immunohistochemical detection of langerin-, CPDs- or double-positive cells, 5μM thick frozen sections were thawed and kept in 70mM NaOH solution in 70% ethanol for 2 minutes to denature nuclear DNA followed by neutralization in 100 mM Tris-HCl (pH 7.5) in 70% ethanol. The sections were washed and permeabilized with 0.2% Triton-X100 in PBS for 20 minutes. After blocking with 5% bovine serum albumin, sections were incubated with antibodies specific for CPDs and biotinylated langerin/CD207. Sections were counterstained with Alexa fluor488 (green color for CPDs) goat anti-mouse IgG1 and streptavidin-Alexa fluor594 (red color for langerin). Sections were mounted with Vectashield mounting medium for fluorescence and stained with DAPI before they were observed under a fluorescence detection equipped Olympus microscope BX51 fitted with a Qcolor DP71 digital camera and photographed.
Southwestern dot-blot analysis of CPDs
Genomic DNA was isolated from the different treatment groups of BM-DC following standard procedures and dot-blot analysis was performed as detailed previously (24, 25). Briefly, genomic DNA (100 ng) was transferred to a positively-charged nitrocellulose membrane by vacuum dot-blotting (Bio-Dot Apparatus, Bio-Rad, Hercules, CA). The membrane was then incubated with antibodies specific for CPDs for 1 hour. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody. The circular dots of CPDs were detected using ECL detection system. The experiment was repeated once.
Isolation of CD11c+ dendritic cells from draining lymph nodes, in vitro stimulation and analysis of cytokines
XPA-KO mice and their wild-type counterparts that were provided a diet supplemented with GSPs (0.5% w/w) or the control diet were UVB irradiated for three consecutive days. Twenty-four hours after the last UVB exposure, mice were sacrificed, draining lymph nodes were harvested and a single-cell suspension prepared, as described previously (11, 15). A MACS system in which the CD11c+ DC were positively selected was used to purify DCs according to the manufacturer’s instructions (Miltenyi Biotec, Inc.). To stimulate cytokine production by the purified DCs, they were incubated or stimulated with LPS (5μg/mL) for 48 hours. After 48 hours, cell culture supernatants were collected, centrifuged and the levels of cytokines, IL-12, IFNγ and IL-10 measured using cytokine-specific ELISA (BioSource International).
In vitro stimulation of CD4+ T cells by DCs and measurement of cytokines level
Mice were UVB irradiated for three consecutive days with or without treatment with GSPs (0.5%, w/w), as described above. Twenty-four hours after the last UVB exposure, mice were sacrificed, the lymph nodes harvested and CD11c+ cells purified as described above. Similarly, CD4+ T cells were isolated from a single cell suspension of spleen cells of normal naïve mice (without any treatment). For this purpose, spleen cells were mixed with ACK buffer (Lonza) and incubated on ice for 5 min to ensure lysis and removal of red blood cells. Remaining cells were mixed with microbeads conjugated to anti-CD4 antibodies. CD4+ T cells were then separated using the MACS system following the instructions of the manufacturer. CD11c+ cells were then placed in culture with CD4+ T cells (1:10 ratio, DC & CD4+ T cells) for 4 days in complete RPMI medium with soluble anti-CD3e (5.0 μg/mL). To examine the ability of the CD11c+ cells to induce T-cell proliferation, CD4+ T cells were pre-labeled with CFSE, a fluorescent dye (26-28). Cells were harvested and then analyzed for proliferation of T cells by FACS analysis, while supernatants from the same treatment groups were used for analysis of cytokines using cytokine-specific ELISA kits.
Effects of adoptive transfer of dendritic cells on the CHS response
The backs of the donor mice were clipper shaved and exposed to UVB radiation for four consecutive days with or without treatment with GSPs in diet (0.5%, w/w). Twenty-four hours after the last UVB exposure, mice were sensitized by painting DNFB on the UVB-irradiated skin site. Twenty-four hours after sensitization, the mice were sacrificed, the regional lymph nodes harvested and single-cell suspensions were prepared. CD11c+ cells were purified or positively selected using MACS system following the manufacturer’s instructions (Miltenyi Biotec, Inc). Greater than 90% of the cells are DCs. These DCs (5× 105 cells/mouse) from wild-type or XPA-KO donor mice were injected subcutaneously into the recipient mice, which were untreated naïve syngeneic mice. Five days after injection of the DCs, the recipient mice were challenged by painting DNFB on the ear skin with ear swelling being determined before and 24 hours and 48 hours after the challenge.
