Abstract
Aging can be viewed as a quasi-programmed phenomenon driven by the overactivation of the nutrient-sensing mTOR gerogene. mTOR-driven aging can be triggered or accelerated by a decline or loss of responsiveness to activation of the energy-sensing protein AMPK, a critical gerosuppressor of mTOR. The occurrence of age-related diseases, therefore, reflects the synergistic interaction between our evolutionary path to sedentarism, which chronically increases a number of mTOR activating gero-promoters (e.g., food, growth factors, cytokines and insulin) and the “defective design” of central metabolic integrators such as mTOR and AMPK. Our laboratories at the Bioactive Food Component Platform in Spain have initiated a systematic approach to molecularly elucidate and clinically explore whether the “xenohormesis hypothesis,” which states that stress-induced synthesis of plant polyphenols and many other phytochemicals provides an environmental chemical signature that upregulates stress-resistance pathways in plant consumers, can be explained in terms of the reactivity of the AMPK/mTOR-axis to so-called xenohormetins. Here, we explore the AMPK/mTOR-xenohormetic nature of complex polyphenols naturally present in extra virgin olive oil (EVOO), a pivotal component of the Mediterranean style diet that has been repeatedly associated with a reduction in age-related morbid conditions and longer life expectancy. Using crude EVOO phenolic extracts highly enriched in the secoiridoids oleuropein aglycon and decarboxymethyl oleuropein aglycon, we show for the first time that (1) the anticancer activity of EVOO secoiridoids is related to the activation of anti-aging/cellular stress-like gene signatures, including endoplasmic reticulum (ER) stress and the unfolded protein response, spermidine and polyamine metabolism, sirtuin-1 (SIRT1) and NRF2 signaling; (2) EVOO secoiridoids activate AMPK and suppress crucial genes involved in the Warburg effect and the self-renewal capacity of “immortal” cancer stem cells; (3) EVOO secoiridoids prevent age-related changes in the cell size, morphological heterogeneity, arrayed cell arrangement and senescence-associated β-galactosidase staining of normal diploid human fibroblasts at the end of their proliferative lifespans. EVOO secoiridoids, which provide an effective defense against plant attack by herbivores and pathogens, are bona fide xenohormetins that are able to activate the gerosuppressor AMPK and trigger numerous resveratrol-like anti-aging transcriptomic signatures. As such, EVOO secoiridoids constitute a new family of plant-produced gerosuppressant agents that molecularly “repair” the aimless (and harmful) AMPK/mTOR-driven quasi-program that leads to aging and aging-related diseases, including cancer.
Keywords: AMPK, aging, cancer, gerogenes, gerosuppression, hormesis, mTOR, olive oil, resveratrol, xenohormesis
Plants have been used for medicinal purposes for thousands of years. A third of the 20 most widely sold drugs on the market are plant-derived, and new molecules that may be beneficial for health are rapidly being discovered.1-4 The global economy and human health both depend in part on the discovery of new and effective medicines. Surprisingly, little effort has been focused on plants that are known to synthesize molecules beneficial to the health of other organisms. One of the reasons for this lack of attention is the ease of patenting new synthetic drugs (known as “new chemical entities”); another is the “impurity” (non-specificity) of plant-derived biocompounds. A compound is considered “non-specific” if it interacts with a number of endogenous proteins. A priori, a plant-derived compound that interacts with several molecular targets may have an imperceptible effect (or even an adverse effect) compared with a pure molecule that interacts specifically with a particular protein.1-4 However, a number of plant molecules interact with enzymes and receptors in ways that are not harmful. By the 5th century B.C.E., Hippocrates had described salicylic acid as “a bitter powder extracted from the willow that relieves pain and reduces fevers.”5,6 In 1763, Reverend Edward Stone experimented with the bark of the white willow (Salix alba) to treat fever and concluded that it was “a very effective remedy.”6,7 Since then, a variety of salicylates have been isolated from plants and used in the treatment of goiter, rheumatic fever, pain and arthritis. Today, 45,000 t of aspirin, an acetylated salicylic acid derivative, is produced each year. Aspirin is just one example of the dozens of plant-derived compounds that are now known to be beneficial to human health and that, furthermore, interact with more than one molecular target. Curiously, salicylate has been shown to activate adenosine monophosphate-activated protein kinase (AMPK),8,9 which is a key therapeutic target for the treatment of obesity, type 2 diabetes and cancer due to its role as a central regulator of lipid and glucose metabolism10-12 and as a critical modulator of aging through its interactions with mTOR, SIRT1 and the sestrins.13-24
Hormesis
Living organisms continually face adverse situations or harmful stimuli. Adaptation to these external aggressors, whether chemical, physical, biological or social, is paramount to survival. In addition, mild exposure to a stimulus that could be harmful at high concentrations might confer subsequent resistance or tolerance to some aggression, even one brought about by the stimulus itself. This adaptive response to stress has been identified as an evolutionarily conserved process. In toxicology, the term hormesis is used to define a two-phase nonlinear biological response in which exposure to a low dose of or weak stimulus by an environmental toxin or harmful substance produces a potentially beneficial effect, while a high dose leads to adverse effects.25-30 In the biomedical field, hormesis refers to an adaptive response of cells and organisms to a moderate or intermittent stressor.31-34 Thus, hormesis could be defined as a process in which exposure to a low dose of an environmental factor or chemical compound that is harmful at high concentrations has a beneficial and adaptive effect on the cell or organism. Furthermore, hormesis represents an essential concept in evolution, because it offers a possible explanation for how life on this planet has adapted to an environment that is at times particularly aggressive. To overcome environmental stresses, organisms might have developed a variety of cell signaling pathways that mediate hormetic responses.35-38 These include transcription factors and the kinases that regulate them, which modulate the expression of genes that encode cytoprotective and stress-response proteins such as chaperones and antioxidant and detoxifying enzymes, among others.
Many animal studies involving dietary restriction (DR) regimens such as caloric restriction (CR), total-nutrient restriction, alternate-day fasting and short-term fasting have shown that DR can increase the resistance of the cells of these animals to various types of stress.39-50 For example, mortality due to natural causes or induced by temperature or specific toxins is significantly reduced in animals subjected to CR compared with animals consuming a normal diet. Reduced caloric intake may also protect animals from various types of cancer, including pancreatic, mammary and prostate cancer.51-57 In humans, alternate-day fasting improves symptoms and reduces markers of inflammation and oxidative stress in asthma patients, retards the growth of tumors and sensitizes a range of cancer cell types to chemotherapy.58-60 By considering CR without malnutrition to be a “mild dietary stress,” the ability of CR to prevent or lessen the severity of cancer, stroke, coronary heart disease, autoimmune disease, allergy, Parkinson disease and Alzheimer disease has been largely considered to represent an “overcompensation” resulting from hormetic mechanisms.61
Xenohormesis
Another well-known example of a hormetic process is exposure to low concentrations of certain phytochemicals. Organisms appear to have evolved the ability to detect stress markers produced by other species in their habitats. In this way, organisms might prepare themselves in anticipation of potential adverse environmental conditions. This inter-species hormesis is known as xenohormesis, the phenomenon in which an organism detects the chemical signals of another species regarding the state of the immediate environment or the availability of food.62-66 This hormetic process generates beneficial effects for the organism. The existence of xenohormesis might explain how chemical compounds produced by plants and other autotrophs to defend against adverse environmental conditions can produce beneficial effects in the heterotrophs (animals and fungi) that consume them. Animals take advantage of the information contained in specific compounds produced by plants in response to stress. In fact, the majority of the known beneficial health effects of edible plants are attributed to molecules produced in response to stress.
Plant stress responses have evolved over millions of years. Because most plants cannot move physically, they must tolerate environmental stresses that may appear at any moment. This type of “sedentary lifestyle” may explain the complexity of the stress response in plants. Plants produce toxins to protect themselves against fungi, insects and predators. Plants cultivated for consumption contain fewer natural toxins than their wild counterparts. When plants grow under aggressive conditions, one observes an increase in the production of natural pesticides (biopesticides) that can produce acute intoxication in humans. Some studies have estimated that more than 90% of pesticides present in the human diet are chemical compounds that are produced by plants to protect themselves. Therefore, xenohormesis could explain how the sophisticated stress response that has evolved as a result of the stationary lifestyle of plants can confer stress resistance and survival benefits to animals that consume bioactive compounds produced by environmentally stressed plants.63 While xenohormetic compounds are harmful to insects and microorganisms, the subtoxic levels at which humans ingest them appear to result in moderate cellular stress responses. This, in turn, might activate stress-response adaptation pathways, leading to increased expression of genes that encode cytoprotective proteins such as antioxidant enzymes, chaperones, growth factors, phase 2 detoxification enzymes and mitochondrial proteins. In this scenario, the ability of a combination of antioxidant/anti-inflammatory polyphenols found in many fruits and vegetables to slow aging67 can be explained by molecular mechanisms that are largely unrelated to any potential antioxidant properties. For example, dietary flavonoids such as quercetin and blueberry polyphenols, among others, have been shown to modulate the lifespan of simple model organisms by activating molecular mechanisms independent of their antioxidant capacity.68-74 Polyphenols found in tea and curcumin interact with dozens of molecular targets, providing many health benefits unrelated to their antioxidant properties.75-85 In this regard, the natural polyphenolic compound resveratrol (3,5,4’-trihydroxystilbene) has emerged as a still-debatable mediator of longevity that certainly delays or attenuates many age-related chronic diseases in animal models.86-106 Currently, activation of AMPK15,107-110 rather than activation of the deacetylase SIRT1 seems to be a/the major effect of resveratrol, providing a plausible explanation for many of the health benefits of this compound that have been reported to date.
Extra Virgin Olive Oil (EVOO) Polyphenols And Xenohormesis: A Forgotten Scenario
We are beginning to accumulate epidemiological, clinical and experimental evidence suggesting that consumption of phenolic-enriched fruits, vegetables and herbs might reduce the risk of chronic diseases, including human malignancies.111-113 In this regard, it has been repeatedly suggested that the ability of the so-called “Mediterranean diet” (i.e., the dietary patterns found in olive-growing areas of the Mediterranean basin) to significantly reduce the incidence of atherosclerosis and cardiovascular disease and decrease the risk of several types of human carcinomas, including breast cancer,114-118 can be largely attributed to the unique characteristics of extra virgin olive oil (EVOO), which is an integral ingredient of the traditional Mediterranean diet and is the juice of the olive obtained solely by mechanical means and consumed without any further refining process other than washing, filtration, decantation or centrifugation. Apart from the health benefits that can be expected from EVOO as the richest source of the monounsaturated fatty acid (MUFA) oleic acid (OA; 18:1n-9),119 cold-pressed EVOO includes minor components such as aliphatic and triterpenic alcohols, sterols, hydrocarbons, volatile compounds and several antioxidants.120-126 Although tocopherols and carotenes are also present, hydrophilic phenolics represent the most abundant family of bioactive EVOO compounds.
As for many plant-derived polyphenols, it has been largely assumed that EVOO-derived complex phenols such as lignans, flavonoids and secoiridoids127-130 provide health benefits, primarily due to their antioxidant activity.131-133 However, the antioxidant capacity of EVOO polyphenols does not directly correlate with their efficacy in terms of bioactivity (e.g., toxicity against cultured cancer cells). Moreover, when EVOO is provided in the diet, plasma concentrations of polyphenols are often lower than the levels required for protection against oxidation. Although the metabolites of EVOO polyphenols may reach concentrations in the bloodstream that are several-fold higher than that found in EVOO, EVOO polyphenol-derived compounds tend to have significantly decreased antioxidant activity compared with the parental compounds.63,134 As an alternative to general mechanisms related to the antioxidant and/or trapping activity of oxygen radicals commonly observed with many plant-derived phenolics, recent studies have demonstrated that complex polyphenols can exert anti-carcinogenic effects by directly modulating the activities of various types of oncoproteins.135-140 The results from our laboratory support the idea that EVOO-derived complex polyphenols constitute a previously unrecognized family of anticancer phytochemicals that have a significant impact on the proliferation and survival of cancer cells, at least in part through the specific suppression of protein activities, gene expression and/or signal transduction events closely related to the malignant phenotype.129,141-149
Although a Mediterranean diet and, more specifically, EVOO consumption have both been associated with increased longevity in the human population,150-156 few studies have attempted to explore in depth the ultimate molecular mechanisms by which EVOO may influence longevity; for the most part, it has been assumed that these effects are the result of the antioxidant potential of its phenolic compounds and other free-radical scavengers, such as vitamin E.157 However, if it is accepted that aging is not caused by reactive oxygen species (ROS), which are instead associated with longevity,40,158-178 why have scientists not yet rejected the commonly accepted view that the anti-aging benefits of EVOO phenolics are simply due to their antioxidant potential? Even more intriguing is the fact that, despite the structural resemblance of EVOO complex polyphenols to some of the hormetic polyphenols mentioned above (e.g., resveratrol), none of the phenolic components naturally present in EVOO have been characterized in terms of their potential to extend lifespan. An exception is a recently published study by Cañuelo and colleagues179 suggesting that tyrosol, a phenol present in EVOO, may increase lifespan and stress resistance in Caenorhabditis elegans, likely through the activation of hormetic mechanisms.
Our laboratories at the Bioactive Food Component Platform (BFCP) in Spain (Fig. 1) have recently begun a systematic approach to evaluate for the first time whether secoiridoids, a family of complex phenols found in Oleacea plants that structurally resemble well-known anti-aging molecules such as resveratrol, are bona fide xenohormetic compounds that significantly impact pivotal signal-transduction pathway(s) (i.e., gerogenes and/or gerosuppressors) that drive(s) most, if not all, aging-related diseases. Dr Blagosklonny has recently proposed that “hormesis does not make sense except in the light of TOR-driven aging.”180 Instead of purposely reconciling hormesis with the conventional view on aging (i.e., aging is a decline and/or a deterioration due to the accumulation of random molecular and cellular damage), Dr Blagosklonny proposes that, because aging is an aimless quasi-programmed phenomenon that is driven by overactivated gerogenes belonging to the nutrient-sensing mTOR (mammalian target of rapamycin) pathway (e.g., mTOR, S6K), the mTOR pathway limits lifespan by accelerating age-related diseases (Fig. 1). Therefore, in humans (and other mammals), age-related diseases represent hyperfunctional phenotypes of mTOR-driven aging that actually limit lifespan. Understandably, if mTOR gerogene activity limits lifespan by accelerating the progression of age-related diseases, such as atherosclerosis or cancer, direct or indirect pharmacological suppression of mTOR-driven aging via the activation of mTOR gerosuppressors such as AMPK would be expected to increase healthy lifespan. In this scenario, Dr Blagosklonny differentiates two types of hormesis, namely, “increasing aging tolerance” or “hormesis B,” which does not affect the aging process itself, and “slowing-down aging” or “hormesis A,” which does affect the aging process by directly inhibiting mTOR activity (e.g., CR, rapamycin, resveratrol, metformin) or by imitating mTOR inhibition (e.g., heat shock). We obviously rejected the idea that EVOO secoiridoids could increase aging tolerance, which may allow an organism to survive catastrophes caused by aging-related diseases. In a “hormesis A” scenario, we hypothesized the following: (1) The anticancer activity of EVOO secoiridoid polyphenols results from the activation of anti-aging-like gene signatures in cancer cells (i.e., the enhancement of cellular stress mechanisms suppresses the hyperfunctional phenotype of immortal cells). (2) EVOO-derived secoiridoids activate the energy-sensing AMPK gerosuppressor (i.e., EVOO secoiridoids operates as AMPK-activating low-energy mimickers). (3) Chronic exposure to EVOO secoiridoid polyphenols efficiently delays the senescence phenotype in normal diploid human fibroblasts (i.e., the agonistic activity of EVOO secoiridoids toward the AMPK gerosuppressor improves the structural and functional integrity of normal cells without promoting their entrance into a potentially deleterious hyperproliferative mode).
