Introduction
Titin is a giant multi-functional sarcomeric filament that provides passive stiffness to cardiac myocytes. At its N-terminus, titin is embedded in the Z-disk of the sarcomere. The rest of the molecule is divided between an elastic I-band region, a thick filament-binding A-band region and the M-band region where the C-terminus is embedded (Fig. 1A, bottom)1. Titin’s extensible I-band region functions as a molecular spring that develops passive force during diastole when sarcomeres are stretched2. This force is important for centering the A-band in the sarcomere3 and, together with the extracellular matrix, for defining diastolic stiffness2. Other regions of titin (Z-disk, A-band, and M-band) are involved in numerous cellular processes including force-dependent signaling4. Here we discuss recently discovered post-transcriptional and post-translational modifications of titin and address their roles in acquired cardiac disease, including dilated cardiomyopathy (DCM) and heart failure with preserved ejection fraction (HFpEF) (often termed diastolic heart failure). (For the purposes of this paper we restrict the term HFpEF to HF patients with left ventricular (LV) EF > 0.50 in the absence of hypertrophic cardiomyopathy or valvular, infiltrative or pericardial disease.) The review also focusses on recent work that reveals mutations in the titin gene (TTN) as a major source of familial cardiomyopathies, including mutations in titin’s spring region linked to arrhythmogenic right ventricular dysplasia5 and mutations in titin’s A-band region responsible for ~30% of DCM cases6. These findings have given rise to the emerging view that TTN is a major disease gene.
Figure 1.
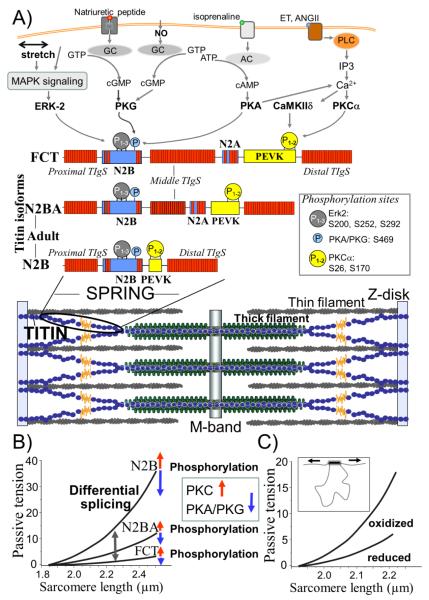
Schematic of titin in the sarcomere (A) and mechanisms for modifying titin-based passive tension (B and C). A) Bottom: Single titin molecules (shown in blue and yellow) span from Z-disk (N-terminus) to M-band (C-terminus). Middle: Composition of extensible I-band region of the N2B, N2BA titin isoforms (found in adults) and the fetal cardiac titin isoform (FCT). Red blocks denote Ig-like domains, blue, unique sequence and yellow, PEVK sequence. Top: Phosphorylation sites in titin’s spring region (the same sites are present in all 3 isoforms) and their upstream signaling pathways (For CamKIIδ sites, see37, 38). Abbreviations: MAPK: mitogen-activated protein kinase; GC: guanylyl cyclase; AC: anelylyl cyclase; NO: nitric oxide; ET: endothelin; ANGII: angiotensin II; IP3: inositol triphosphate. See text for additional abbreviations and details. B) Schematic of force-extension curves of titin isoforms and the effects of phosphorylation on passive tension. C) Schematic of effect of oxidation to form cysteine disulfide crosslinks in the N2B region of titin (inset) and its effect on passive tension.