Results
Prevention of UVB-induced immunosuppression by GSPs requires an NER mechanism
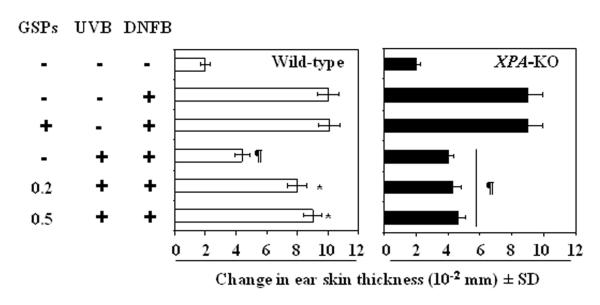
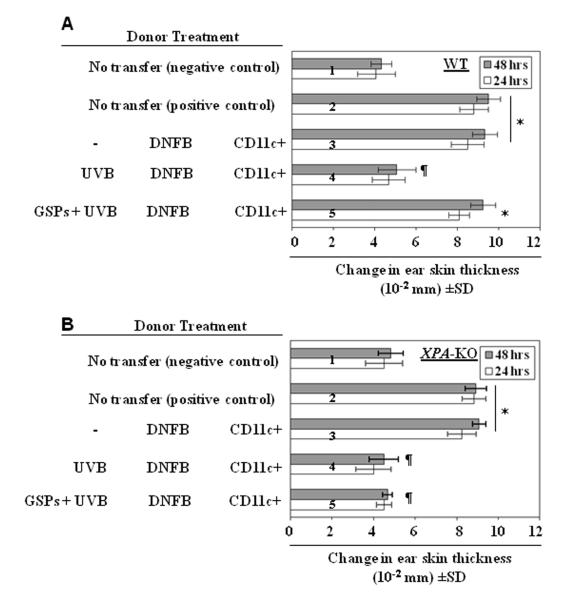
UVB-induced DNA damage in the form of CPDs has been associated with immunosuppression and initiation of photocarcinogenesis (18, 21, 22). Therefore, we determined whether GSPs prevent UVB-induced immunosuppression in XPA-deficient or XPA-KO mice which do not have the ability to repair UVB-induced DNA damage in the form of CPDs because of the absence of functional NER. For this purpose, contact hypersensitivity was assessed in XPA-KO and their wild-type counterparts that had been provided GSPs in the diet (0.2% and 0.5%, w/w). Consistent with previous reports, the contact sensitization reaction and ear swelling response to DNFB occurred in UVB-irradiated wild-type mice, which are XPA-proficient, and the responses were significantly higher in the UVB-irradiated wild-type mice that were fed GSPs (P<0.005; Left panel, two bars from the bottom of the panel) than UVB-irradiated wild-type mice that were not fed GSPs. Indeed, the ear swelling response in the UVB-irradiated wild-type mice that were fed GSPs was comparable to the response of wild-type mice that were not UVB-irradiated (Figure 1, Left panel, 2nd bar from the top). As would be anticipated, the CHS response was significantly lower (P<0.001; right panel, 4th bar from the top) in XPA-KO mice that were UVB-irradiated than those XPA-KO mice that were not UVB-irradiated (Figure 1, Right panel, 2nd bar from the top, positive control), confirming that UVB radiation is immunosuppressive in XPA-KO mice. However, significant UVB-induced suppression of the CHS response (P<0.001) also was observed in the UVB-exposed XPA-KO mice that were fed GSPs, which suggests that the prevention of UVB-induced immunosuppression by dietary GSPs requires intact NER mechanisms.
Figure 1.
Dietary GSPs do not prevent UVB-induced suppression of the CHS response in XPA-deficient mice but do so in their wild-type counterparts. Mice were exposed to UVB radiation on four consecutive days. Twenty-four hours after the last UVB exposure, the mice were sensitized with DNFB. Five days after sensitization, the mice were challenged by painting DNFB on the ear skin, and ear thickness was measured. The change in ear thickness is reported in millimeters (mm x10−2) as the mean ± SD, n=5 per group. The experiment was repeated once with similar results. ¶Significant inhibition versus positive control, P<0.001.
*Significant increase in ear skin thickness versus non-GSPs-treated and UVB-exposed wild-type mice; P<0.005.