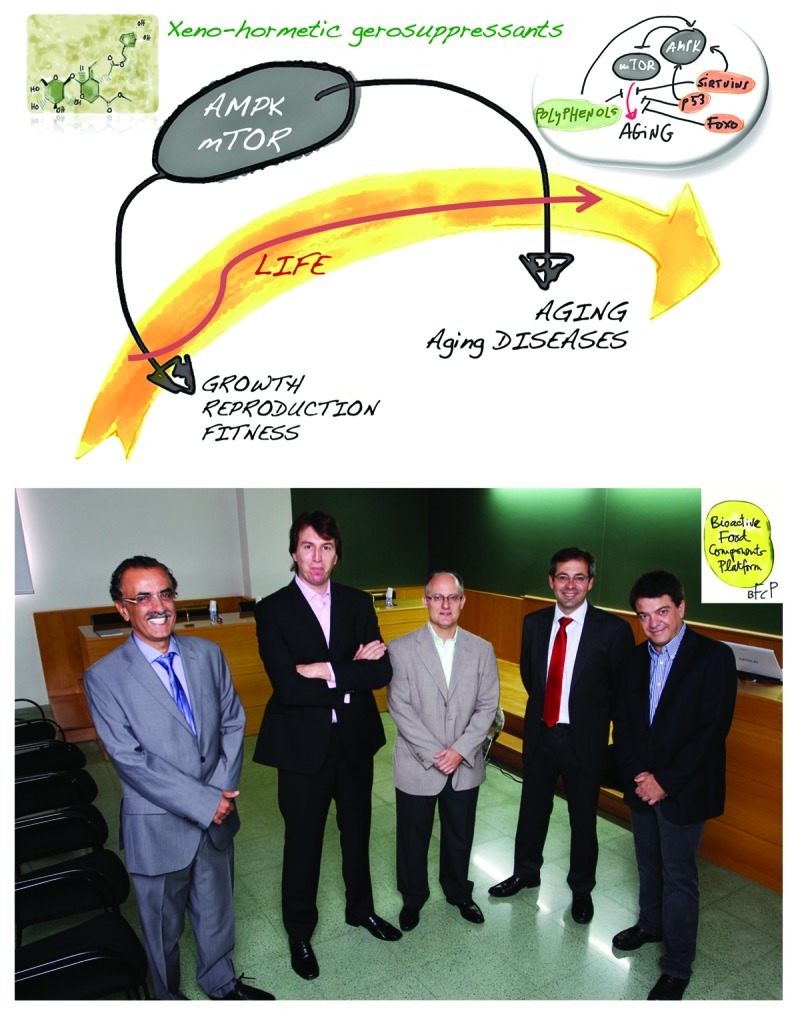
Figure 1. The Bioactive Food Component Platform (BFCP), Spain. The Spanish BFCP has two main goals: first, to molecularly elucidate the cellular and physiological abilities of humans to take advantage of the health benefits chemically encrypted within plant-derived biocompounds; second, to translate the sophisticated stress response of plants, which has evolved as a result of their stationary lifestyle, to the clinical arena to combat human aging and age-related diseases. Both goals of the BFCP revolve around the assumption that age-related diseases (e.g., atherosclerosis, diabetes, cancer, and others) reflect the synergistic interaction between our evolutionary path to sedentarism, which chronically increases a number of mTOR activating gero-promoting factors (e.g., nutrients, growth factors, cytokines, insulin), and the “defective design” of central metabolic integrators such as mTOR and AMPK. Design defects in the metabolic nature of the antagonistic pleiotropy model of aging involve both the ability of the mTOR gerogene to continue, in an aimless (and harmful) manner, a developmental program that was beneficial early in life but was not switched off upon its completion, and the necessary weakness of gerosuppressor genes such as AMPK that antagonize the gerogenic mTOR pathway (i.e., the responsiveness of AMPK signaling should clearly decline with aging, because robust, continuous activation of AMPK in response to cellular stresses will result in accelerated aging).354,355 The BFCP therefore aims to revisit the xenohormesis hypothesis in terms of clinically valuable plant-produced gerosuppressant agents that molecularly “repair” the aimless (and harmful) AMPK/mTOR-driven quasi-program of aging and aging-related diseases (top panel). The BFCP integrates five multidisciplinary teams of biologists, biochemists, chemists, pharmacists, physicians, and engineers to research, design, and develop anti-aging biomedical strategies based on plant-derived gerosuppressants. From left to right in the bottom photograph are Dr Jorge Joven (Universitat Rovira I Virgili, Reus, Spain), Dr Javier A. Menendez (Catalan Institute of Oncology, Girona, Spain, Dr Vicente Micol (Miguel Hernández University, Elche, Spain), Dr Antonio Segura-Carretero (University of Granada, Granada, Spain), and Dr Carlos Alonso-Villaverde (Universitat Rovira i Virgili, Reus, Spain). (The original painting in the top panel is from Dr Jorge Joven based on Fig. 3, ref. 311 by Dr Mikhail v. Blagosklonny; the BCFP team photograph in the bottom panel is by photographer Pere Ferré, Tarragona, Spain.)
In this paper, we present the first body of experimental evidence suggesting that EVOO secoiridoid polyphenols, by acting as biocompounds that belong to the recently defined group of “hormesis A” compounds, can efficiently promote cytotoxicity in human cancer cells through the paradoxical activation of anti-aging/cellular stress-like gene signatures, which, in turn, significantly weaken age-related effects (e.g., cellular senescence) in normal human diploid fibroblasts.
Secoiridoid-Rich EVOO Phenolic Fractions Activate Resveratrol-Like Anti-Aging Transcriptomic Signatures in Cancer Cells
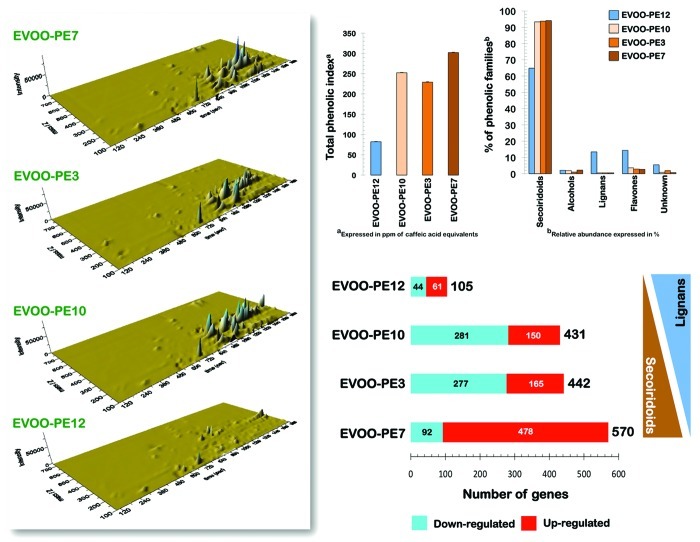
We previously reported that the cytotoxic potencies of individual phenolic extracts (PE) from a variety of EVOO monovarietals were positively related to the relative content of secoiridoids, a group of complex polyphenols.145,146 Highly active EVOO PEs were notably enriched in secoiridoids (Fig. 2), whereas substitution of secoiridoids by other complex polyphenols, such as lignans, in PE mixtures was related to a loss of tumoricidal activity. To identify the key pathways and functions associated with the anti-tumoral activity of crude PE isolated from individual EVOO monovarietals, we performed genome-wide analyses in which we compared the global transcriptomic profiles of JIMT1 breast cancer cells using whole human genome microarrays. RNA was extracted and prepared from metastatic JIMT1 breast cancer cells that had been cultured for 6 h at 70% confluence in the absence or presence of four different EVOO PEs exhibiting the following cytotoxic potencies: EVOO-PE7 > EVOO-PE3 > EVOO-PE10 > > EVOO-PE12, as determined by MTT-based cell viability assays after 5 d exposure to EVOO PEs.145,146 After RNA hybridization to an Agilent 44K (double-density) Whole Human Genome Oligo Microarray containing 45,220 features (probes) representing 41,000 unique human genes and transcripts, the normalized and filtered data from all experimental groups were analyzed simultaneously using the SAM algorithm. We set the significance cut-off at a median FDR of < 5.0%. When we used a 2.0-fold change cut-off relative to the transcriptome of untreated control cells to identify specific effects of EVOO PEs on gene expression, we observed that JIMT1 cancer cells treated with the EVOO PE with the lowest secoiridoid content (PE12) had the lowest number of altered genes (Fig. 3). Of note, while the total number of altered genes was similar (~400 to 600) after exposure to EVOO PEs with higher secoiridoid content (EVOO-PE7, EVOO-PE3 and EVOO-PE10), there was a trend toward enhanced levels of transcripts of more genes in response to EVOO PEs with higher secoiridoid contents. Intriguingly, the majority of the altered genes (~84%) were upregulated following treatment with EVOO-PE7, the phenolic extract with the highest relative secoiridoid content (Fig. 2; Table S1) and the highest anti-tumoral activity.146
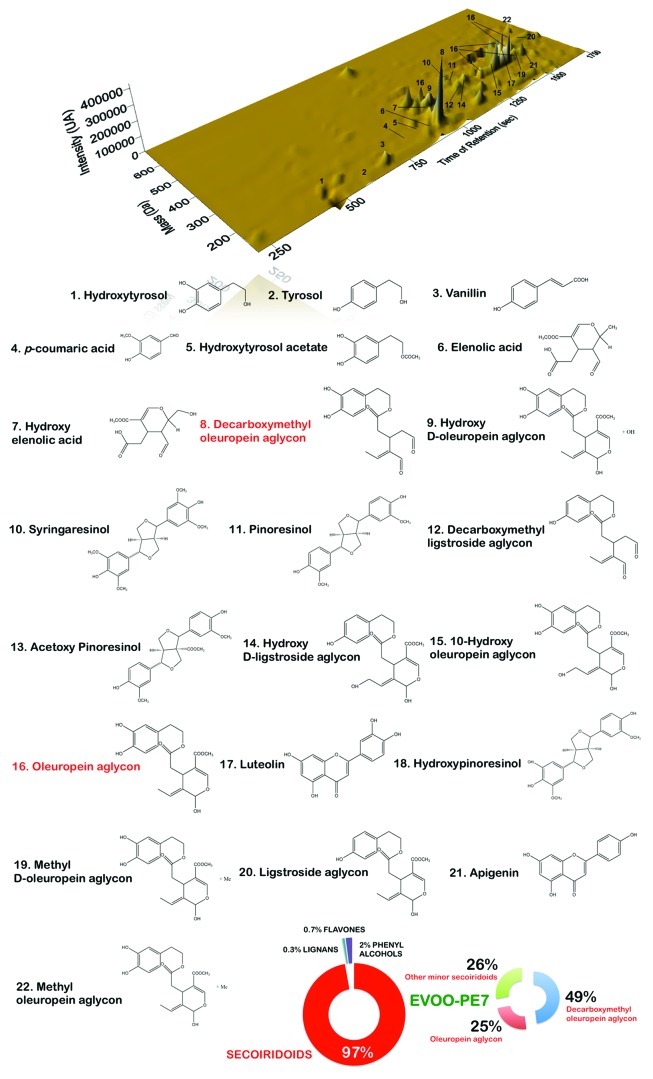
Figure 2. Main phenolic compounds identified in EVOO phenolic extracts by HPLC-DAD-ESI-TOF. The figure shows the chemical structures of the main phenolic compounds compounds identified in secoiridoids-rich Picual EVOO variety following protocols described in reference 147. The figure shows also the percent distribution of the main phenolic families identified in the Picual EVOO-PE7.
Figure 3. Relationship between the distribution of phenolic families in EVOO-PEs and their impact on the whole-genome transcription profile of human breast cancer cells. Total RNA isolated from JIMT1 cells grown in the absence or presence of EVOO-PE12, EVOO-PE10, EVOO-PE3 or EVOO-PE7 (2 μg/mL) for 6 h was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA quantity and quality were determined using the RNA 6000 Nano Assay kit on an Agilent 2100 BioAnalyzer (Agilent Technologies) as recommended. Whole Human Genome Oligo Microarrays (G4112F) were then hybridized. Briefly, 500 ng of total RNA from each sample was amplified by Oligo-dT-T7 reverse transcription and labeled by in vitro transcription with T7 RNA polymerase in the presence of Cy5-CTP or Cy3-CTP using the Quick Amp Labeling Kit (Agilent) and purified using RNAeasy columns (Qiagen). After fragmentation, 825 ng of labeled cRNA from each of the two samples were co-hybridized in in situ hybridization buffer (Agilent) for 17 h at 65°C and washed at room temperature for 1 min in Gene Expression Wash Buffer 1 (Agilent) and 1 min at 37°C in Gene Expression Wash Buffer 2 (Agilent). The images were generated on a confocal microarray scanner (G2565BA, Agilent) at 5 μm resolution and quantified using GenePix 6.0 (Molecular Dynamics). Spots with signal intensities of at least twice the local background that were not saturated and not flagged by GenePix were considered reliable. Extracted intensities were background-corrected, and the log2 ratios were normalized in an intensity-dependent fashion by the global LOWESS method (intra-chip normalization). Normalized log2 ratios were scaled between arrays to make all data comparable. Raw data were processed using MMARGE, a web implementation of LIMMA, a microarray analysis library developed within the Bioconductor project in the R statistical environment. To identify genes that are differentially expressed, the multiclass SAM procedure (significance analysis of microarrays) was applied. Genes with a q-value (FDR) below 5% and a fold change exceeding 2.0 in absolute value were selected as relevant (see also Table S2).