Titin isoforms and heart disease
The extensible I-band region of titin is comprised of three distinct elements: 1) tandem Ig segments that consist of serially-linked immunoglobulin(Ig)-like domains, 2) the springlike PEVK (containing a high percentage of proline, glutamic acid, valine, and lysine residues), and 3) the N2B element with its extensible unique sequence (N2B-Us) (Fig. 1A, middle)7, 8. Titin is encoded by a single gene. Variable mRNA splice pathways result in distinct titin isoform classes8. In cardiac muscle three classes of titin isoforms are present: adult N2BA, adult N2B, and the fetal cardiac titin (FCT) isoforms (Fig. 1A middle)8-10. These three titin isoform classes differ in their I-band region; the rest of the molecule (Z-disk, A-band, M-line localized regions) is largely identical8. The tandem Ig segments can be visualized as “beads on a string” with folded Ig domains with a diameter of 4-5 nm separated by short peptide linkers11. All isoforms contain a proximal tandem Ig segment (Ig1-15) and a distal tandem Ig segment (Ig84-105)7, 8. The N2BA and FCT titin isoforms also contain a middle tandem Ig segment that contains a variable number of Ig domains (Fig. 1A, middle)9. The N2B element is found in all cardiac titin isoforms. In addition to behaving as a large molecular spring, the N2B-Us is a substrate for various kinases that affect its mechanical properties (see below). While the N2B element is found in both adult cardiac titin isoforms, the N2A element is only found in the N2BA isoform (hence its name) and FCT isoforms 7, 8. Similar to the N2B element, N2A contains Ig domains and a unique sequence that binds the calpain protease p94 and proteins that belong to the muscle ankyrin-repeat protein family (MARPs), which relocate from the I-band of the sarcomere to the nucleus to regulate transcription following mechanical stress12. Like the N2B-Us, the PEVK region of titin also behaves as a molecular spring13. The PEVK sequence is encoded by 114 exons, seven of which are found in the N2B titin isoform while the PEVK region of N2BA titin contains additional exons and is much larger14. The additional Ig domains and PEVK sequence and the inclusion of the N2A element make the N2BA titin isoform larger than the N2B isoform (~3.3 MDa vs. 2.97 MDa)8. The FCT class of titin isoforms (3.5-3.6 MDa,) contains the largest middle tandem Ig segment and the largest PEVK sequence of all the cardiac titin isoforms9.
The force required to stretch a titin molecule depends non-linearly on its fractional extension15. At a given SL the extension of N2B and N2BA titin (physical distance between the end of the thin filament-binding region of titin and beginning of the thick filament-binding region) is the same. However, since N2B titin has a shorter contour length (due to fewer Ig domains, a shorter PEVK segment, and absence of the N2A element), the fractional extension of the N2B isoform is greater16. Therefore, more force is needed to stretch the N2B titin isoform—it is stiffer because it is shorter. For this reason, sarcomeres that express different titin isoforms develop levels of passive force that greatly differ (Fig. 1B).
Adult cardiac muscle co-express N2B and N2BA cardiac titin at the level of the half sarcomere17. The number of titin molecules per thick filament is likely to be constant (6 per half thick filament) but the expression ratio of compliant (N2BA) to stiff (N2B) titin is variable (in human control LV myocardium ~40:6018). Because of the intimate relationship between the size of titin’s I-band region and titin-based passive tension, with larger elastic I-band regions corresponding to lower passive tension, the titin isoform expression ratio in the heart is a crucial determinant of titin-based passive tension (Fig. 1B).
Variable titin expression ratios have been found in patients with cardiac disease. Patients with ischemic cardiomyopathy have been shown to express increased levels of N2BA titin that was accompanied by decreased stiffness at the myofibrillar level 18. Changes in titin isoform expression have also been found in patients with end-stage HF due to non-ischemic DCM, where the compliant N2BA isoform was up-regulated and associated with decreased passive stiffness and increased chamber compliance 19, 20. The study by Nagueh et al19 also suggested a physiological benefit of this change in titin expression via correlation between the titin isoform shift and improved exercise tolerance. Upregulation of compliant titin isoforms has also been found in patients with HFpEF, a group that accounts for about half of all HF cases and is characterized by increased diastolic stiffness21, 22. An adaptive change in isoform expression towards increased expression of compliant titin isoforms also occurs in mice with pathological hypertrophy23 and rats with long-term hypothyroidism24. Overall these studies suggest that upregulation of the more compliant N2BA titin isoform is an important compensatory adaptation to counteract the increased stiffness of the extracellular matrix 19.