GSPs enhance repair of UVB-induced DNA damage in BM-DCs obtained from wild-type mice but not in BM-DCs from XPA-KO mice
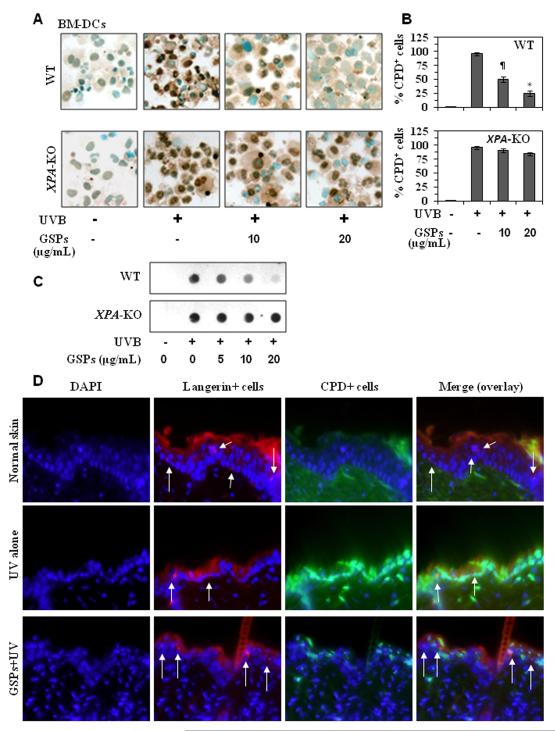
To determine whether GSPs have the ability to enhance repair of UVB-induced DNA damage in DCs, DCs were purified from the bone marrow of XPA-KO mice and their wild-type counterparts, treated with GSPs and exposed to UVB radiation. Cells were harvested either immediately or 24 hours after UVB irradiation and the presence of DNA damage in the form of CPDs determined by cytostaining. CPDs were not detectable in the DCs that were not UVB irradiated, regardless of their source (Figure 2A). CPDs were detectable in the DCs from XPA-KO mice and their wild-type counterparts immediately after UVB-exposure and treatment with GSPs did not affect the numbers of CPD+ DCs immediately after UVB exposure (data not shown). This suggests that GSPs do not act to prevent UVB-induced formation of CPDs and also excludes the possibility that the GSPs in the culture medium are acting to filter the UVB radiation. When the DCs obtained from wild-type mice were analyzed 24 hours after UVB irradiation, however, there were significantly fewer CPD+ cells in the DCs that had been treated with GSPs as compared to the non-GSPs-treated UVB-irradiated DCs (P<0.01- P<0.001; Figure 2A & 2B), suggesting that GSPs might accelerate the repair of UVB-induced CPDs. This was verified using dot blot analysis, and that this form of DNA damage was not apparent in the DCs that were not UVB-irradiated whether or not they were treated with GSPs (Figure 2C). In contrast to the effects of GSPs on the DCs obtained from the wild-type mice, GSPs-mediated repair was not observed in the DCs obtained from XPA-deficient mice 24 hours after UVB irradiation (Figure 2A, 2B and 2C). These results suggest that GSPs have the ability to enhance repair of UVB-induced DNA damage in DCs and that this requires XPA activity or NER mechanisms.
Figure 2.
GSPs significantly repair UVB-induced DNA damage in BM-DCs obtained from wild-type mice but do not repair damaged DNA in BM-DCs from XPA-KO mice. A, BM-DCs were exposed to UVB (5 mJ/cm2) with or without the pretreatment with GSPs (0, 5, 10 and 20 μg/mL) and harvested either immediately or 24 hours later, cytospun, and subjected to cytostaining to detect CPD+ cells. CPD+ cells are dark brown. Magnification, x400. B, The numbers of UVB-induced CPD+ cells in wild-type and XPA-deficient BM-DCs are expressed in terms of the percentage of CPD+ cells as a mean ± SD of the results of three independent experiments. Significant difference vs GSPs-treated and UVB-irradiated cells, *P<0.001; ¶P<0.01. C, Analysis of CPDs by dot-blot assay. Results are shown from a single experiment that is a representative of two separate experiments. D, Treatment of mice with dietary GSPs enhanced the repair of UV-induced DNA damage in epidermal DCs (langerin-positive cells). Red fluorescence indicates langerin+ cells and green fluorescence indicates CPD+ cells. Arrows indicate langerin-positive and CPDs-negative or less intense cells in GSPs-fed group under overlay (merge) panel. Representative photomicrographs are shown, n=3/group. Magnification, x400.
GSPs enhance repair of UVB-induced DNA damage in epidermal DCs of wild-type mice but not in DCs of XPA-KO mice
We further verified DNA repair ability of GSPs in DC in vivo mouse model. As shown in Figure 2D, compared to non-UV-exposed mouse skin, UV exposure to the skin of wild-type mice induced a larger number of CPD-positive cells (green color) while decreased the number of langerin-positive DCs (red color) in the epidermis of the skin. In contrast, the numbers of CPD-positive cells were less while the number of langerin-positive cells were higher in the skin of GSPs-fed group of mice compared to UV alone exposed mouse skin. The data on overlay panel indicate that the majority of langerin-positive cells did not show the presence of CPDs in GSPs-fed group of mice compared to the cells detected in the skin of mice which were not fed GSPs in diet but exposed to UV. These results suggest that GSPs enhanced the repair of UV-induced DNA damage in langerin-positive subset of DCs (Figure 2D). In contrast, this effect of GSPs was not observed in the DCs of XPA-KO mouse skin under identical conditions (data not shown).