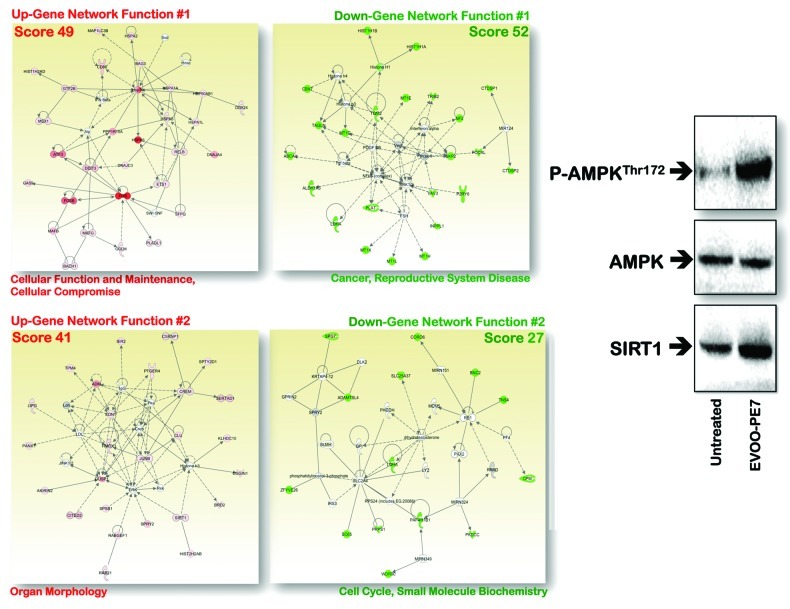
When we previously screened the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database by performing Gene Set Enrichment Analysis (GSEA) to identify key pathways and functions potentially associated with the anti-tumoral activity of EVOO PEs, we observed that the highly active EVOO-PE7 had a dramatic differential impact on the expression of the GADD45 stress-response gene family; by contrast, the expression of this family of genes remained largely unchanged upon treatment with EVOO-PE3 or EVOO-PE10.146 We thus speculated that naturally occurring phenolic mixtures highly enriched in the complex polyphenols oleuropein aglycon (OA) and decarboxymethyl oleuropein aglycon (DOA) (Fig. 2) could lead to enhanced transcript levels of genes that are upregulated by stress. To test this hypothesis, we utilized the “core analysis” function included in the analysis software package ingenuity pathway analysis (IPA, Ingenuity Systems Inc.) to interpret EVOO-PE7-induced global transcriptomic profiles in the context of biological processes, networks and pathways. The IPA software algorithmically generates networks of up- and downregulated functionally related annotated genes based on their connectivity and assigns a score (i.e., a numerical value that takes into consideration both the number of focus genes in a network and the size of the network to approximate how relevant each network is to the original list of focus genes). Figure 4 illustrates graphically the two gene network functions that were most significantly (score ≥ 3) upregulated (red) and downregulated (green) within the EVOO secoiridoid-induced stress transcriptomic signature in human breast cancer cells.
Figure 4. Network analysis of EVOO secoiridoids-regulated genes in human breast cancer cells. Left: Gene networks were constructed using ingenuity pathway analysis (Ingenuity® Systems). Data sets containing identifiers of genes with > 2.0-fold up- or downregulatory changes were uploaded into the application. These “focus genes” were overlaid onto a global molecular network developed from information contained in the ingenuity pathway knowledge base. Networks of these “focus genes” (nodes) are algorithmically generated based on the principle that highly connected gene networks are most biologically meaningful. All edges are supported by at least one reference from the literature stored in the ingenuity pathway knowledge base (the IPA interaction database is manually curated by scientists and updated quarterly). Briefly, the user-input or “‘focus genes” gene list is compared with the “global molecular network” (GMN) database, which consists of thousands of genes and interactions. The focus genes are sorted based on highest to lowest connectivity within the GMN; networks of approximately 35 genes are then constructed beginning with the most highly connected focus gene. IPA assigns a p-value for a network of size n and an input focus gene list of size f by calculating the probability of identifying f or more focus genes in a randomly selected set of n genes from the GMN. The intensity of the node color indicates the degree of expression (green scale for downregulated nodes; red scale for upregulated nodes). The score indicates the likelihood that the genes in a network are found together by random chance. Using a 99% confidence interval, scores of ≥ 3 are significant. Nodes are displayed using various shapes that represent the functional class of the gene product (diamonds, enzymes; ovals, transcription factors; triangles, kinases; circles, others). A solid line indicates a direct interaction; a dashed line indicates an indirect interaction. A line without an arrowhead indicates binding, and a plus sign indicates that other networks contain this gene product. Figure shows up- and downregulated networks with the two highest IPA score (a composite measure that indicates statistical significance that molecules depicted in the network are interconnected). Right: Representative western blot analyses of SIRT1, total AMPK, and phosphorylated AMPK (fosfo-AMPKαThr172) in EVOO untreated- (control) and secoiridoids-treated JIMT1 breast cancer cells.
EVOO secoiridoids activate endoplasmic reticulum (ER) stress chaperones and unfolded protein response (UPR) genes
The primary function of the gene networks that were upregulated by EVOO secoiridoids was related to “cellular function and maintenance and cellular compromise” (score = 49). These gene networks include numerous genes encoding isoforms of constitutevely expressed and stress-induced 70-kDa heat shock proteins (Hsp70s), which are chaperones involved in crucial cellular functions in all kingdoms of life.181-184 While constitutively expressed Hsp70 chaperones have housekeeping functions (e.g., folding of nascent polypeptides, protein translocation between cellular compartments and degradation of unstable and misfolded proteins), stress-induced Hsp70s prevent the accumulation of proteins that have become denatured in response to various cellular stresses (e.g., heat stress, radiation, ischemia, heavy metals or other stimuli that activate stress transcription factors). Treatment with the secoiridoid-rich EVOO-PE7 extract markedly (~11-fold) upregulated the expression of the HSPA6 (Hsp70B’) gene, which is strictly inducible with no detectable basal expression.185,186 Indeed, HSPA6 protein induction is a sensitive biomarker of cellular stress that appears transiently in response to heat stress, whereas levels of HSPA1A (Hsp72), which was also induced by EVOO-PE7, persist for days.187 EVOO-PE7 secoiridoids enhanced the expression of the HSPA1L gene (Hsp70-hom or Hsp70t), which encodes a constitutively expressed, non-inducible cytosolic protein that is highly abundant in testis,189,190 and of HSPA2 (Hsp70–2), a constitutively expressed gene that is expressed at high levels in testis, is essential for the maturation of male gametocytes and is linked to male infertility.191-194 Importantly, EVOO-PE7 secoiridoids also upregulated the transcriptional expression of the DNAJA4 and DNAJC3 genes, which encodes the endoplasmic reticulum (ER)-localized DnaJ family of proteins (ERdj proteins).195 The induced transcription of DNAJ genes is part of a specific pathway that is collectively termed the “unfolded protein response” (UPR) and is evoked when unfolded proteins accumulate in the ER.196-199 The UPR ultimately leads to reduced import of proteins into the ER and upregulation of genes encoding ER chaperones and other components of the ER-associated degradation pathway.200
The previously unrecognized ability of EVOO secoiridoids to upregulate a set of genes involved in the ER stress response to unfolded proteins may appear to conflict with the demonstrated ability of these compounds to strongly inhibit the growth of highly aggressive breast cancer cells.145,146 The UPR is the major protective and compensatory mechanism that enables cells to survive during ER stress. While UPR induction initially results in a general decrease in protein synthesis, which reduces the influx of nascent proteins into the ER, activation of the UPR also results in the enhanced transcription of ER resident chaperones, folding enzymes and other components of the protein degradation machinery, thus preventing aggregation of the accumulating misfolded proteins. This cell protective mechanism, which is also elicited upon induction of Hsp70s,201,202 results in a transient induction of cell cycle arrest and in the accumulation of molecular chaperones that bind and recover unfolded proteins. However, prolonged exposure of cells to ER stress can induce a switch from cell survival to cell death, because the protective function of these mechanisms appears to be temporally restricted.203,204 In this scenario, it is reasonable to suggest that exposure to EVOO secoiridoids promotes cell death-UPR branch signaling by impeding the alleviation of ER stress. Moreover, the coupling of EVOO secoiridoid-activated ER stress and UPR with EVOO secoiridoid-induced cytotoxicity in cancer cells appears to recapitulate the molecular mechanism by which the well-known defense molecule resveratrol simultaneously exerts anti-proliferative and chemopreventive effects.205,206 First, induction of GADD153/CHOP (DDIT3), one of the pivotal components of the ER stress pathway that is significantly upregulated by EVOO secoiridoids, has been shown to be involved in resveratrol-induced cell death in cancer cells.207 Second, resveratrol has been shown to upregulate genes involved in the ER stress response to unfolded proteins.208 Third, because resveratrol can trigger ER stress-induced cell death, UPR could be a potential mechanism of resveratrol cytotoxicity.206,209 EVOO secoiridoids and resveratrol could also share mechanism(s) through which they activate ER stress-like responses. Like resveratrol, EVOO secoiridoid polyphenols paradoxically have a propensity to stimulate the formation of ROS,210-213 which can cause oxidation of nascent proteins, leading to misfolding of proteins and ER stress.214 In addition, EVOO secoiridoid polyphenols can operate in a resveratrol-like manner to molecularly mimic a CR-like situation involving ATP deficiency15,107-110,215-217
EVOO secoiridoids induce c-Fos and modify the expression of genes related to polyamine metabolism
The most prominent EVOO secoiridoid-activated “cellular function and maintenance and cellular compromise” gene network involves not only the chaperone genes HSPA6 and Hsp70, but also c-Fos, a key resveratrol-targeted proto-oncogene.218,219 Indeed, the gene whose expression was most enhanced after treatment with EVOO secoiridoids was c-Fos (FOS; ~20-fold). FOSB, another member of the Fos family of transcription factors, which includes c-Fos, FosB, Fra1 and Fra2, was also one of the 10 most upregulated genes (~11-fold). The Fos family proteins heterodimerize with Jun family proteins (c-Jun, JunB and JunD) to form active AP-1 (activator protein-1) transcription factors, which bind to AP-1 sites present in the promoters of certain genes and regulate their transcription. Of note, treatment of human breast cancer cells with EVOO secoiridoids significantly (~3-fold) upregulated the expression of JUNB. There is increasing evidence that the AP-1 complex plays an important role not only in the proliferation but also in the differentiation of several cell types; several chemopreventive agents (e.g., 1,25-dihydroxyvitamin D3 and butyrate) stimulate cell differentiation in an AP-1-dependent manner.220,221 Resveratrol also stimulates the AP-1 constituents c-Fos and c-Jun to inhibit cancer cell growth.218 The data imply that EVOO secoiridoid-induced upregulation of AP-1 is not associated with tumorigenesis, but rather with growth inhibition and/or differentiation of breast cancer cells.
Because resveratrol-induced c-Fos is functionally related to resveratrol’s ability to modify polyamine metabolism,218 we speculated that the previously unrecognized ability of EVOO secoiridoids to induce c-Fos might involve changes in the expression of genes associated with polyamine synthesis and/or polyamine catabolism. Similar to resveratrol, EVOO secoiridoids significantly upregulated (~4-fold) the expression of spermidine/spermine N1-acetyltransferease (SSAT), the rate-limiting enzyme in polyamine catabolism (Table S1). This enzyme converts spermine to spermidine and the latter to putrescine in cooperation with polyamine oxidase (PAOX). Increased polyamine catabolism in response to EVOO secoiridoids was also suggested by the significant upregulation of the spermine oxidase (SMO) gene. Unlike resveratrol, EVOO secoiridoids downregulated PAOX gene expression, whereas they upregulated some genes involved in polyamine biosynthesis, such as arginase (ARG2) and ornithine decarboxylase (ODC). Although we lacked experimental approaches for measuring the intracellular levels of spermine, spermidine, putrescine and acetyl-spermidine following exposure to EVOO secoiridoids, our data indicate that the mechanism of the growth inhibitory action of EVOO-derived complex polyphenols likely involves an increase in polyamine catabolism with simultaneous induction of c-Fos and its AP-1-related DNA binding activity.
The previously unrecognized ability of EVOO secoiridoids to upregulate key genes directly involved in the conversion of arginine to ornithine (i.e., arginase) and in the conversion of ornithine to putrescine (i.e., ornithine decarboxylase) suggests that the augmentation of polyamine catabolism observed after exposure of cells to EVOO secoiridoids could be related to growth inhibition processes, whereas the augmentation of polyamine synthesis could be related to bona fide anti-aging effects. This appears likely, because polyamine levels decline continuously with age and polyamine (spermidine or high-polyamine diet) supplementation increases life span in model organisms.101,222-231 Because autophagy is required for the cytoprotective and/or anti-aging effects of resveratrol and spermidine, experiments are currently underway in our laboratories at the Bioactive Food Component Platform in Spain to determine if regulation of polyamine metabolism by EVOO-derived secoiridoids differentially impacts the fitness of cancer vs. normal cells undergoing metabolic stress. Because pro-autophagic polyphenols have been shown to reduce the acetylation of cytoplasmic proteins,232 we are also investigating whether EVOO secoiridoids might impact the activation status of autophagy while differentially affecting the acetylproteome of cancer vs. normal cells.
EVOO secoiridoids upregulate SIRT1 and inhibit cancer-promoting genes
In the above-mentioned transcriptome scenario and considering that resveratrol and spermidine increase lifespan by activating the histone deacetylase Sirtuin 1 (SIRT1) and inhibiting histone acetylases, respectively,101 we determined if the resveratrol-like actions of EVOO secoiridoids involve changes in the expression of the SIRT1 gene. Of note, not only was SIRT1 significantly upregulated by EVOO secoiridoids, SIRT1 was also part of the second most significant gene network activated by EVOO secoiridoids, the “organ morphology” gene network (score = 41) (Fig. 4). Although SIRT1 has long been thought to play a role in cancer, the debate regarding its role as an oncogene or tumor suppressor continues.19,232,233 As an inducer of cell survival, it might appear reasonable to suggest that SIRT1 fits the definition of an oncogene; conversely, because SIRT1 is considered important in organism survival, a tumor suppressor function might also be anticipated. Genetic and drug-induced activation of SIRT1 has been shown to inhibit growth and/or induce apoptosis in certain cancer models,234,235 while super-SIRT1 mice exhibiting moderate SIRT1 overexpression (a ~3-fold increase) are generally healthier than control mice and are partially protected from certain solid tumors.236-239
Because neoplastic cells are thought to recapitulate many stem cell characteristics, including metabolic ones,17,20,240-245 the oncogenic vs. tumor-suppressive activities of SIRT1 can be viewed in terms of the specific contribution of SIRT1 to maintaining or impeding “stemness-like” status in cell populations involved in tissue regeneration or cancer tissue heterogeneity, respectively. To preliminarily assess whether EVOO secoiridoid-induced upregulation of SIRT1 is related to the activation of onco-suppressive transcriptional events in highly aggressive cancer cells, we evaluated whether well-known oncogenes were among the 92 genes that were significantly downregulated by EVOO secoiridoids (Table S1; Fig. 4). Interestingly, the EVOO secoiridoids most frequently downregulated gene networks related to “cancer and reproductive system disease” (score = 52), including numerous metallothionein (MT) gene isoforms (MT1E, MT1G, MT1X, MT1L, MT1H). MT belongs to a family of metal-binding proteins whose roles range from heavy metal detoxification to the promotion of tumorigenesis. MT has been reported to be highly expressed in many tumors, including breast cancer, and is known to regulate key processes such as cell proliferation, apoptosis and even chemoresistance.246-250 Because the role of MT in metal ion homeostasis is fundamental for controlling the activation of stem/progenitor cells, we speculated that MT downregulation by EVOO secoiridoids might be part of a broader genetic network involving key cancer stem cell (CSC)-related genes. Consistent with this hypothesis, the “cancer and reproductive system disease” gene network downregulated by EVOO secoiridoids includes the ALDH1A3 gene, a biomarker of primitive normal human mammary luminal cells that shows high activity specifically in breast carcinomas. In such tumors, expression of the ALDH1A3 gene identifies the tumorigenic cell fraction that is capable of self-renewal and of generating tumors by recapitulating the heterogeneity of the parental tumor (i.e., breast CSCs).251-254 We are currently investigating whether treatment with EVOO secoiridoids impedes the propensity of breast CSCs to form multicellular “microtumors” under non-adherent and non-differentiating conditions (i.e., mammospheres).