Mechanisms that underlie changes in titin isoform expression are not well understood, although a possible breakthrough is the recent discovery of the role of the splice factor RBM2025. Naturally occurring RBM20 mutations in both human patients and in a rat model result in low expression levels of RBM20 and expression of large and highly compliant titin isoforms25. It is thus possible that a reduction in expression level of RBM20 in cardiac disease states underlies the upregulation of compliant titin isoforms. Clearly more work is needed to understand the mechanisms that drive titin isoform expression, with a focus on RBM20, and to explore whether experimentally upregulating complaint titin isoforms can be used to ameliorate increased myocardial stiffness in HF patients.
Post-translational modifications and disease
It is well-known that post-translational modifications (PTMs) of contractile and regulatory proteins greatly affect cardiac function. Recent single molecule force spectroscopy studies of titin have discovered that kinases phosphorylate the extensible region of titin (Fig. 1A, top) and significantly alter the stiffness of the PEVK and N2B-Us spring elements. This allows for rapid adjustment of titin stiffness (Fig. 1B) and adaptations of cardiac performance to meet hemodynamic loads.
The PEVK spring element has been found to be phosphorylated by protein kinase Cα (PKCα) (see Fig. 1A top). PKC is activated by the α1-adrenergic signaling pathway and PKCα, the predominant isozyme in the heart, is a key player in contractile dysfunction and heart failure26, 27; PKCα phosphorylates the PEVK element of titin which leads to increased passive tension 28. The primary sites of phosphorylation are two highly-conserved serine residues (S26 and S170) within the constitutive PEVK element. 28 Phosphorylation of these conserved serine residues reduces the bending rigidity of the PEVK region 29, which is consistent with the increased passive tension in response to PKC phosphorylation seen at the tissue level 28. The link between PKCα, PEVK phosphorylation, and passive tension was further established by a study that showed that PKCα had no effect on passive tension in mice in whom the PEVK sites were genetically removed 30.
The N2B element of titin is also a kinase substrate whose mechanical properties change following phosphorylation. Protein kinase A (PKA), which is stimulated by the β-adrenergic pathway, reduces passive tension in cardiac myocytes 31, 32 (see Fig. 1B). A more pronounced effect is present when PP1 de-phosphorylation is performed prior to PKA treatment, which indicates that the basal level of phosphorylation plays an important role in determining passive tension.
Similar to PKA, protein kinase G, a cGMP-dependent kinase that is part of signaling cascades initiated by nitric oxide (NO) and natriuretic peptides (NPs), phosphorylates the unique sequence of the N2B element and reduces passive tension; the PKG phosphorylation site (S469) is also a residue targeted by PKA 33, see Fig. 1A, top.
Whether the basal PKA/PKG phosphorylation level of titin is altered in cardiac disease has been addressed in several recent studies. Comparing end-stage DCM patients with non-failing donor hearts revealed a trend towards a reduced basal level of phosphorylation of the PKA/PKG sites 33. Another study employing endomyocardial biopsies also provided evidence for hypo-phosphorylation of titin in patients with both HFpEF and DCM; mechanical experiments revealed increased passive tension of cardiac myocytes that was partially normalized after PKA or PKG treatment of the cells21. However, passive tension was not fully normalized by either PKA or PKG phosphorylation and remained higher than in controls (considering the aforementioned titin isoform shift toward the more compliant N2BA isoform in HFpEF, passive tension was expected to be lower than in the controls21, 22). This higher passive tension following normalization of the PKA/PKG phosphorylation sites of titin could be explained by a change in the basal phosphorylation level of the PKCα sites found in the PEVK spring, but this was not investigated. Support for the idea that titin’s PKCα sites may be at play was provided by a study in mice with increased after-load induced heart failure where PEVK’sS26 was hyperphoshorylated relative to sham controls and, importantly, PP1 treatment normalized the phosphorylation level as well as the passive tension23.