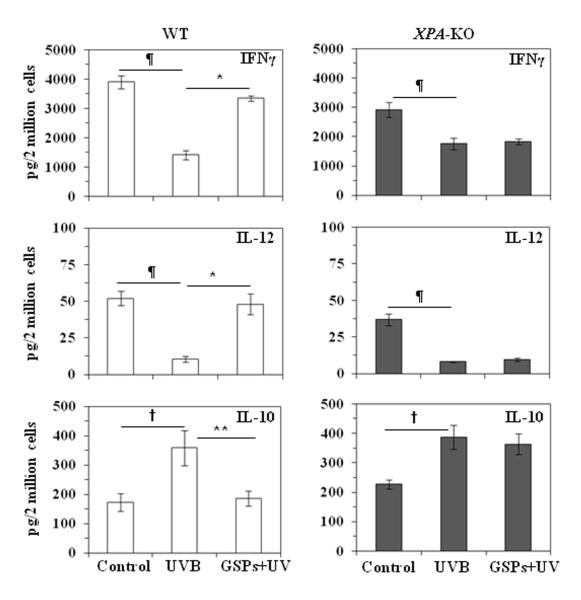
Dietary GSPs increase the levels of IFNγ and IL-12 in CD11c+ DCs obtained from UVB-exposed wild-type mice but not DCs from UVB-exposed XPA-deficient mice
To determine whether the repair of the DNA damage in the DCs is associated with restoration of the functional activity of DCs, we investigated whether the repair of DNA damage is associated with their ability to enhance Th1-type cytokines (i.e., IFNγ and IL-12). For this purpose, wild-type and XPA-KO mice were UVB exposed (wild-type, 150 mJ/cm2; XPA-KO, 20 mJ/cm2) on 3 consecutive days. DCs (CD11c+ cells) were then isolated from the lymph nodes of the mice, stimulated with LPS for 48 hours, and the supernatants collected for analysis of the cytokines by ELISA (Figure 3). As would be anticipated, the levels of IFNγ and IL-12 were significantly lower (P<0.01) in the supernatants from DCs that had been obtained from the wild-type mice exposed to UVB as compared with the levels in the supernatants of DCs obtained from the control mice that had not been exposed to UVB. DCs obtained from UVB-irradiated wild-type mice that had been fed GSPs produced significantly higher levels of IFNγ and IL-12 as compared with DCs obtained from non-GSPs-treated UVB-exposed wild-type mice (P<0.01). Additionally, the production of IL-10, which is considered as an immunosuppressive cytokine, was significantly lower (P<0.01) in DCs obtained from the UVB-exposed mice that had been fed GSPs than those that were fed the standard diet. These results suggest that dietary GSPs can promote or restore the function of DCs after UVB-irradiation. An association of the ability of the GSPs to mediate restoration of DC function with their ability to repair DNA was suggested by the lack of a significant elevation in the production of IFNγ and IL-12 or reduction in the production of IL-10, in DCs obtained from UVB-exposed XPA-KO mice that were fed GSPs.
Figure 3.
Dietary GSPs enhanced the production of IL-12 and IFNγ in DCs obtained from UVB-exposed wild-type mice but not DCs from XPA-KO mice. Mice were exposed to UVB three times on consecutive days. Mice were sacrificed 24 hours after the last UVB exposure, and DCs (CD11c+ cells) were isolated from the lymph nodes. DCs were stimulated with LPS (5μg/mL) for 48 hours, then harvested and supernatants collected. The concentrations of cytokines in the cell supernatants were estimated by ELISA and are presented as the mean ±SD in terms of pg/2 million cells. Experiment was repeated once; n=5. Significant decrease versus non-UVB control, ¶P<0.01; significant increase versus UVB alone, *P<0.01. Significant increase versus non-UVB control, †P<0.01, significant decrease versus UVB alone, **P<0.01.