In addition to the CSC marker ALDH1A3, treatment with EVOO secoiridoids notably downregulated the expression of SKP2, the gene that encodes the F-box protein S-phase kinase-associated protein 2 (Skp2). Skp2 belongs to the ubiquitin-proteasome system (UPS), which plays a vital role in regulating many biological processes by controlling the timely turnover of proteins. Because Skp2 is responsible for the degradation of several tumor suppressor proteins (e.g., p27, p57, p21, p130, FOXO1), it is thought to function as an oncoprotein that interacts with other major signaling pathways (e.g., PI3K/Akt, mTOR, PPARγ, ERK, FoxP3 and IGF) in breast cancer.255-259 As such, there is renewed interest in developing Skp2 inhibitors as a general approach for cancer prevention and therapy.259 Although specific drugs that inactivate Skp2 in cancer cells have not been identified, it is noteworthy that several naturally occurring compounds (1,2,3,4,6-penta-O-galloyl-β-D-glucose [PGG], gallic acid, epigallocatechin-gallate [EGCG], quercetin, curcumin, and lycopene) downregulate Skp2 expression in human cancers, including breast cancer.260-262 We now add the complex polyphenols secoiridoids to the growing list of natural polyphenols that can function as potent inhibitors of Skp2.
The function of the second most downregulated gene network in response to EVOO secoiridoids is related to “cell cycle, amino acid metabolism and small molecule biochemistry” (score = 27) and was identified based on LDHA, a gene that was also overrepresented in the most downregulated gene network described above (Fig. 4). Lactate dehydrogenase (LDH) acts at a critical branch point in the metabolism of major nutrients; it is also active in the tricarboxylic acid (TCA) cycle and in determining tumor pH.263 Glucose and glutamine are the major carbon sources for rapidly proliferating tumors and provide precursors for nucleic acids, proteins and lipids as well as reducing capability (NADPH). Pyruvate is largely derived from glucose and glutamine metabolism; it can be converted to lactate by the LDH complex and/or enter the TCA cycle for conversion to CO2 and ATP. The conversion of pyruvate to lactate is also catalyzed by LDH in a reversible reaction that results in the formation of NAD+, which is necessary for further glycolysis. LDH is a tetrameric enzyme containing two major subunits (A and B) that are coded by the LDHA and LDHB genes; together, these subunits form five different isoenzymes.264 Although all five isoenzymes can catalyze the forward and backward conversion of pyruvate and lactate, LDHA kinetically favors the conversion of pyruvate to lactate, whereas LDHB predominantly converts lactate to pyruvate, which is further oxidized through the TCA cycle. Serum LDH levels are often increased in cancer patients, and LDHA protein expression is often upregulated in tumors.265-273 Like high lactate levels, which are a key feature of the aerobic glycolysis (Warburg effect) in tumor cells and are associated with the subsequent development of metastases,274,275 the presence of high LDH levels in tumors has been linked to poor prognosis and greater metastatic potential. Because the LDHA protein is required for the maintenance and progression of many tumors, it also represents a potential target for cancer therapy.276 Our findings suggest a potential inhibitory role of EVOO secoiridoids against the Warburg effect in tumor cells.
EVOO secoiridoids are resveratrol transcriptional mimickers that activate the energy sensor AMPK
Previous studies have shown that resveratrol efficiently induces changes in the transcriptional profiles of key metabolic tissues that closely resemble the changes induced by CR.91,277 The data presented here demonstrate that EVOO secoiridoids appear to mimic key features of resveratrol-induced gene expression patterns to inhibit the growth of cancer cells, whose aberrant bioenergetic and biosynthetic metabolism is unambiguously required for proliferation and/or survival. To further confirm that administration of EVOO secoiridoids functionally mimics resveratrol via activation of AMPK-related stress signaling pathways, we employed two additional complementary approaches. First, systematic and integrative analyses of EVOO secoiridoid-regulated gene lists were conducted using the DAVID (Database for Annotation, Visualization and Integrated Discovery) bioinformatics resource (National Institute of Allergy and Infectious Diseases, NIH), a web-based public database capable of uncovering biological features and meaning associated with large gene lists, regardless of which genomic platform or software package was used to generate the list (http://david.abcc.ncifcrf.gov/). DAVID uses a set of fuzzy classification algorithms to group genes based on their co-occurrence in annotation terms and ranks the gene groups using an internal (EASE) score.278,279 DAVID was used to evaluate the enrichment distribution across the “biological processes” in the gene ontology (GO) tree. The threshold value of the enrichment score was set at 1.0 instead of 1.3, thereby avoiding the loss of important information. The gene list was organized and condensed into biologically meaningful modules using the DAVID gene functional classification tool at the medium level of statistical stringency. When ranking the importance of annotation groups with enrichment scores ≥ 1.0, DAVID term-centric modular enrichment analysis revealed that the “biological modules” significantly enhanced by EVOO secoiridoids paradoxically included positive regulation of “developmental and biological processes,” “response to stress,” “organ morphogenesis,” “response to chemical stimulus (unfolded protein),” “response to wounding” and “chromatin assembly,” among others (Table S2). The activation of anti-aging biological modules was concomitant with the significant downregulation of “hexose catabolic processes,” “cell cycle” and “cellular carbohydrate metabolic processes,” among others (Table S2). Therefore, highly aggressive cancer cells appear to react to EVOO secoiridoid-triggered cellular stress signals by evoking cell survival programs that ultimately result in cancer cell death.
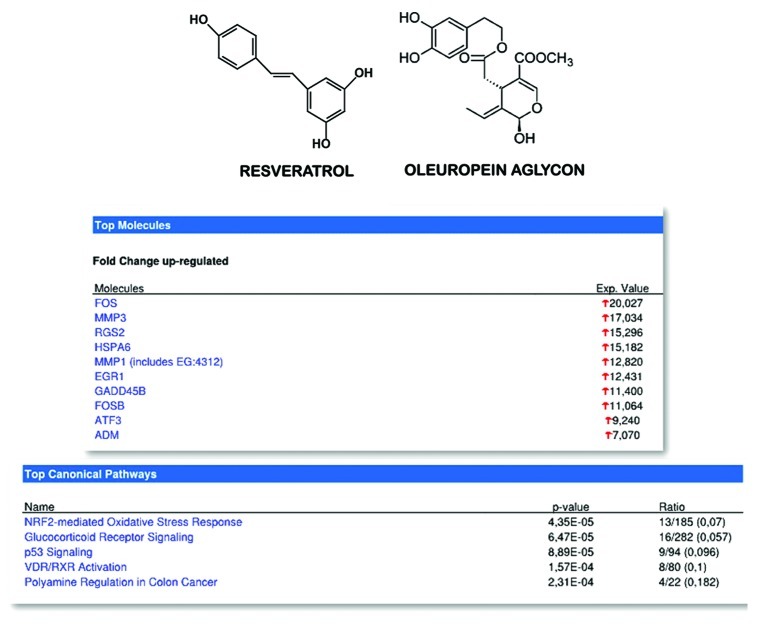
Second, to unambiguously determine whether the crucial signaling pathways that are significantly altered in the presence of EVOO secoiridoids are similar to those previously recognized for resveratrol, we used the “canonical pathway analysis” function included in the IPA analysis software. This analysis associates probe sets with the canonical pathways included in Ingenuity’s Knowledge Base and returns two measures of association: (1) the ratio of the number of genes from the list that map to the pathway to the total number of genes that map to the same pathway, and (2) a p-value based on Fisher’s exact test to ascertain enrichment. Notably, when the canonical pathways induced by EVOO secoiridoids were ordered by p-value (p < 0.05; the ratio value is also shown), all of the molecular mechanisms underlying resveratrol’s recognized anti-aging effects were over-represented in the five canonical pathways that were most significantly upregulated by EVOO secoiridoids in cancer cells (Fig. 5). First, the above-mentioned resveratrol-induced FOS-dependent inhibition of polyamine synthesis and increased polyamine catabolism218,219 was mirrored in the EVOO secoiridoid-induced “polyamine regulation in colon cancer” pathway (p-value = 2.31E-04). Second, the resveratrol-related vitamin-D/retinoic acid-like differentiation-induced effects280-284 were mirrored in the EVOO secoiridoid-induced “VDR/RXR activation” pathway (p-value = 1.57E-04). Third, the resveratrol-induced p53-related engagement of cell cycle arrest and/or apoptotic signals285-290 was mirrored in the EVOO secoiridoid-induced “p53 signaling” pathway (p-value = 8.89E-05). Fourth, resveratrol’s anti-inflammatory effects related to epigenetic and chaperone-dependent activation of the glucocorticoid receptor134,291-294 were mirrored in the EVOO secoiridoid-induced “glucocorticoid receptor signaling” pathway (p-value = 6.47E-05). Fifth, resveratrol’s ability to protect against oxidative stress damage by modulating nuclear redox factor 2 (NRF2) signaling295-302 was mirrored in the EVOO secoiridoid-induced “NRF2-mediated oxidative stress response” pathway (p-value = 4.35E-05). Considering that most of the beneficial effects of CR on the carcinogenic process are likely mediated by NRF2303,304 and that recent studies have shown that a diet rich in EVOO phenolics (e.g., hydroxytyrosol, which is mainly formed from the hydrolysis of the secoiridoid oleuropein aglycone) induces SIRT1 and NRF2-dependent gene expression of anti-stress targets [e.g., glutathione-S-transferase (GST), γ-glutamyl cysteine synthetase (γ-GCS), nicotinamide adenine dinucleotide phosphate [NAD(P)H]:quinone oxidoreductase (NQO1) and paraoxonase-2 (PON2) mRNAs as well as paraoxonase-1 (PON1) activity] in senescence-accelerated (SAMP8) mice,305 our findings strongly support the idea that the ability of secoiridoids to activate NRF2 signaling in somatic cells constitutes a mechanism through which EVOO complex polyphenols could lead to a delay in or the prevention of the onset of some forms of human cancers (e.g., breast cancer) and subsequently contribute to improved human health and lifespan.
Figure 5. Top: Structural similarities between resveratrol and the EVOO secoiridoid oleuropein aglycon. Bottom: IPA-identified top individual genes and top canonical pathways affected by EVOO secoiridoids.
Finally, we sought to investigate the unexplored possibility that EVOO secoiridoids might attenuate the adaptable aging-accelerating mTOR signaling pathway in cancer cells. Incubation of JIMT1 breast cancer cells (Fig. 4) and PC9 lung carcinoma cells (data not shown) with increasing concentrations of an EVOO PE rich in secoiridoids resulted in increasing activation of the mTOR gerosuppressor AMPK.306 Activation of AMPK was associated with phosphorylation of the α-catalytic subunit of the enzyme at Thr-172, as assessed using a phosphospecific antibody. Minimal changes in total AMPK protein levels were detected with anti-α1/-α2 AMPK antibodies. When EVOO phenolic extracts highly enriched with secoiridoids were replaced by EVOO phenolic extracts with a similar content of total polyphenols but enriched with lignans, we observed a drastic decrease in the ability of the EVOO PEs to activate AMPK (data not shown). These results demonstrate for the first time that AMPK becomes significantly activated by EVOO-derived phenolic extracts when the amount of complex polyphenol secoiridoids but not lignans exceeds a critical threshold.
EVOO Secoiridoid Polyphenols Induce Senescence Delay In Human Diploid Fibroblasts
Well-accepted hormetic strategies such as repeated mild heat stress (RMHS) significantly affect several age-related phenomena in human skin fibroblasts, e.g., cell size and cell morphology, but do not modify the proliferative capacity of these cells.32,33,78 We decided to investigate whether repeated exposure to non-cytotoxic concentrations of EVOO secoiridoids might improve the structural and functional integrity of skin fibroblasts in vitro without promoting entrance of these cells into a potentially deleterious hyper-proliferative mode. Thus, using the well-established in vitro senescence model of human diploid fibroblasts (HDFs), we determined for the first time whether senescence-associated changes occur in response to chronic exposure to crude EVOO PEs. Cell viability (MTT assays) confirmed that, at the concentration employed in our studies, EVOO secoiridoids did not exhibit highly toxic effects. Compared with untreated control fibroblasts, we observed almost no cell death of young HDFs after 72 h of incubation with 200 ng/mL EVOO-PE7. After 10 d of treatment, however, 20–25% of the cells cultured in the presence of the secoiridoid-rich EVOO PE were metabolically nonviable (data not shown).