A recently discovered novel phosphorylation pathway involves the extracellular-signal-regulated kinase- 2 (ERK2), that phosphorylates the N2B-Us at 3 conserved serines34(see Fig. 1A, top). It was surmised that ERK2-based phosphorylation lowers titin-based passive tension (increased compliance), but experimental evidence for this proposal is still required. Furthermore ERK2 phosphorylation was shown to be inhibited by binding of the 4 and a half LIM protein 1 (FHL1) to the N2B-Us34. FHL1 has previously been shown to bind to the N2B-Us and assemble a stretch sensing signalosome that consists of components of the mitogen activated signaling pathway35. These new findings suggest a possible link between stretch sensing and phosphorylation based regulation of passive stiffness. Another novel pathway involves CaMKII, a Ca2+ and calmodulin dependent serine/threonine kinase that is activated by increases in cellular Ca2+. Four isoforms have been described (α, β, γ, and δ) of which CaMKIIδ is the predominant isoform in the heart36. Hidalgo and colleagues have shown that CaMKIIδ phosphorylates titin in skinned and intact myocardium and that the titin N2B and PEVK spring elements, but not Ig domains are phosphorylated by CaMKIIδ37, 38. Furthermore, the phosphorylation sites overlap with the PKC sites (including the PKC sites S26 and S170 of the PEVK element, see Fig. 1A, top)38. The effect of CaMKIIδ phosphorylation of the PEVK sites is likely to be similar to that reported for PKC phosphorylation, i.e., an increase in passive tension and that of phosphorylation of the N2B element a reduction in passive tension; Western blot studies with phosho-specific antibodies suggest that CaMKIIδ phosphorylation of the N2B element might be the dominant process 38. Considering that the ERK2 and CaMKIIδ signaling pathways play important roles in cardiac health and disease36, 39, additional research is warranted that focusses on the roles of ERK2 and CaMKIIδ phosphorylation of titin.
The mechanical properties of the N2B-Us can be altered by more than just phosphorylation status. For example, there are six cysteine residues in the human N2B-Us that have the potential to form disulfide bonds with one another, depending on the oxidative state within the sarcomere. A disulfide bond would reduce the contour length of the sequence and change its mechanical response to stretch. The effect of cysteine cross-linking on the mechanics of the N2B-Us was shown at both the single molecule level40 and the tissue level, where oxidative stress increased passive tension and hysteresis in wild-type tissue41 (Fig. 1D) but had an attenuated effect in tissue from a mouse model where the entire N2B element was removed 41. The study of oxidative conditions and changes in passive tension is important considering that oxidative stress is elevated in HF patients and has been correlated with myocardial dysfunction42.
In summary, titin-based myocardial stiffness is determined by the titin isoform composition and the phosphorylation state of titin’s elastic I-band, with different kinases affecting titin elasticity in disparate ways. Comprehensive studies of titin isoform expression and phosphorylation status is mandatory for determining the mechanisms by which titin stiffness changes during acute and chronic disease.
Titin mutations and disease
Until recently, a small number of titin mutations had been documented in association with human cardiomyopathies 5, 43-48. It was predicted that the difficulty of complete sequencing of such a large gene in many patients might be responsible for this dearth of identified mutations12. Most of these mutations were associated with DCM. Associations with hypertrophic cardiomyopathy (HCM) were rare. Missense HCM mutations have included Arg740Leu, which increases titin-αactinin binding, and Ser3799Tyr, which increases four and a half LIM protein 2 (FHL2) binding 43, 45. Interestingly, mutations of genes encoding proteins known to interact with titin, including myomesin49, cardiac ankyrin repeat protein (ANKRD-1) 50, 51, FHL252 and telethonin (TCAP) 53 have been found to be associated with both HCM and DCM.
Recently, using several large familial registries of cardiomyopathy patients Herman et al 6 published a landmark study in which both next-generation and dideoxy sequencing were employed to sequence the titin gene in a large number of patients. They focused on mutations (nonsense, frameshift, splicing and copy number) that are likely to have an important effect on titin’s full-length structure, as opposed to single amino acid missense mutations. Their results provide a much more complete picture of the frequency and nature of titin mutations in these patients and are in accord with the idea that titin mutations are indeed much more common than previously known. Specifically, they found that ~30% of DCM patients have mutations in the titin gene, whereas only 1% of HCM mutations were localized to titin. Of the matched subjects without evidence of heart disease 3% also had titin mutations. The penetrance of these DCM mutations was very high in patients older than 40 years. Based on these results mutations of titin appear to be a rare cause of HCM whereas they are by far the most common genetic cause of DCM.