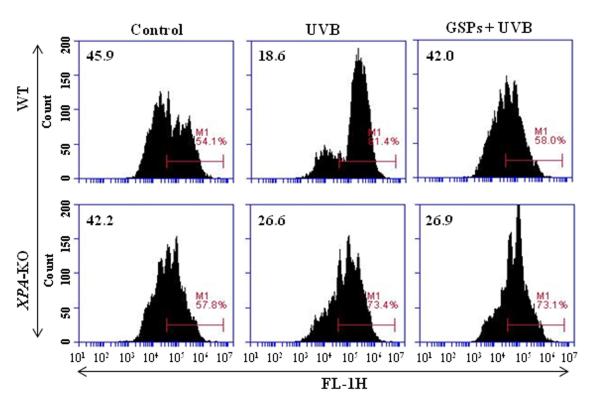
Dietary GSPs enhance the ability of DCs from UVB-irradiated wild-type mice, but not DCs from XPA-deficient mice, to stimulate T cells in vitro
To verify that dietary GSPs can promote the functions of dendritic cells from UVB-irradiated mice and that this contributes to the prevention of UVB-induced immunosuppression, we first tested whether DCs from GSPs-treated mice can stimulate the proliferation of T cells. For this purpose, CD4+ T cells were isolated from the spleens of naïve wild-type mice and labeled with CFSE. These CFSE-labeled CD4+ T cells were then co-incubated for 4 days with CD11c+ cells isolated from the lymph nodes of various treatment groups of wild-type and XPA-KO mice. The proliferation index of the CD4+ T cells was then determined using FACS analysis and the levels of cytokines in the supernatants measured using cytokine-specific ELISA. Consistent with UVB-induced immune suppression, the proliferation of the CD4+ T cells was significantly lower when they were co-incubated with DCs prepared from UVB-irradiated wild-type mice than when they were incubated with DCs from wild-type mice that were not UVB-irradiated (Figure 4). The proliferation of CD4+ T cells was significantly greater on co-incubation with DCs from UVB-irradiated wild-type mice that had been fed GSPs than on co-incubation with DCs from UVB-exposed wild-type mice that had not been fed GSPs, as shown in Figure 4 (Upper panels). In contrast, the proliferation of CD4+ T cells was not enhanced when DCs were obtained from UVB-exposed XPA-KO mice that had been fed GSPs (Figure 4, Lower panels).
Figure 4.
Dietary GSPs improve the functional ability of DCs from UVB-irradiated wild-type mice, but not XPA-KO mice, to enhance the proliferation of CD4+ T cells. CD4+ T cells isolated from the spleens of naïve mice (wild-type) were labeled with CFSE and co-incubated with CD11c+ cells (DCs) isolated from lymph nodes of the different treatment groups of wild-type and XPA-KO mice in the presence of anti-CD3e (5.0 μg/mL). After 4 days, cells were harvested and analyzed for their proliferation index using FACS. Representative histograms from one experiment are shown from a total of three independent experiments with numerical values indicating percent proliferating CD4+ T cells.
The ability of the GSPs to promote the function of UVB-irradiated DCs was supported by the analysis of the levels of cytokines in the supernatants of the co-cultures. The immune suppressive effects of UV radiation were indicated by the finding that the levels of IFNγ (a Th1-type cytokine) were significantly lower (P<0.01) in the supernatants obtained from the co-cultures in which the DCs were obtained from UVB-irradiated wild-type mice as compared to the supernatants from the co-cultures in which the DCs were obtained from wild-type mice that were not UVB-irradiated. Moreover, the levels of IFNγ in the supernatants from the co-cultures in which the DCs were obtained from UVB-irradiated wild-type mice that had been fed GSPs were significantly higher (more than 2-fold, P<0.001) than when the DCs were obtained from UVB-irradiated wild-type mice that had not been fed GSPs (Table 1). The levels of IL-10 and IL-4 also were significantly lower (P<0.01) in the supernatants from the co-cultures in which the DCs were obtained from UVB-irradiated wild-type mice that had been fed GSPs than when the DCs were obtained from UVB-irradiated wild-type mice that had not been fed GSPs.
Table 1.
Effect of co-culture of dendritic cells from wild-type and XPA-KO mice with CD4+ T cells on cytokine production
| Cytokines | Wild-type |
XPA-KO |
||||
|---|---|---|---|---|---|---|
| Control | UV | GSPs +UV | Control | UV | GSPs+ UV | |
| IFNγ | 320 | 152 (53) | 353 (132)* | 212 | 131 (38) | 152 |
| IL-4 | 215 | 296 (38) | 197 (33)* | 208 | 328 (57) | 250 |
| IL-10 | 456 | 844 (85) | 659 (22)* | 308 | 532 (73) | 487 |
Dendritic cells were isolated from the draining lymph nodes of different treatment groups, as described in Materials and Methods. CD4+ T cells isolated from the spleens of naïve wild-type mice were labeled with CFSE and co-cultured with CD11c+ cells in the ratio of 10:1 for 4 days. Cells were harvested, and cell supernatants were subjected to the analysis of cytokines by ELISA. Values in parentheses under UV-exposed groups indicate percent reduction (IFNγ) or percent increase (IL-4, IL-10) in the amount of cytokines secreted by CD4+ T cells compared to controls. Asterisks (*) indicate that co-culture of DCs from GSPs-treated wild-type mice with CD4+ T cells enhanced the production of IFNγ (132%) and reduced the production of IL-4 (33%) and IL-10 (22%) compared to non-GSPs-treated UVB-irradiated mice in wild-type group. Cytokine concentration is reported in terms of mean values and pg/2 million cells, n=2. Standard deviation to mean values of cytokine content was in the range of 4-8% in each group.