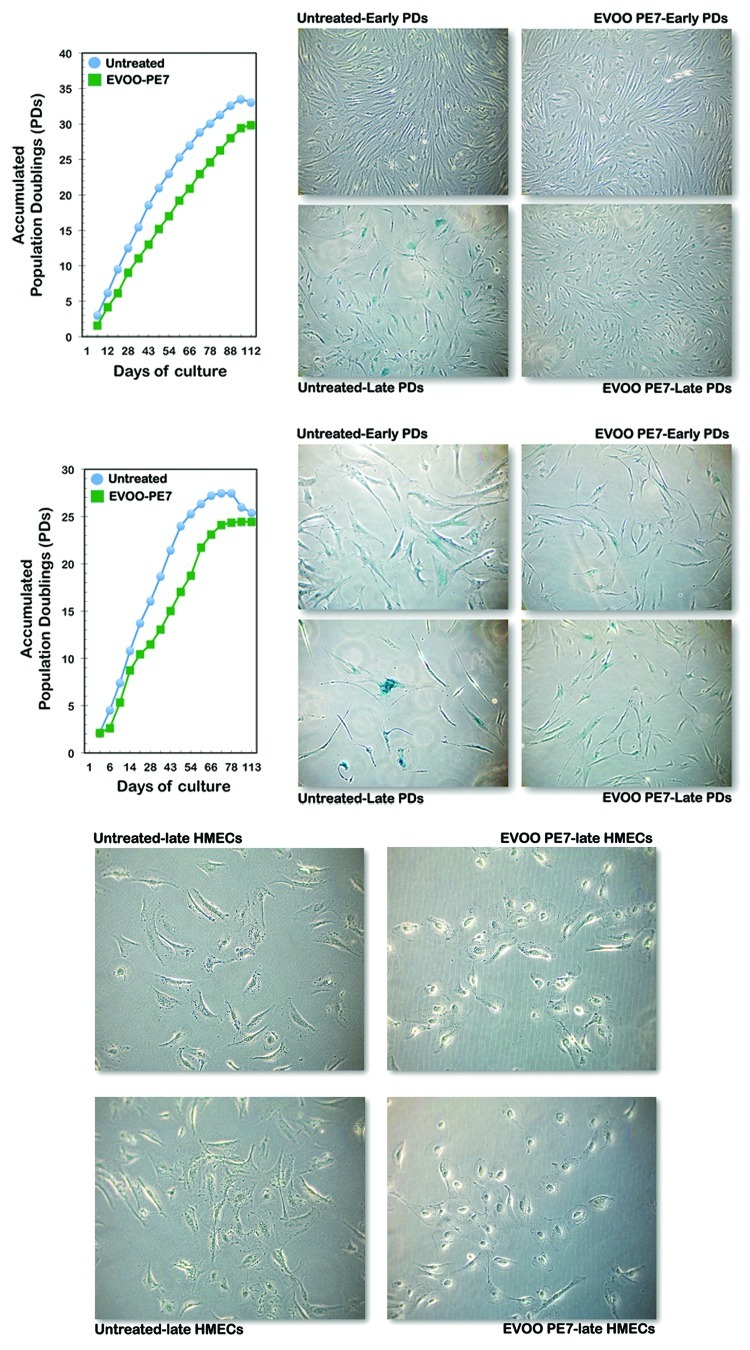
Low-passage p16INK4a-positive WI-38 fetal lung HDFs and p16INK4a-negative BJ-1 neonatal foreskin HDFs were exposed to low concentrations of EVOO secoiridoids or to the same volume of vehicle twice a week during serial passaging throughout their entire replicative life spans. Chronic exposure to EVOO secoiridoids failed to lengthen the proliferative lifespans of WI-38 and BJ-1 HDFs (Fig. 6). However, whereas the growth rates, population doubling rates and cumulative population doubling (PD) levels of the cells were mostly unaffected by repeated exposure to EVOO secoiridoids, age-related changes in cell size, cellular morphology and senescence-associated β-galactosidase (SA-β-gal) staining were significantly altered. Age-related alterations in the morphology of fibroblasts, which is one of the most obvious changes that occurs during cellular aging, was significantly reduced in EVOO secoiridoid-treated HDFs. At the end of their proliferative lifespans, untreated control cultures underwent a significant increase in cell size, taking on a flattened appearance; their morphological heterogeneity also increased, and they suffered a complete loss of arrayed arrangement and accumulated significant amounts of intracellular and extracellular debris, with concomitant increases in the sizes of their nuclei and nucleoli (Fig. 6, left). Indeed, a short-term treatment (3 d) with EVOO secoiridoids notably ameliorated the intense vacuolization and abundante accumulation of cell debris in nearly senescent human mammary epithelial cells (HMECs) (Fig. 6, right). Moreover, we noted significantly higher proportions of β-gal-positive WI-38 and BJ-1 cells in old HDF cultures than in young HDF cultures. At the end of their proliferative lifespans, HDF cultures grown continuously in the presence of EVOO secoiridoids demonstrated significantly reduced age-related morphological alterations and displayed relatively young-like morphologies. Old HDF cultures chronically exposed to EVOO secoiridoids did not undergo significant cell enlargement and largely maintained the thin, long, spindle shapes observed in younger HDF cell populations. In contrast to control cultures, EVOO secoiridoid-treated HDFs maintained an arrayed arrangement of morphologically homogeneous cells (Fig. 6), with reduced accumulation of lysosomal residual bodies and an almost complete absence of multinucleated cells. When the proliferation rate of the untreated control cultures began to decrease as cellular senescence approached, SA-β-gal activity was measured; we notably observed significantly fewer β-gal-positive cells in EVOO secoiridoid-treated HDFs than in vehicle-treated HDFs (Fig. 6).
Figure 6. Impact of chronic exposure to EVOO secoiridoids in age-related changes of cultured human diploid fibroblasts (HDFs) and human mammary epithelial cells (HMECs). Left: Graphs showing cumulative population doubling for BJ-1 (top) and WI-38 (bottom) HDFs continuously cultured in the absence or presence of 200 ng/mL EVOO-PE7. Representative microphotographs illustrate the differential acquisition of age-related biomarkers including changes in cell morphology and SA-β-gal activity (blue staining) in response to EVOO secoiridoids. Right: Representative microphotographs illustrate the impact of short-term treatment (3 d) with EVOO secoiridoids in the vacuolization and abundant accumulation of cell debris in pre-senescent HMECs.
Taken together, these findings demonstrate for the first time that complex mixtures of crude EVOO PEs antagonize cellular senescence without modifying the proliferative capacity of HDFs. Katsiki and colleagues307 previously reported that oleuropein-treated cultures of normal human fibroblasts exhibited a significant delay in the appearance of senescence morphology. In their hands, however, oleuropein treatment of human embryonic fibroblasts conferred a life span extension of approximately 15%. It is plausible that the presence of numerous phenolic molecules within a crude EVOO PE would not preclude the ability of “diluted” secoiridoids to suppress senescence as efficiently as a single purified secoiridoid (e.g., oleuropein) and that, at concentrations such as those used in our experiment, the slightly cytotoxic effects of the crude EVOO phenolic mixture would prevent a plausible anti-aging (preservation of proliferative capacity) effect. Of note, when older HDFs chronically cultured in the presence of 200 ng/mL EVOO-PE7 were challenged with higher concentrations of the same PE, they were notably refractory to the cytotoxic effects observed when EVOO secoiridoid-naive young HDFs were treated with the same high dose of polyphenols (data not shown). This finding supports the idea that continuous exposure to hormetic stresses (e.g., low-dose secoiridoids) can protect cells from stronger stresses (e.g., high-dose secoiridoids) but that these stronger stresses do not cause aging, as aging is not caused by any stress.180
EVOO Secoiridoid Polyphenols: A New Family Of “Xenohormetic” Compounds
The previously unrecognized ability of EVOO secoiridoids to activate endogenous cellular defense pathways (e.g., the evolutionarily conserved NAD-dependent deacetylase sirtuin-1 and NRF2 pathways) that integrate the adaptive stress response and positively control the expression of a battery of stress response proteins in human cells support the original “xenohormesis hypothesis” of Howitz and Sinclair63,89,134 invoking the interspecies communication of stress signals. The literature on sirtuin focuses on pharmacological activators of SIRT1 (e.g., resveratrol, SRT1720), which have been proposed as therapeutics for diabetes, neurodegeneration, inflammation and other diseases. However, many compounds may have been identified as SIRT1 activators due to artifacts in the assay methodology (i.e., the use of fluorescently tagged substrates). By performing the first comprehensive analysis of gene expression and transcriptome dynamics of human breast cancer cells grown in the presence of crude phenolic EVOO extracts, we present compelling data that suggest that the stress response of Oleacea plants, which has evolved as a result of their stationary lifestyle, might confer stress resistance and “anti-aging benefits” to animals such as humans that consume bioactive secoiridoids produced by Oleacea.
In highly proliferative cancer cells that possess aberrant bioenergetic and biosynthetic metabolism, EVOO secoiridoid-imposed metabolic reprogramming would be expected to promote growth inhibition and cell death; however, the ability of relatively non-toxic secoiridoids to upregulate a variety of transcriptomic programs involved in regulating stress responses should result in increased longevity of normal cells. This apparent metabolic paradox can easily be resolved in the context of an evolutionary view of the “AMPK/mTOR-xenohormetic” model. AMPK, whose ancestral role may have been related to the response to starvation for the preferred carbon source, glucose, appears to have arisen very early during eukaryotic evolution. Rapid cell growth requires the active synthesis of proteins, rRNA and lipids, all of which are switched off by the activation of AMPK (and, likely, by downstream inactivation of mTOR). Indeed, one reason for the high glycolytic rate of rapidly proliferating cells, including tumor cells, is that the TCA cycle ceases to be a purely catabolic pathway and becomes at least partially anabolic, actively providing precursors for biosynthesis, particularly citrate for lipid synthesis.275 Accordingly, both tumor cells and viruses (and likely other pathogens) appear to have developed mechanisms to downregulate the energy sensor AMPK and escape from its restraining influence on growth and biosynthesis.9,10,107 In an “AMPK/mTOR-xenohormetic” model, there is no need to assume that animals and fungi have retained an ability to be activated by certain plant stress molecules, because they provide useful advance warning of a deteriorating environment or food supply. Soil bacteria do not produce the macrocyclic lactone rapamycin as an anticancer drug or a pro-longevity medicine but as an antibiotic that inhibits the growth of fungal competitors. The French lilac or goat’s rue (Galega officinalis) produces galegine, the bioactive starting material from which metformin was developed, as a defense compound to deter grazing by herbivores, not as a gerosuppressant that delays aging and suppresses tumorigenesis. Grapes produce resveratrol in response to fungal infection but not as a longevity nutrient with anticancer properties. Similarly, upon activation and conversion to oleuropein aglycon by deglycosylation, phenolic secoiridoid glycosides such as oleuropein can induce a loss of nutritive value via the loss of lysine and inactivation of enzymes by functioning as a unique multivalent alkylator that acts as a protein crosslinker, providing plants with an effective defense against attack by herbivores and possibly by pathogens.307-310 The fact that numerous and apparently unrelated “nutraceuticals,” “xenobiotics” and other biocompounds derived from traditional herbal medicines act as xenohormetic compounds merely reflects their common ability to inhibit the aging-driven activity of mTOR gerogenes and/or to activate key gerosuppressors of the mTOR pathway (i.e., AMPK).180,311-322 Considering that mitochondria became the main cellular power source during the evolutionary development of eukaryotes,323 AMPK plausibly arose very early during eukaryotic evolution due to the requirement for sensing energy status in the cytoplasm and providing a signal to modulate mitochondrial function. Indeed, the ancestral function of AMPK in plants and animals was likely to orchestrate resistance responses to the effects of carbohydrate starvation (e.g., to trigger a switch back to oxidative metabolism in response to deprivation of the preferred carbon source, glucose). Of note, most of the potent activators of AMPK are plant defense compounds that inhibit mitochondrial ATP synthesis. Forthcoming studies should definitively elucidate whether EVOO secoiridoids and other xenohormetic compounds impact both mitochondrial functionality and AMPK-like metabolic sensors across different species (e.g., olive and human) during times of stress; in this scenario, xenohormesis should be viewed as providing a shared ability to molecularly connect mitochondria and AMPK during evolution. Curiously, SIRT1 has been shown to play an essential role in the ability of moderate doses of resveratrol to stimulate AMPK and improve mitochondrial function both in vitro and in vivo.324
EVOO Secoiridoid Polyphenols: A New Family Of “Gerosuppressant” Compounds
Despite the high degree of structural resemblance between EVOO-derived complex polyphenols and well-recognized CR-like polyphenols that are known to experimentally extend lifespan (i.e., resveratrol), no studies have explored the actual molecular function of EVOO secoiridoids in retarding human aging. As for many other polyphenols, it has been erroneously assumed that EVOO-derived complex phenols provide health benefits, including higher longevity, largely because of their antioxidant activity. Our laboratories at the Spanish BFCP have been studying for the first time whether secoiridoids, a family of complex phenolics characteristic of Oleacea plants, by functioning as biocompounds belonging to the recently defined group of “hormesis A” compounds (i.e., inhibitors of the pro-aging activity of mTOR gerogenes and/or activators of mTOR gerosuppressors such as AMPK) can, like resveratrol, affect anti-aging signaling pathways in ways that significantly promote cytotoxicity in immortal tumor cells and that weaken age-related pro-senescence effects in normal cells Because changes in the expression of significant numbers of genes have been linked to the anticancer and lifespan effects of all known lifespan interventions (CR- and the so-called CR-mimetics), we hypothesized that global changes in the human transcriptome detectable by the use of high-density microarrays could be used for the preliminary identification of candidate EVOO secoiridoids-induced anti-aging/anticancer gene signatures. The strength of evidence supporting the xenohormetic activity of EVOO secoiridoids was tested by assuming that their tumoricidal activity results from the paradoxical activation of cellular stress-like, anti-aging transcriptomic signatures in cancer cells. By following this genome-wide analysis approach in highly aggressive human breast cancer cells that were briefly exposed to crude EVOO phenolic extracts highly enriched in the secoiridoids oleuropein aglycone and decarboxymethyl oleuropein aglycone, we demonstrated that Oleacea plant defense molecules, which are able to exert strong protein-denaturing/protein-crosslinking/lysine-alkylating activities against herbivores, can efficiently induce in human cells intracellular signaling pathways that may respond to biological stress at the molecular/cellular level. We confirmed that the stress pathways activated by EVOO secoiridoids might defend cells and tissues in a hormetic-like manner, because they regulate energy metabolism in a way that would be expected to enhance cellular survival during times of stress. Thus, the anticancer activity of EVOO secoiridoids was found to be related to the activation of anti-aging/cellular stress-like gene signatures, including endoplasmic reticulum (ER) stress and the unfolded protein response, spermidine and polyamine metabolism, sirtuin-1 (SIRT1),325-351 and NRF2 signaling. EVOO secoiridoids activated the gerosuppressor AMPK and inhibited crucial metabolic genes involved in the Warburg effect and the self-renewal capacity of “immortal” cancer stem cells and EVOO secoiridoids significantly prevented age-related changes in cell size, morphological heterogeneity, arrayed arrangement and senescence-associated β-galactosidase staining of normal diploid human fibroblasts at the end of their proliferative lifespan.
Aging can be viewed as a quasi-programmed phenomenon driven by the overactivation of the nutrient-sensing mTOR gerogene. Complementing this idea, emerging studies indicate that the responsiveness of AMPK signaling clearly declines with aging.306 The loss of sensitivity of AMPK activation to cellular stress impairs metabolic regulation, increases oxidative stress and reduces autophagic clearance. Not surprisingly, very recent studies have illuminated the central role that loss of the AMPK gerosuppressor plays in dictating the unique metabotype that drives tumorigenesis.352,353 Given that AMPK is a crucial gerosuppressor and tumor-suppressor that suppresses mTOR-driven geroconversion (as well as mTOR oncogenic transformation), age-related diseases reflect a synergistic interaction between our evolutionary path to sedentarism, which chronically increases a number of gero-promoting factors, e.g., mTOR activators such as nutrients (glucose, amino acids, fatty acids), growth factors, cytokines and insulin and the “defective design” of central metabolic integrators such as AMPK and mTOR. In this scenario, xenohormesis should be viewed in terms of plant-produced gerosuppressants that molecularly “repair” the aimless (and harmful) AMPK/mTOR-driven quasi-program of aging and aging-related diseases (Fig. 7).