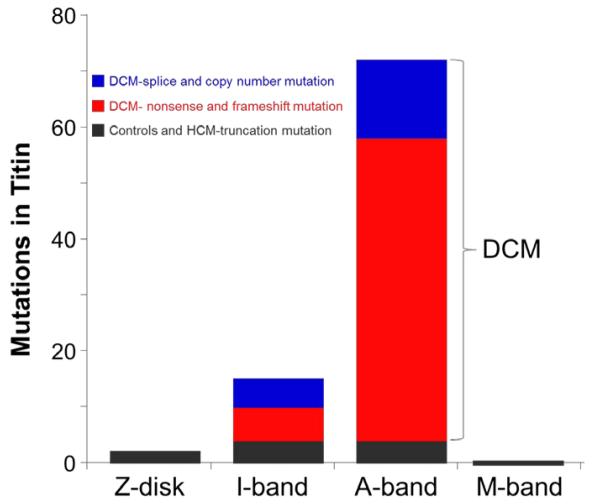
The DCM causing mutations are not randomly distributed along the titin gene; instead, the bulk of the mutations are located in titin’s large A band region that associates with the thick filament 6 (Fig. 2). Strikingly, there were no mutations in the Z-disk or M-band regions. The A band portion of the protein is thought to be critical for biomechanical sensing and signaling and contains the titin kinase domain as well as binding/interacting sites for a number of key proteins, including the thick filament associated protein that crosslinks the thick filament with titin’s C terminus, myomesin, obscurin, protease calpain-3, myosin binding protein C, FHL2/DRAL, and muscle specific ring finger protein-154. Since the kinase domain in particular may play a key role in strain sensitive signaling and communications with the nucleus in conjunction with the other proteins in this region, it is intriguing to consider that these mutations may result in diverse effects on gene expression and cardiac remodeling in DCM.
Figure 2.
Spatial distribution of mutations in titin. Titin molecule is divided into Z-disk, I-band, A-band, and M-band regions. DCM-causing mutations are absent from the Z-disk and M-band region and are most prominent tin the A-band region of the molecule. (Figure based on data from Herman et al. 6.)
Absence of mutations in the Z-disk and paucity of mutations in the I-band region could indicate that mutations in these regions do not cause DCM or HCM (the patient populations that were studied6). Alternatively, mutations in the Z-disk and I-band regions of titin could be highly detrimental to sarcomere function and be incompatible with life, and therefore are not seen in patient populations. Truncation of titin in the Z-disk and I-band regions would result in proteins that are insufficiently long to span to the A-band region, abolishing the mechanical functions of titin. In contrast, truncations in the A-band region of titin result in mutant titins that should be able to incorporate in the Z-disk, span from Z-disk to A-band and be able to make connections to thick filament proteins. Indeed, in a mouse model that conditionally deletes titin’s M-band exons MEx1 and MEx2, the mutant titin does incorporate into the sarcomere and the A-band is relatively normal except for structural defects in the M-band region55. Consistent with this, histo-pathologic examination of hearts with truncated titin in the study of Herman et al6 did not suggest marked sarcomere disorganization. Furthermore, it is possible that increased production and/or incorporation of non-mutated titin can compensate in affected individuals, as was suggested in a mouse heterozygous for a truncation mutation in the middle of the A-band region56. These mice appeared to have normal cardiac function and morphology until they were subjected to the stress of exposure to angiotensin II or isoproterenol. Perhaps a similar mechanism occurs in patients with A-band titin truncations who also are apparently normal until middle age when they develop DCM6, suggesting that stresses encountered as adults act as a trigger for development of clinical disease. A similar mechanism could also underlie the gender effects that were noted, with more adverse events at earlier ages in men than in women6.