In contrast, the levels of IFNγ were not significantly elevated in the supernatants obtained from co-cultures in which the DCs were obtained from UVB-exposed XPA-KO mice that were fed GSPs. Additionally, the levels of IL-10 and IL-4 were modestly lower in the supernatants from co-cultures in which the DCs were obtained from UVB-exposed XPA-KO mice that were fed GSPs (Table 1). The production of higher levels of Th1-type and lower levels of Th2-type cytokines by the DCs obtained from GSPs-treated wild-type mice further suggests that dietary GSPs promotes the functional activation of DCs in wild-type mice and this may have contributed to the stimulation of the proliferation potential of CD4+ T cells observed in vitro.
GSPs prevent UVB-induced immunosuppression by enhancing the functional activation of DCs in UVB-exposed mice: Adoptive transfer experiments with CD11c+ cells
It has been shown that UVB-induced suppression of CHS responses is dependent on the function of DCs (29). Our current studies revealed that GSPs increase the ability of UVB-irradiated DCs to stimulate Th1-type cytokines and stimulate the proliferation of T cells (Figures 2-4 and Table 1). Based on these results, we carried out adoptive transfer experiments. The donor mice (wild-type and XPA-KO) were provided a diet supplemented with GSPs (0.5%, w/w) or the control diet, exposed to UVB, and sensitized with DNFB. Twenty-four hours after sensitization, mice were sacrificed and the regional lymph nodes collected. Single-cell suspensions were prepared and the CD11c+ cells positively selected using the MACS system. Purified CD11c+ cells (5× 105/mouse) were injected subcutaneously into naïve wild-type mice, which were then challenged by application of DNFB on the ear skin 5 days later. The ear thickness was measured before challenge and 24 hours and 48 hours after challenge. As shown in Figure 5A, the naïve mice that received CD11c+ cells from UVB-exposed, wild-type donor mice that had been fed GSPs showed a greater CHS response (5th bar from the top) than the naïve mice that received DCs from the UVB-exposed wild-type mice that were not fed GSPs (4th bar from the top). The CHS response after challenge with DNFB was a little higher 48 hours after challenge than 24 hours after challenge, but the difference was not statistically significant. Collectively, these results suggest that the prevention of UVB-induced immunosuppression by GSPs is mediated through their functional activation of dendritic cells. This chemopreventive effect of GSPs on the CHS response was not seen in the naïve mice which had received DCs from UVB-irradiated and GSPs-fed XPA-KO mice (Figure 5B), suggesting that the GSPs-mediated promotion of the function of UVB-damaged DC is dependent on their effects on DNA repair in the DCs.
Figure 5.
Dietary GSPs improve the ability of DCs to induce CHS responses in wild-type mice, but not in XPA-KO mice. Donor mice (wild-type and XPA-KO) were UVB-irradiated, sensitized with DNFB 24 hours after the last UVB exposure. Mice were sacrificed 24 hours after sensitization, and lymph nodes were harvested, single cell suspension was made, and CD11c+ cells were positively selected using MACS system. Syngeneic recipient mice were injected subcutaneously with 5× 105 CD11c+ cells obtained from donor mice. Recipient mice were ear challenged with DNFB 5 days after injection of cells, and ear skin thickness was measured before and 24 and 48 hours after challenge. The change in ear thickness is reported as the mean of millimeters (x 10−2) ± SD, n=5 per group. *Significantly greater CHS response versus recipient of CD11c+ from UVB+ DNFB treated mice, P<0.001; ¶Significantly lower CHS response versus the positive control (DNFB-sensitized) group, P<0.001.