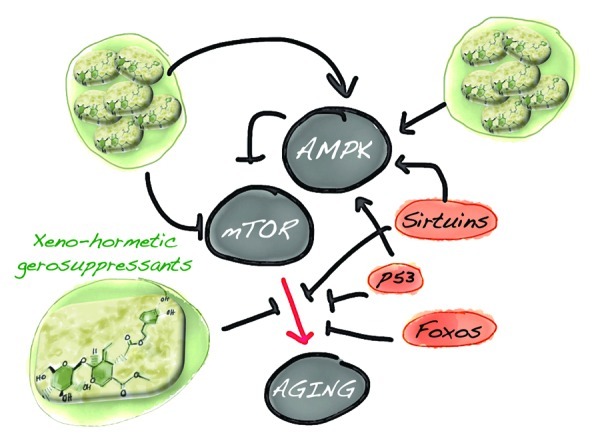
Figure 7. EVOO secoiridoids: A new family of plant-produced gerosuppressant agents that molecularly “repair” the aimless (and harmful) AMPK/mTOR-driven quasi-program that leads to aging and aging-related diseases, including cancer.
Supplementary Material
Acknowledgments
This work was financially supported by the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, grants CP05–00090, PI06–0778 and RD06–0020–0028), the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain) and the Ministerio de Ciencia e Innovación (SAF2009–11579, Plan Nacional de I+D+ I, MICINN, Spain). Alejandro Vazquez-Martin received the Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria -FIS-, Spain). Sílvia Cufí received a research fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN, Spain).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23756
References
- 1.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–20. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.McChesney JD, Venkataraman SK, Henri JT. Plant natural products: back to the future or into extinction? Phytochemistry. 2007;68:2015–22. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Molinari G. Natural products in drug discovery: present status and perspectives. Adv Exp Med Biol. 2009;655:13–27. doi: 10.1007/978-1-4419-1132-2_2. [DOI] [PubMed] [Google Scholar]
- 4.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–5. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 5.Lévesque H, Lafont O. [Aspirin throughout the ages: a historical review] Rev Med Interne. 2000;21(Suppl 1):8s–17s. doi: 10.1016/s0248-8663(00)88720-2. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR. The fight against rheumatism: from willow bark to COX-1 sparing drugs. J Physiol Pharmacol. 2000;51:573–86. [PubMed] [Google Scholar]
- 7.Hedner T, Everts B. The early clinical history of salicylates in rheumatology and pain. Clin Rheumatol. 1998;17:17–25. doi: 10.1007/BF01450953. [DOI] [PubMed] [Google Scholar]
- 8.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–36. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Ji J, Yan XH. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52:373–81. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- 12.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Mousa SA, Gallati C, Simone T, Dier E, Yalcin M, Dyskin E, et al. Dual targeting of the antagonistic pathways mediated by Sirt1 and TXNIP as a putative approach to enhance the efficacy of anti-aging interventions. Aging (Albany NY) 2009;1:412–24. doi: 10.18632/aging.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Bodmer R, Bier E, Karin M. Sestrins at the crossroad between stress and aging. Aging (Albany NY) 2010;2:369–74. doi: 10.18632/aging.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–8. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–91. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvanese V, Fraga MF. SirT1 brings stemness closer to cancer and aging. Aging (Albany NY) 2011;3:162–7. doi: 10.18632/aging.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez JA, Cufí S, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Vellon L, et al. Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging (Albany NY) 2011;3:1063–77. doi: 10.18632/aging.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardo PS, Boriek AM. The physiological roles of Sirt1 in skeletal muscle. Aging (Albany NY) 2011;3:430–7. doi: 10.18632/aging.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corominas-Faja B, Quirantes-Piné R, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Martin-Castillo B, et al. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging (Albany NY) 2012;4:480–98. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget. 2012;3:1061–3. doi: 10.18632/oncotarget.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–58. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany NY) 2009;1:887–902. doi: 10.18632/aging.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese EJ. Hormesis is central to toxicology, pharmacology and risk assessment. Hum Exp Toxicol. 2010;29:249–61. doi: 10.1177/0960327109363973. [DOI] [PubMed] [Google Scholar]
- 27.Kendig EL, Le HH, Belcher SM. Defining hormesis: evaluation of a complex concentration response phenomenon. Int J Toxicol. 2010;29:235–46. doi: 10.1177/1091581810363012. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY) 2011;3:821–8. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13:215–35. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- 31.Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–5. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 32.Rattan SIS. Anti-ageing strategies: prevention or therapy? Showing ageing from within. EMBO Rep. 2005;6(Spec No):S25–9. doi: 10.1038/sj.embor.7400401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rattan SI. Hormetic modulation of aging and longevity by mild heat stress. Dose Response. 2005;3:533–46. doi: 10.2203/dose-response.003.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masoro EJ. Role of hormesis in life extension by caloric restriction. Dose Response. 2007;5:163–73. doi: 10.2203/dose-response.06-005.Masoro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yashin AI. Hormesis against aging and diseases: using properties of biological adaptation for health and survival improvement. Dose Response. 2009;8:41–7. doi: 10.2203/dose-response.09-024.Yashin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, et al. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta. 2012;1822:753–83. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese EJ. Hormesis: Toxicological foundations and role in aging research. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.02.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–36. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–19. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9:4474–6. doi: 10.4161/cc.9.22.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blagosklonny MV. Rapamycin-induced glucose intolerance: hunger or starvation diabetes. Cell Cycle. 2011;10:4217–24. doi: 10.4161/cc.10.24.18595. [DOI] [PubMed] [Google Scholar]
- 44.Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012;4:350–8. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang D, Liu Y, Chen D. SIRT-ain relief from age-inducing stress. Aging (Albany NY) 2011;3:158–61. doi: 10.18632/aging.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 2011;3:223–36. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY) 2011;3:374–9. doi: 10.18632/aging.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–40. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–16. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 50.Chung JH. Using PDE inhibitors to harness the benefits of calorie restriction: lessons from resveratrol. Aging (Albany NY) 2012;4:144–5. doi: 10.18632/aging.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, et al. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9:372–82. doi: 10.1111/j.1474-9726.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- 53.Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760–74. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–9. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 55.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martín-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–20. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson HJ, McTiernan A. Weight cycling and cancer: weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res (Phila) 2011;4:1736–42. doi: 10.1158/1940-6207.CAPR-11-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erkekol FO, Celik GE, Keskin O, Güllü E, Mungan D, Misirligil Z. Fasting: an important issue in asthma management compliance. Ann Allergy Asthma Immunol. 2006;97:370–4. doi: 10.1016/S1081-1206(10)60803-4. [DOI] [PubMed] [Google Scholar]
- 59.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:24ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7:e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kouda K, Iki M. Beneficial effects of mild stress (hormetic effects): dietary restriction and health. J Physiol Anthropol. 2010;29:127–32. doi: 10.2114/jpa2.29.127. [DOI] [PubMed] [Google Scholar]
- 62.Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–9. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- 63.Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–91. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooper PL, Hooper PL, Tytell M, Vígh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–70. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–46. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surh YJ. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann N Y Acad Sci. 2011;1229:1–6. doi: 10.1111/j.1749-6632.2011.06097.x. [DOI] [PubMed] [Google Scholar]
- 67.Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81(Suppl):313S–6S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 68.Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 69.Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–77. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 70.Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45:763–71. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Klappan AK, Hones S, Mylonas I, Brüning A. Proteasome inhibition by quercetin triggers macroautophagy and blocks mTOR activity. Histochem Cell Biol. 2012;137:25–36. doi: 10.1007/s00418-011-0869-0. [DOI] [PubMed] [Google Scholar]
- 72.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1:515–28. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saul N, Pietsch K, Menzel R, Steinberg CE. Quercetin-mediated longevity in Caenorhabditis elegans: is DAF-16 involved? Mech Ageing Dev. 2008;129:611–3. doi: 10.1016/j.mad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Speciale A, Chirafisi J, Saija A, Cimino F. Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med. 2011;11:770–89. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- 76.Rockenfeller P, Madeo F. Ageing and eating. Biochim Biophys Acta. 2010;1803:499–506. doi: 10.1016/j.bbamcr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Rattan SI, Ali RE. Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann N Y Acad Sci. 2007;1100:424–30. doi: 10.1196/annals.1395.047. [DOI] [PubMed] [Google Scholar]
- 79.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–8. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiang L, Nakamura Y, Lim YM, Yamasaki Y, Kurokawa-Nose Y, Maruyama W, et al. Tetrahydrocurcumin extends life span and inhibits the oxidative stress response by regulating the FOXO forkhead transcription factor. Aging (Albany NY) 2011;3:1098–109. doi: 10.18632/aging.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pramanik D, Campbell NR, Das S, Gupta S, Chenna V, Bisht S, et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget. 2012;3:640–50. doi: 10.18632/oncotarget.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J Cell Mol Med. 2010;14:2543–51. doi: 10.1111/j.1582-4934.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sikora E, Bielak-Zmijewska A, Mosieniak G, Piwocka K. The promise of slow down ageing may come from curcumin. Curr Pharm Des. 2010;16:884–92. doi: 10.2174/138161210790883507. [DOI] [PubMed] [Google Scholar]
- 85.Queen BL, Tollefsbol TO. Polyphenols and aging. Curr Aging Sci. 2010;3:34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 87.Liu BL, Zhang X, Zhang W, Zhen HN. New enlightenment of French Paradox: resveratrol’s potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007;6:1833–6. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- 88.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 89.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 90.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 94.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–43. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 95.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–52. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 96.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 97.Blagosklonny MV. Inhibition of S6K by resveratrol: in search of the purpose. Aging (Albany NY) 2009;1:511–4. doi: 10.18632/aging.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8:1901–4. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 99.Rascón B, Hubbard BP, Sinclair DA, Amdam GV. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging (Albany NY) 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–58. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, et al. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 2009;1:961–70. doi: 10.18632/aging.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hofseth LJ, Singh UP, Singh NP, Nagarkatti M, Nagarkatti PS. Taming the beast within: resveratrol suppresses colitis and prevents colon cancer. Aging (Albany NY) 2010;2:183–4. doi: 10.18632/aging.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vetterli L, Maechler P. Resveratrol-activated SIRT1 in liver and pancreatic β-cells: a Janus head looking to the same direction of metabolic homeostasis. Aging (Albany NY) 2011;3:444–9. doi: 10.18632/aging.100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antosh M, Fox D, Helfand SL, Cooper LN, Neretti N. New comparative genomics approach reveals a conserved health span signature across species. Aging (Albany NY) 2011;3:576–83. doi: 10.18632/aging.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung JH. Using PDE inhibitors to harness the benefits of calorie restriction: lessons from resveratrol. Aging (Albany NY) 2012;4:144–5. doi: 10.18632/aging.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–6. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 108.Do GM, Jung UJ, Park HJ, Kwon EY, Jeon SM, McGregor RA, et al. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282–91. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- 109.Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, et al. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem. 2012;23:1100–12. doi: 10.1016/j.jnutbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 110.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu CL, Yen GC. Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008;52:53–61. doi: 10.1002/mnfr.200700393. [DOI] [PubMed] [Google Scholar]
- 112.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–43. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 113.Cicerale S, Conlan XA, Sinclair AJ, Keast RS. Chemistry and health of olive oil phenolics. Crit Rev Food Sci Nutr. 2009;49:218–36. doi: 10.1080/10408390701856223. [DOI] [PubMed] [Google Scholar]
- 114.Colomer R, Menéndez JA. Mediterranean diet, olive oil and cancer. Clin Transl Oncol. 2006;8:15–21. doi: 10.1007/s12094-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 115.Menendez JA, Lupu R. Mediterranean dietary traditions for the molecular treatment of human cancer: anti-oncogenic actions of the main olive oil’s monounsaturated fatty acid oleic acid (18:1n-9) Curr Pharm Biotechnol. 2006;7:495–502. doi: 10.2174/138920106779116900. [DOI] [PubMed] [Google Scholar]
- 116.Escrich E, Moral R, Grau L, Costa I, Solanas M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol Nutr Food Res. 2007;51:1279–92. doi: 10.1002/mnfr.200700213. [DOI] [PubMed] [Google Scholar]
- 117.Colomer R, Lupu R, Papadimitropoulou A, Vellón L, Vázquez-Martín A, Brunet J, et al. Giacomo Castelvetro’s salads. Anti-HER2 oncogene nutraceuticals since the 17th century? Clin Transl Oncol. 2008;10:30–4. doi: 10.1007/s12094-008-0151-7. [DOI] [PubMed] [Google Scholar]
- 118.López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284–94. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 119.Escrich E, Solanas M, Moral R, Costa I, Grau L. Are the olive oil and other dietary lipids related to cancer? Experimental evidence. Clin Transl Oncol. 2006;8:868–83. doi: 10.1007/s12094-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 120.Galli C, Visioli F. Antioxidant and other activities of phenolics in olives/olive oil, typical components of the Mediterranean diet. Lipids. 1999;34:23–6. doi: 10.1007/BF02562224. [DOI] [PubMed] [Google Scholar]
- 121.Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer. 2000;36:1235–47. doi: 10.1016/S0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 122.Owen RW, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin Chem. 2000;46:976–88. [PubMed] [Google Scholar]
- 123.Visioli F, Galli C. Phenolics from olive oil and its waste products. Biological activities in in vitro and in vivo studies. World Rev Nutr Diet. 2001;88:233–7. doi: 10.1159/000059757. [DOI] [PubMed] [Google Scholar]
- 124.Visioli F, Poli A, Gall C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev. 2002;22:65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 125.Visioli F, Galli C. Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr. 2002;42:209–21. doi: 10.1080/10408690290825529. [DOI] [PubMed] [Google Scholar]
- 126.Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, et al. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–6. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- 127.Carrasco-Pancorbo A, Cerretani L, Bendini A, Segura-Carretero A, Gallina-Toschi T, Fernandez-Gutiérrez A. Analytical determination of polyphenols in olive oils. J Sep Sci. 2005;28:837–58. doi: 10.1002/jssc.200500032. [DOI] [PubMed] [Google Scholar]
- 128.Bendini A, Cerretani L, Carrasco-Pancorbo A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, et al. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007;12:1679–719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.García-Villalba R, Carrasco-Pancorbo A, Oliveras-Ferraros C, Vázquez-Martín A, Menéndez JA, Segura-Carretero A, et al. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J Pharm Biomed Anal. 2010;51:416–29. doi: 10.1016/j.jpba.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 130.García-Villalba R, Carrasco-Pancorbo A, Vázquez-Martín A, Oliveras-Ferraros C, Menéndez JA, Segura-Carretero A, et al. A 2-D-HPLC-CE platform coupled to ESI-TOF-MS to characterize the phenolic fraction in olive oil. Electrophoresis. 2009;30:2688–701. doi: 10.1002/elps.200800807. [DOI] [PubMed] [Google Scholar]