Discussion
In the present study, we demonstrate a novel cellular target of GSPs that prevents UVB-induced immunosuppression in mice. UV-induced photodamage of epidermal LC, a subpopulation of DCs in the skin, is considered to be an important mechanism or cellular target in UV-induced immune suppression (8, 19). There is evidence indicating that DNA repair mechanisms are related directly to the function of LCs in the stimulation of T cells and the induction of immune response (17, 18). It has been shown that a reduction in CPD+ LCs is correlated with increased function of the LCs as assessed by the induction of CHS and the production of IFNγ by T cells (17). It also has been reported that the repair of CPDs in UV-exposed skin requires NER mechanisms or the XPA gene (17, 25). Therefore, we tested the effect of dietary GSPs on UVB-induced immunosuppression in XPA-KO mice and the resultant data were compared with the CHS response in their wild-type counterparts. We found that dietary GSPs prevent UV-induced suppression of the CHS response to the contact sensitizer, DNFB, in wild-type mice but this effect of GSPs was not observed in XPA-KO mice, suggesting the involvement of XPA or NER mechanisms in the prevention of UV-induced immunosuppression by GSPs in mice. To determine and verify whether GSPs can act to enhance DNA repair in DCs, we subjected GSPs-treated or untreated mouse BM-DC to UV irradiation. The cells were harvested immediately or 24 hours after UV irradiation and CPD+ cells were identified by immuno-cytostaining. We found that treatment of UV-irradiated DC with GSPs resulted in repair of CPDs; however, GSPs were not able to repair UV-induced DNA damage in DCs obtained from XPA-KO mice. Further, dietary GSPs were found to enhance the repair of UV-induced DNA damage in the form of CPDs in epidermal DCs (langerin-positive cells) in wild-type mice, but this effect of GSPs was not observed in the epidermal DCs of UV-exposed skin of XPA-KO mice. These results suggest that GSPs do not have the ability to repair UV-induced CPDs in DCs from XPA-KO mice and indicate that GSPs-mediated DNA repair in DCs is mediated through XPA and is an important mechanism in their prevention of UV-induced immunosuppression. Studies by Li et al (30) also have shown the role of XPA in the repair of UV-induced DNA damage. They have demonstrated that upon DNA damage in S phase, nucleotide excision repair is regulated by the ATR/p53-dependent checkpoint; however this repair mechanism is independent of p53 in G1 or G2 phase. Roy et al (31) have shown that GSPs induce apoptosis in JB6 C141 keratinocytes and this effect of GSPs was p53-depednent because apoptosis occurred mainly in cells expressing wild-type p53 than in cells which were p53-deficient. These studies suggest the involvement of p53 in repair of damaged DNA, and needs further studies on this aspect and its role in UV-induced immunosuppression and its prevention.
It is known that the production of specific cytokines by DCs plays a major role in their ability to stimulate specific populations of T cells. DCs can produce both IL-12, an immunostimulatory cytokine that is a prominent stimulator of Th1 cells, and IL-10, an immunosuppressive cytokine that induces Treg cells (32-35). UV-irradiation suppresses the production of IL-12, whereas it increases the production of IL-10 in the skin (36-38). The UV-irradiation reduction in the production of IL-12 by the DCs, suggests that this may be a mechanism by which UV radiation stimulates the development of tolerogenic DCs (39, 40). Based on this information, we analyzed the effects of dietary GSPs on the production of various cytokines by DCs obtained from UV-exposed XPA-KO and their wild-type counterparts. We found that dietary GSPs enhanced the production of IL-12 and IFNγ while reducing the levels of IL-10 in DCs obtained from UVB-exposed wild-type mice. This ameliorating effect of GSPs was not found in the DCs obtained from UVB-exposed XPA-KO mice. Collectively, these data suggest that GSPs can rescue the regulated production of IL-12 and IL-10 by the UV-damaged dendritic cells and that the modulation of cytokines in DCs is associated with the GSPs-mediated repair of the UVB-induced DNA damage.
This concept was supported by analysis of the ability of GSPs to restore the function of DCs in terms of their ability to activate T-cell subpopulations. To address this issue, the DCs obtained from different treatment groups of XPA-KO and their wild-type counterparts were co-cultured with CFSE-labeled CD4+ T cells. The results of FACS analysis revealed that DCs obtained from UVB-irradiated wild-type mice that were fed GSPs significantly stimulate T-cell proliferation whereas DCs obtained from UVB-irradiated wild-type mice that were not fed GSPs suppressed T-cell proliferation. These results indicate that GSPs enhance the function of DCs in terms of their ability to enhance the proliferation ability of T cells. In the same set of experiments, GSPs also enhanced the production of IFNγ while suppressing the levels of IL-10 and IL-4 by the DCs, which further supports the concept that dietary GSPs enhance the functional activity of DCs. Under identical conditions, dietary GSPs failed to activate DCs obtained from UV-exposed XPA-KO mice. The DCs obtained from the UVB-irradiated XPA-KO mice that were fed GSPs were not able to stimulate the proliferation potential of T cells or the levels of IFNγ. These results support our hypothesis that GSPs prevent UVB-induced immunosuppression and it does so by repair of CPDs in DCs that is associated with functional activation of UVB-irradiated DCs.
Our studies suggest that GSPs increase the ability of UV-irradiated DCs to stimulate T-cell activation/proliferation. Therefore, we tested whether dietary GSPs improve the ability of UVB-exposed DCs to induce CHS responses following adoptive transfer. Transfer of DCs from UVB-irradiated wild-type mice that had been fed GSPs to naïve mice resulted in a higher CHS response to DNFB than that observed in naïve mice that received DCs from UV-exposed wild-type mice that were not fed GSPs. Under identical conditions, adoptive transfer of DCs from UVB-irradiated XPA-KO mice that were fed GSPs to naïve mice did not induce a CHS response to the contact sensitizer, DNFB. This may be associated with the inability of GSPs to protect DCs from UV-induced DNA damage in these mice. It is to note that dendritic cells in the skin consist of different subpopulations. It is uninvestigated, and therefore, not excluded that DC subpopulations may have different sensitivities to UV induced DNA damage and they may respond differently to GSPs mediated preventive effects. These issues need further exploration and may be examined in our future studies.
Collectively, the results of this study identify a previously un-described mechanism by which dietary GSPs prevent UV-induced immunosuppression in experimental mice. We clearly show that the GSPs prevention of UV radiation-induced immunosuppression is mediated through DNA repair-dependent functional activation of dendritic cells. These findings have important implications for the use of GSPs in the chemoprevention of UV radiation-induced melanoma and non-melanoma skin cancers.
Acknowledgments
Financial support: This work was supported by the Veterans Administration Merit Review Award (5I01BX001059 & 1I01BX001410) to S.K.K. and C.A.E., and the UAB Skin Diseases Research Center (AR050948-01).
Footnotes
Disclosure of Potential Conflicts of Interest There is no conflict of interest to declare.
Authors’ Contributions: Conception and design: M. Vaid, R. Prasad, T. Singh, H. Xu, S.K. Katiyar
Development of methodology: M. Vaid, R. Prasad, T. Singh, H. Xu, S.K. Katiyar
Analysis and interpretation of data: M. Vaid, C.A. Elmets, H. Xu, S.K. Katiyar
Writing, review and/or revision of the manuscript: M. Vaid, S.K. Katiyar
Study supervision: H. Xu, S.K. Katiyar
References
- 1.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 2.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 3.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–31. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 5.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 6.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983;158:781–94. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kripke ML. Photoimmunology. Photochem Photobiol. 1990;52:919–24. doi: 10.1111/j.1751-1097.1990.tb08703.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89:8497–501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–43. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 10.Kripke ML. Immunologic unresponsiveness induced by UV radiation. Immunol Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–87. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–88. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 14.Meeran SM, Vaid M, Punathil T, Katiyar SK. Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl phorbol-13-acetate-caused skin tumor promotion in 7, 12-dimethylbenz(a)anthracene-initiated mouse skin, which is associated with the inhibition of inflammatory responses. Carcinogenesis. 2009;30:520–8. doi: 10.1093/carcin/bgp019. [DOI] [PubMed] [Google Scholar]
- 15.Vaid M, Singh T, Li A, Katiyar N, Sharma S, Elmets CA, et al. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev Res. 2011;4:238–47. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–20. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, et al. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc Natl Acad Sci USA. 1997;94:5255–60. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, et al. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 20.Meunier L. Ultraviolet light and dendritic cells. Eur J Dermatol. 1999;9:269–75. [PubMed] [Google Scholar]
- 21.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res (Phila) 2010;3:179–89. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries A, van Oostrom CT, Hofhuis FM, Dortant PM, Berg RJ, de Gruijl FR, et al. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature. 1995;377:169–73. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE. 2011;6:e21410. doi: 10.1371/journal.pone.0021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum Group A-dependent mechanism. Cancer Prev Res. 2010;3:1621–9. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 26.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 27.Parish CR, Glidden MH, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0409s84. Chapter 4:Unit4.9. [DOI] [PubMed] [Google Scholar]
- 28.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J Med. 2005;54:165–71. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Musich PR, Serrano MA, Dong Z, Zou Y. XPA-mediated regulation of global nucleotide excision repair by ATR is p53-dependent and occurs primarily in S-phase. PLoS ONE. 2011;6:e28326. doi: 10.1371/journal.pone.0028326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy AM, Baliga MS, Elmets CA, Katiyar SK. Grape seed proanthocyanidins induce apoptosis through p53, Bax and caspase 3 pathways. Neoplasia. 2005;7:24–36. doi: 10.1593/neo.04412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 33.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 34.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Ann Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 35.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunological Rev. 2003;196:125–46. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 36.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–71. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–20. [PubMed] [Google Scholar]
- 38.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicol Applied Pharmacol. 2007;224:220–7. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation. Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J Immunol. 1994;152:3410–6. [PubMed] [Google Scholar]
- 40.Ullrich SE, Schmitt DA. The role of cytokines in UV-induced systemic immune suppression. J Dermatol Sci. 2000;23:S10–2. doi: 10.1016/s0923-1811(99)00073-0. [DOI] [PubMed] [Google Scholar]