- 131.Owen RW, Giacosa A, Hull WE, Haubner R, Würtele G, Spiegelhalder B, et al. Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol. 2000;1:107–12. doi: 10.1016/S1470-2045(00)00015-2. [DOI] [PubMed] [Google Scholar]
- 132.Servili M, Esposto S, Fabiani R, Urbani S, Taticchi A, Mariucci F, et al. Phenolic compounds in olive oil: antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- 133.Raederstorff D. Antioxidant activity of olive polyphenols in humans: a review. Int J Vitam Nutr Res. 2009;79:152–65. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- 134.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 135.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–89. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 136.Way TD, Kao MC, Lin JK. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cells. FEBS Lett. 2005;579:145–52. doi: 10.1016/j.febslet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 137.Shimizu M, Deguchi A, Joe AK, Mckoy JF, Moriwaki H, Weinstein IB. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J Exp Ther Oncol. 2005;5:69–78. [PubMed] [Google Scholar]
- 138.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–53. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 139.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 140.Chiang CT, Way TD, Lin JK. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol Cancer Ther. 2007;6:2127–38. doi: 10.1158/1535-7163.MCT-07-0107. [DOI] [PubMed] [Google Scholar]
- 141.Menendez JA, Vazquez-Martin A, Colomer R, Brunet J, Carrasco-Pancorbo A, Garcia-Villalba R, et al. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer. 2007;7:80. doi: 10.1186/1471-2407-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menendez JA, Vazquez-Martin A, Oliveras-Ferraros C, Garcia-Villalba R, Carrasco-Pancorbo A, Fernandez-Gutierrez A, et al. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int J Mol Med. 2008;22:433–9. [PubMed] [Google Scholar]
- 143.Menendez JA, Vazquez-Martin A, Oliveras-Ferraros C, Garcia-Villalba R, Carrasco-Pancorbo A, Fernandez-Gutierrez A, et al. Extra-virgin olive oil polyphenols inhibit HER2 (erbB-2)-induced malignant transformation in human breast epithelial cells: relationship between the chemical structures of extra-virgin olive oil secoiridoids and lignans and their inhibitory activities on the tyrosine kinase activity of HER2. Int J Oncol. 2009;34:43–51. [PubMed] [Google Scholar]
- 144.Menendez JA, Vazquez-Martin A, Garcia-Villalba R, Carrasco-Pancorbo A, Oliveras-Ferraros C, Fernandez-Gutierrez A, et al. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra-Virgin Olive Oil (EVOO) BMC Cancer. 2008;8:377. doi: 10.1186/1471-2407-8-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lozano-Sánchez J, Segura-Carretero A, Menendez JA, Oliveras-Ferraros C, Cerretani L, Fernández-Gutiérrez A. Prediction of extra virgin olive oil varieties through their phenolic profile. Potential cytotoxic activity against human breast cancer cells. J Agric Food Chem. 2010;58:9942–55. doi: 10.1021/jf101502q. [DOI] [PubMed] [Google Scholar]
- 146.Oliveras-Ferraros C, Fernández-Arroyo S, Vazquez-Martin A, Lozano-Sánchez J, Cufí S, Joven J, et al. Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2-targeting drugs by inducing GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of Histone H3. Int J Oncol. 2011;38:1533–47. doi: 10.3892/ijo.2011.993. [DOI] [PubMed] [Google Scholar]
- 147.Vazquez-Martin A, Fernández-Arroyo S, Cufí S, Oliveras-Ferraros C, Lozano-Sánchez J, Vellón L, et al. Phenolic secoiridoids in extra virgin olive oil impede fibrogenic and oncogenic epithelial-to-mesenchymal transition: extra virgin olive oil as a source of novel antiaging phytochemicals. Rejuvenation Res. 2012;15:3–21. doi: 10.1089/rej.2011.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.García-Villalba R, Carrasco-Pancorbo A, Oliveras-Ferraros C, Menéndez JA, Segura-Carretero A, Fernández-Gutiérrez A. Uptake and metabolism of olive oil polyphenols in human breast cancer cells using nano-liquid chromatography coupled to electrospray ionization-time of flight-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;898:69–77. doi: 10.1016/j.jchromb.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 149.Roldán C, de la Torre A, Mota S, Morales-Soto A, Menéndez J, Segura-Carretero A. Identification of active compounds in vegetal extracts based on correlation between activity and HPLC-MS data. Food Chem. 2013;136:392–9. doi: 10.1016/j.foodchem.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 150.Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br J Nutr. 2011;106:1581–91. doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
- 151.Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, et al. Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr. 2006;96:384–92. doi: 10.1079/BJN20061824. [DOI] [PubMed] [Google Scholar]
- 152.Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocké MC, Peeters PH, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330:991. doi: 10.1136/bmj.38415.644155.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trichopoulou A, Dilis V. Olive oil and longevity. Mol Nutr Food Res. 2007;51:1275–8. doi: 10.1002/mnfr.200700134. [DOI] [PubMed] [Google Scholar]
- 154.Trichopoulou A. Traditional Mediterranean diet and longevity in the elderly: a review. Public Health Nutr. 2004;7:943–7. doi: 10.1079/PHN2004558. [DOI] [PubMed] [Google Scholar]
- 155.Trichopoulou A, Critselis E. Mediterranean diet and longevity. Eur J Cancer Prev. 2004;13:453–6. doi: 10.1097/00008469-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 156.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 157.Frankel EN. Nutritional and biological properties of extra virgin olive oil. J Agric Food Chem. 2011;59:785–92. doi: 10.1021/jf103813t. [DOI] [PubMed] [Google Scholar]
- 158.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–54. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 159.Hunt PR, Son TG, Wilson MA, Yu QS, Wood WH, Zhang Y, et al. Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One. 2011;6:e21922. doi: 10.1371/journal.pone.0021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hwang AB, Lee SJ. Regulation of life span by mitochondrial respiration: the HIF-1 and ROS connection. Aging (Albany NY) 2011;3:304–10. doi: 10.18632/aging.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic Biol Med. 2004;37:239–50. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 162.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–41. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gems D, Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–7. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- 165.Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Edman U, Garcia AM, Busuttil RA, Sorensen D, Lundell M, Kapahi P, et al. Lifespan extension by dietary restriction is not linked to protection against somatic DNA damage in Drosophila melanogaster. Aging Cell. 2009;8:331–8. doi: 10.1111/j.1474-9726.2009.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Raamsdonk JM, Meng Y, Camp D, Yang W, Jia X, Bénard C, et al. Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics. 2010;185:559–71. doi: 10.1534/genetics.110.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Van Raamsdonk JM, Hekimi S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid Redox Signal. 2010;13:1911–53. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 169.Yang W, Li J, Hekimi S. A Measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics. 2007;177:2063–74. doi: 10.1534/genetics.107.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guachalla LM, Rudolph KL. ROS induced DNA damage and checkpoint responses: influences on aging? Cell Cycle. 2010;9:4058–60. doi: 10.4161/cc.9.20.13577. [DOI] [PubMed] [Google Scholar]
- 171.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–76. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sanz A, Fernández-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2010;2:200–23. doi: 10.18632/aging.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pani G. P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2010;2:514–8. doi: 10.18632/aging.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, et al. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med. 2011;51:1575–82. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Speakman JR, Selman C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–9. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- 177.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–6. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rodriguez KA, Wywial E, Perez VI, Lambert AJ, Edrey YH, Lewis KN, et al. Walking the oxidative stress tightrope: a perspective from the naked mole-rat, the longest-living rodent. Curr Pharm Des. 2011;17:2290–307. doi: 10.2174/138161211797052457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cañuelo A, Gilbert-López B, Pacheco-Liñán P, Martínez-Lara E, Siles E, Miranda-Vizuete A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech Ageing Dev. 2012;133:563–74. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 180.Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–62. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brocchieri L, Conway de Macario E, Macario AJ. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 184.Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV. Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget. 2011;2:209–21. doi: 10.18632/oncotarget.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leung TKC, Rajendran MY, Monfries C, Hall C, Lim L. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B’) and isolation of its cDNA and genomic DNA. Biochem J. 1990;267:125–32. doi: 10.1042/bj2670125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leung TKC, Hall C, Rajendran M, Spurr NK, Lim L. The human heat-shock genes HSPA6 and HSPA7 are both expressed and localize to chromosome 1. Genomics. 1992;12:74–9. doi: 10.1016/0888-7543(92)90409-L. [DOI] [PubMed] [Google Scholar]
- 187.Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B’ regulation and function. Cell Stress Chaperones. 2007;12:219–29. doi: 10.1379/CSC-278.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jäättelä M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–82. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–51. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 190.Sargent CA, Dunham I, Trowsdale J, Campbell RD. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989;86:1968–72. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Son WY, Hwang SH, Han CT, Lee JH, Kim S, Kim YC. Specific expression of heat shock protein HspA2 in human male germ cells. Mol Hum Reprod. 1999;5:1122–6. doi: 10.1093/molehr/5.12.1122. [DOI] [PubMed] [Google Scholar]
- 192.Govin J, Caron C, Escoffier E, Ferro M, Kuhn L, Rousseaux S, et al. Post-meiotic shifts in HSPA2/HSP70.2 chaperone activity during mouse spermatogenesis. J Biol Chem. 2006;281:37888–92. doi: 10.1074/jbc.M608147200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhu D, Dix DJ, Eddy EM. HSP70-2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development. 1997;124:3007–14. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]
- 194.Feng HL, Sandlow JI, Sparks AET. Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertil Steril. 2001;76:1136–9. doi: 10.1016/S0015-0282(01)02892-8. [DOI] [PubMed] [Google Scholar]
- 195.Svärd M, Biterova EI, Bourhis JM, Guy JE. The crystal structure of the human co-chaperone P58(IPK) PLoS One. 2011;6:e22337. doi: 10.1371/journal.pone.0022337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A. 2002;99:15920–5. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wiseman RL, Haynes CM, Ron D. SnapShot: The unfolded protein response. Cell. 2010;140:590–590, e2. doi: 10.1016/j.cell.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 198.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 199.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66(Suppl 1):S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 200.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Endo S, Hiramatsu N, Hayakawa K, Okamura M, Kasai A, Tagawa Y, et al. Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol. 2007;72:1337–48. doi: 10.1124/mol.107.039164. [DOI] [PubMed] [Google Scholar]
- 202.Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123:3849–55. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 203.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- 205.Liu BQ, Gao YY, Niu XF, Xie JS, Meng X, Guan Y, et al. Implication of unfolded protein response in resveratrol-induced inhibition of K562 cell proliferation. Biochem Biophys Res Commun. 2010;391:778–82. doi: 10.1016/j.bbrc.2009.11.137. [DOI] [PubMed] [Google Scholar]
- 206.Yan Y, Gao YY, Liu BQ, Niu XF, Zhuang Y, Wang HQ. Resveratrol-induced cytotoxicity in human Burkitt’s lymphoma cells is coupled to the unfolded protein response. BMC Cancer. 2010;10:445. doi: 10.1186/1471-2407-10-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Woo KJ, Lee TJ, Lee SH, Lee JM, Seo JH, Jeong YJ, et al. Elevated gadd153/chop expression during resveratrol-induced apoptosis in human colon cancer cells. Biochem Pharmacol. 2007;73:68–76. doi: 10.1016/j.bcp.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 208.Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–15. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 209.Chinta SJ, Poksay KS, Kaundinya G, Hart M, Bredesen DE, Andersen JK, et al. Endoplasmic reticulum stress-induced cell death in dopaminergic cells: effect of resveratrol. J Mol Neurosci. 2009;39:157–68. doi: 10.1007/s12031-008-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441–8. doi: 10.1196/annals.1397.047. [DOI] [PubMed] [Google Scholar]
- 211.Heiss EH, Schilder YD, Dirsch VM. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J Biol Chem. 2007;282:26759–66. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 212.Low IC, Chen ZX, Pervaiz S. Bcl-2 modulates resveratrol-induced ROS production by regulating mitochondrial respiration in tumor cells. Antioxid Redox Signal. 2010;13:807–19. doi: 10.1089/ars.2009.3050. [DOI] [PubMed] [Google Scholar]
- 213.Hussain AR, Uddin S, Bu R, Khan OS, Ahmed SO, Ahmed M, et al. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS One. 2011;6:e24703. doi: 10.1371/journal.pone.0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 215.Khanal P, Oh WK, Yun HJ, Namgoong GM, Ahn SG, Kwon SM, et al. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis. 2011;32:545–53. doi: 10.1093/carcin/bgr001. [DOI] [PubMed] [Google Scholar]
- 216.Hao J, Shen W, Yu G, Jia H, Li X, Feng Z, et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem. 2010;21:634–44. doi: 10.1016/j.jnutbio.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 217.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wolter F, Turchanowa L, Stein J. Resveratrol-induced modification of polyamine metabolism is accompanied by induction of c-Fos. Carcinogenesis. 2003;24:469–74. doi: 10.1093/carcin/24.3.469. [DOI] [PubMed] [Google Scholar]
- 219.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen A, Davis BH, Bissonnette M, Scaglione-Sewell B, Brasitus TA. 1,25-Dihydroxyvitamin D(3) stimulates activator protein-1-dependent Caco-2 cell differentiation. J Biol Chem. 1999;274:35505–13. doi: 10.1074/jbc.274.50.35505. [DOI] [PubMed] [Google Scholar]
- 221.Souleimani A, Asselin C. Regulation of C-fos expression by sodium butyrate in the human colon carcinoma cell line Caco-2. Biochem Biophys Res Commun. 1993;193:330–6. doi: 10.1006/bbrc.1993.1628. [DOI] [PubMed] [Google Scholar]
- 222.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–32. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 224.Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160–2. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 225.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–6. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 226.Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–29. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mariño G, Morselli E, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, et al. Longevity-relevant regulation of autophagy at the level of the acetylproteome. Autophagy. 2011;7:647–9. doi: 10.4161/auto.7.6.15191. [DOI] [PubMed] [Google Scholar]
- 228.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–95. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 229.Bennetzen MV, Mariño G, Pultz D, Morselli E, Færgeman NJ, Kroemer G, et al. Phosphoproteomic analysis of cells treated with longevity-related autophagy inducers. Cell Cycle. 2012;11:1827–40. doi: 10.4161/cc.20233. [DOI] [PubMed] [Google Scholar]
- 230.Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, et al. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bauer MA, Carmona-Gutiérrez D, Ruckenstuhl C, Reisenbichler A, Megalou EV, Eisenberg T, et al. Spermidine promotes mating and fertilization efficiency in model organisms. Cell Cycle. 2012;12 doi: 10.4161/cc.23199. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–5. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 233.Nelson LE, Valentine RJ, Cacicedo JM, Gauthier MS, Ido Y, Ruderman NB. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. Am J Physiol Cell Physiol. 2012;303:C4–13. doi: 10.1152/ajpcell.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–23. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–35. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Herranz D, Serrano M. Impact of Sirt1 on mammalian aging. Aging (Albany NY) 2010;2:315–6. doi: 10.18632/aging.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819–23. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 241.Menendez JA, Vellon L, Oliveras-Ferraros C, Cufí S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle. 2011;10:3658–77. doi: 10.4161/cc.10.21.18128. [DOI] [PubMed] [Google Scholar]
- 242.Menendez JA, Cufí S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. Gerosuppressant metformin: less is more. Aging (Albany NY) 2011;3:348–62. doi: 10.18632/aging.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Quirantes R, Segura-Carretero A, Micol V, et al. Metformin lowers the threshold for stress-induced senescence: a role for the microRNA-200 family and miR-205. Cell Cycle. 2012;11:1235–46. doi: 10.4161/cc.11.6.19665. [DOI] [PubMed] [Google Scholar]
- 244.Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, et al. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–89. doi: 10.4161/cc.11.5.19450. [DOI] [PubMed] [Google Scholar]
- 245.Vazquez-Martin A, Corominas-Faja B, Cufí S, Vellon L, Oliveras-Ferraros C, Menendez OJ, et al. The mitochondrial H (+) -ATP synthase and the lipogenic switch: New core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle. 2012;12 doi: 10.4161/cc.23352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lai Y, Yip GW, Bay BH. Targeting metallothionein for prognosis and treatment of breast cancer. Recent Pat Anticancer Drug Discov. 2011;6:178–85. doi: 10.2174/157489211795328495. [DOI] [PubMed] [Google Scholar]
- 247.Bay BH, Jin R, Huang J, Tan PH. Metallothionein as a prognostic biomarker in breast cancer. Exp Biol Med (Maywood) 2006;231:1516–21. doi: 10.1177/153537020623100910. [DOI] [PubMed] [Google Scholar]
- 248.Eckschlager T, Adam V, Hrabeta J, Figova K, Kizek R. Metallothioneins and cancer. Curr Protein Pept Sci. 2009;10:360–75. doi: 10.2174/138920309788922243. [DOI] [PubMed] [Google Scholar]
- 249.Pedersen MØ, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 250.Nielsen AE, Bohr A, Penkowa M. The Balance between Life and Death of Cells: Roles of Metallothioneins. Biomark Insights. 2007;1:99–111. [PMC free article] [PubMed] [Google Scholar]
- 251.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Eirew P, Kannan N, Knapp DJ, Vaillant F, Emerman JT, Lindeman GJ, et al. Aldehyde dehydrogenase activity is a biomarker of primitive normal human mammary luminal cells. Stem Cells. 2012;30:344–8. doi: 10.1002/stem.1001. [DOI] [PubMed] [Google Scholar]
- 253.Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 254.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–84. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 255.Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–24. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- 256.Chan CH, Lee SW, Wang J, Lin HK. Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal. 2010;10:1001–15. doi: 10.1100/tsw.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang Z, Fukushima H, Inuzuka H, Wan L, Liu P, Gao D, et al. Skp2 is a promising therapeutic target in breast cancer. Front Oncol. 2012;1:18702. doi: 10.3389/fonc.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang Z, Inuzuka H, Zhong J, Liu P, Sarkar FH, Sun Y, et al. Identification of acetylation-dependent regulatory mechanisms that govern the oncogenic functions of Skp2. Oncotarget. 2012;3:1294–300. doi: 10.18632/oncotarget.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–9. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-β-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem. 2011;59:6765–75. doi: 10.1021/jf201096v. [DOI] [PubMed] [Google Scholar]
- 261.Huang HC, Way TD, Lin CL, Lin JK. EGCG stabilizes p27kip1 in E2-stimulated MCF-7 cells through down-regulation of the Skp2 protein. Endocrinology. 2008;149:5972–83. doi: 10.1210/en.2008-0408. [DOI] [PubMed] [Google Scholar]
- 262.Hsu JD, Kao SH, Ou TT, Chen YJ, Li YJ, Wang CJ. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27(Kip1) attributed to disruption of p27(Kip1)/Skp2 complex. J Agric Food Chem. 2011;59:1996–2003. doi: 10.1021/jf103656v. [DOI] [PubMed] [Google Scholar]
- 263.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 264.Everse J, Kaplan NO. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- 265.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 266.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 267.Serganova I, Rizwan A, Ni X, Thakur SB, Vider J, Russell J, et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res. 2011;17:6250–61. doi: 10.1158/1078-0432.CCR-11-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10:1271–86. doi: 10.4161/cc.10.8.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Balliet RM, Capparelli C, Guido C, Pestell TG, Martinez-Outschoorn UE, Lin Z, et al. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–73. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–35. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Guido C, Whitaker-Menezes D, Lin Z, Pestell RG, Howell A, Zimmers TA, et al. Mitochondrial fission induces glycolytic reprogramming in cancer-associated myofibroblasts, driving stromal lactate production, and early tumor growth. Oncotarget. 2012;3:798–810. doi: 10.18632/oncotarget.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Carito V, Bonuccelli G, Martinez-Outschoorn UE, Whitaker-Menezes D, Caroleo MC, Cione E, et al. Metabolic remodeling of the tumor microenvironment: migration stimulating factor (MSF) reprograms myofibroblasts toward lactate production, fueling anabolic tumor growth. Cell Cycle. 2012;11:3403–14. doi: 10.4161/cc.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–74. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 275.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 280.Wietzke JA, Welsh J. Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1,25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol. 2003;84:149–57. doi: 10.1016/S0960-0760(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 281.Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaissé JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–52. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- 282.Hayes DP. Resveratrol and vitamin D: significant potential interpretative problems arising from their mutual processes, interactions and effects. Med Hypotheses. 2011;77:765–72. doi: 10.1016/j.mehy.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 283.Singh CK, Kumar A, LaVoie HA, DiPette DJ, Singh US. Resveratrol prevents impairment in activation of retinoic acid receptors and MAP kinases in the embryos of a rodent model of diabetic embryopathy. Reprod Sci. 2012;19:949–61. doi: 10.1177/1933719112438972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Stefanska B, Salamé P, Bednarek A, Fabianowska-Majewska K. Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br J Nutr. 2012;107:781–90. doi: 10.1017/S0007114511003631. [DOI] [PubMed] [Google Scholar]
- 285.Huang C, Ma WY, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20:237–42. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 286.Lu J, Ho CH, Ghai G, Chen KY. Resveratrol analog, 3,4,5,4′-tetrahydroxystilbene, differentially induces pro-apoptotic p53/Bax gene expression and inhibits the growth of transformed cells but not their normal counterparts. Carcinogenesis. 2001;22:321–8. doi: 10.1093/carcin/22.2.321. [DOI] [PubMed] [Google Scholar]
- 287.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–10. [PubMed] [Google Scholar]
- 288.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–34. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 289.Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80:134–43. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 290.Gosslau A, Pabbaraja S, Knapp S, Chen KY. Trans- and cis-stilbene polyphenols induced rapid perinuclear mitochondrial clustering and p53-independent apoptosis in cancer cells but not normal cells. Eur J Pharmacol. 2008;587:25–34. doi: 10.1016/j.ejphar.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 291.Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–83. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 292.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 293.Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–76. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- 294.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–73. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 295.Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–9. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 296.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–88. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 297.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 298.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bishayee A, Barnes KF, Bhatia D, Darvesh AS, Carroll RT. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res (Phila) 2010;3:753–63. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 300.Sahin K, Orhan C, Akdemir F, Tuzcu M, Iben C, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr (Berl) 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 301.He X, Wang L, Szklarz G, Bi Y, Ma Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J Pharmacol Exp Ther. 2012;342:81–90. doi: 10.1124/jpet.112.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cheng AS, Cheng YH, Chiou CH, Chang TL. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem. 2012;60:9180–7. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- 303.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–30. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Martín-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–20. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bayram B, Ozcelik B, Grimm S, Roeder T, Schrader C, Ernst IM, et al. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012;15:71–81. doi: 10.1089/rej.2011.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 307.Katsiki M, Chondrogianni N, Chinou I, Rivett AJ, Gonos ES. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10:157–72. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 308.Konno K, Hirayama C, Yasui H, Nakamura M. Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc Natl Acad Sci U S A. 1999;96:9159–64. doi: 10.1073/pnas.96.16.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Báidez AG, Gómez P, Del Río JA, Ortuño A. Dysfunctionality of the xylem in Olea europaea L. Plants associated with the infection process by Verticillium dahliae Kleb. Role of phenolic compounds in plant defense mechanism. J Agric Food Chem. 2007;55:3373–7. doi: 10.1021/jf063166d. [DOI] [PubMed] [Google Scholar]
- 310.Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101:3751–4. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 311.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–6. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 312.Blagosklonny MV. NCI’s provocative questions on cancer: some answers to ignite discussion. Oncotarget. 2011;2:1352–67. doi: 10.18632/oncotarget.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–6. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 314.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–91. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011;3:1130–41. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–65. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Pospelova TV, Leontieva OV, Bykova TV, Zubova SG, Pospelov VA, Blagosklonny MV. Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle. 2012;11:2402–7. doi: 10.4161/cc.20882. [DOI] [PubMed] [Google Scholar]
- 318.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY) 2012;4:709–14. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, et al. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY) 2012;4:715–22. doi: 10.18632/aging.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Leontieva OV, Blagosklonny MV. Hypoxia and gerosuppression: the mTOR saga continues. Cell Cycle. 2012;11:3926–31. doi: 10.4161/cc.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012;4:547–52. doi: 10.18632/aging.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–6. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 323.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–34. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 324.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–90. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2:415–31. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pardo PS, Boriek AM. An autoregulatory loop reverts the mechanosensitive Sirt1 induction by EGR1 in skeletal muscle cells. Aging (Albany NY) 2012;4:456–61. doi: 10.18632/aging.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Purushotham A, Schug TT, Li X. SIRT1 performs a balancing act on the tight-rope toward longevity. Aging (Albany NY) 2009;1:669–73. doi: 10.18632/aging.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Brooks CL, Gu W. Anti-aging protein SIRT1: a role in cervical cancer? Aging (Albany NY) 2009;1:278–80. doi: 10.18632/aging.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Stein S, Schäfer N, Breitenstein A, Besler C, Winnik S, Lohmann C, et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging (Albany NY) 2010;2:353–60. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY) 2010;2:527–34. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yang Z, Ming XF. The vascular SIRTainty. Aging (Albany NY) 2010;2:331–2. doi: 10.18632/aging.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bolasco G, Calogero R, Carrara M, Banchaabouchi MA, Bilbao D, Mazzoccoli G, et al. Cardioprotective mIGF-1/SIRT1 signaling induces hypertension, leukocytosis and fear response in mice. Aging (Albany NY) 2012;4:402–16. doi: 10.18632/aging.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Halperin-Sheinfeld M, Gertler A, Okun E, Sredni B, Cohen HY. The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes. Aging (Albany NY) 2012;4:436–47. doi: 10.18632/aging.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cantó C, Auwerx J. Interference between PARPs and SIRT1: a novel approach to healthy ageing? Aging (Albany NY) 2011;3:543–7. doi: 10.18632/aging.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2010;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Schug TT, Li X. Surprising sirtuin crosstalk in the heart. Aging (Albany NY) 2010;2:129–32. doi: 10.18632/aging.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ramadori G, Coppari R. Does hypothalamic SIRT1 regulate aging? Aging (Albany NY) 2011;3:325–8. doi: 10.18632/aging.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E. SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1,2 and HMGCS1,2. Aging (Albany NY) 2011;3:635–42. doi: 10.18632/aging.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mai A. Identification of specific and semi-specific SIRT inhibitors through computer-aided studies. Aging (Albany NY) 2011;3:819–20. doi: 10.18632/aging.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Schlicker C, Boanca G, Lakshminarasimhan M, Steegborn C. Structure-based development of novel sirtuin inhibitors. Aging (Albany NY) 2011;3:852–72. doi: 10.18632/aging.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Banerjee KK, Ayyub C, Sengupta S, Kolthur-Seetharam U. dSir2 deficiency in the fatbody, but not muscles, affects systemic insulin signaling, fat mobilization and starvation survival in flies. Aging (Albany NY) 2012;4:206–23. doi: 10.18632/aging.100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Naiman S, Kanfi Y, Cohen HY. Sirtuins as regulators of mammalian aging. Aging (Albany NY) 2012;4:521–2. doi: 10.18632/aging.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Antosh M, Fox D, Helfand SL, Cooper LN, Neretti N. New comparative genomics approach reveals a conserved health span signature across species. Aging (Albany NY) 2011;3:576–83. doi: 10.18632/aging.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Borrás C, Monleón D, López-Grueso R, Gambini J, Orlando L, Pallardó FV, et al. RasGrf1 deficiency delays aging in mice. Aging (Albany NY) 2011;3:262–76. doi: 10.18632/aging.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Vendrell A, Posas F. Sir2 plays a key role in cell fate determination upon SAPK activation. Aging (Albany NY) 2011;3:1163–8. doi: 10.18632/aging.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhang D, Liu Y, Chen D. SIRT-ain relief from age-inducing stress. Aging (Albany NY) 2011;3:158–61. doi: 10.18632/aging.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ha CW, Huh WK. The implication of Sir2 in replicative aging and senescence in Saccharomyces cerevisiae. Aging (Albany NY) 2011;3:319–24. doi: 10.18632/aging.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Tucci P. Caloric restriction: is mammalian life extension linked to p53? Aging (Albany NY) 2012;4:525–34. doi: 10.18632/aging.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Burks TN, Cohn RD. One size may not fit all: anti-aging therapies and sarcopenia. Aging (Albany NY) 2011;3:1142–53. doi: 10.18632/aging.100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Panieri E, Toietta G, Mele M, Labate V, Ranieri SC, Fusco S, et al. Nutrient withdrawal rescues growth factor-deprived cells from mTOR-dependent damage. Aging (Albany NY) 2010;2:487–503. doi: 10.18632/aging.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Vazquez-Martin A, Cufi S, Lopez-Bonet E, Corominas-Faja B, Oliveras-Ferraros C, Martin-Castillo B, et al. Metformin limits the tumourigenicity of iPS cells without affecting their pluripotency. Sci Rep. 2012;2:964. doi: 10.1038/srep00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK Is a Negative Regulator of the Warburg Effect and Suppresses Tumor Growth In vivo. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.12.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mariño G, Ugalde AP, Salvador-Montoliu N, Varela I, Quirós PM, Cadiñanos J, et al. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008;17:2196–211. doi: 10.1093/hmg/ddn120. [DOI] [PubMed] [Google Scholar]
- 355.Mariño G, López-Otín C. Autophagy and aging: new lessons from progeroid mice. Autophagy. 2008;4:807–9. doi: 10.4161/auto.6478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.