Finally, titin mutations also appear to cause arrhythmogenic right ventricular dysplasia (ARVD)5. In a study of 38 affected families, seven were found to have unique titin variants, including a prominent Thr2896lle mutation which completely segregated with the ARVD phenotype in a single large family. This mutation locates to the 10th Ig domain in the proximal tandem Ig. It is surprising that a single point mutation in an Ig domain leads to cardiomyopathy, but the combination of a variety of experimental techniques has suggested a hypothesis that links altered Ig10 dynamics with degradation of healthy myocardium. Nuclear magnetic resonance (NMR) data and proteolysis assays have shown that Ig10 domains harboring the disease-linked mutation are structurally compromised and more prone to degradation 5; AFM data also show that mutant Ig10 unfolds at a lower force compared to native Ig10 6, which is consistent with the idea that the mutation weakens the domain’s β-barrel structure and results in a higher percentage of unfolded mutant Ig10 compared to native Ig10. This propensity to exist in an unfolded structure combined with the increased rate of degradation suggests that the mutation leads to cleaved titins, which would abolish titin’s essential force-generating mechanism and likely lead to further titin degradation and possibly even apoptosis.
A high prevalence of titin mutations was also recently reported by Golbus et al57 who analyzed the ‘1000 Genomes’ cohort. A cumulative frequency of titin indels of 9% was found, with just over 5% of the general population having a 18bp in frame deletion in the PEVK region of titin57. As suggested by the authors57, the discovered titin variants might not cause diseases on their own but may modify the phenotype of mutations in other genes. If correct this would be an important consideration for future genetic testing and study of genotype-phenotype relationships. Thus, titin may be an important disease gene not only because it causes diseases on its own but because it may modify the phenotype of mutations in other genes.
Future Directions
Therapeutic modalities targeting titin are at present largely theoretical, but the previous discussion suggests some possibilities for consideration. Increasing the expression of compliant N2BA titin or inducing the production of even more compliant fetal isoforms in disease states where myocardial passive stiffness is increased (for example HFpEF), offers the possibility of improving diastolic compliance and relieving symptoms. As discussed earlier, such an approach might be possible by manipulation of splicing of titin through reducing the expression or activity of RBM2025 . A potential negative effect of increasing compliant titin isoforms, however, is a reduction in titin-dependent diastolic recoil with impairment of early diastolic filling. Thus, it will be important to experimentally determine whether the net effect of such an intervention is positive with respective to diastolic function. This also underscores the possibility that limiting fibrosis by manipulation of the extra-cellular matrix might also provide a means to improve compliance.
Post-translational modifications offer additional possibilities. Beta-adrenergic blockers have a well-established role in the treatment of ischemic and non-ischemic DCM. Although there is no evidence-based rationale, many patients with HFpEF also receive beta-adrenergic blocking drugs despite the fact that they could reduce phosphorylation of titin’s PKA/PKG sites and further increase myocardial passive stiffness. While chronic administration of catecholamines that increase PKA activity and heart rate cannot be recommended in patients with heart failure, it is possible that beta-blockers should be avoided in HFpEF patients because of their effect on titin. It is also at least possible that drugs that increase PKA activity without causing major changes in heart rate, e.g., phospodiesterase inhibitors such as milrinone, could be beneficial in HFpEF. Similarly, interventions that improve endothelial function in HFpEF with resultant increased PKG activity might also be useful. The results of the soon to be completed RELAX trial of sildenafil in HFpEF58 will be of great interest in this regard, especially in light of the recent finding that sildenafil improves diastolic LV distensibility and increases titin phosphorylation in the dog59. Correspondingly, if phosphorylation of titin’s PKCα sites is increased in HF, this would increase passive stiffness and suggests the possibility of interventions that reduce PKC activity. Endothelial activation, which is present in both heart failure and pulmonary hypertension60, augments PKC activity and could also contribute to increased diastolic stiffness and thus be a target for therapeutic intervention. It will also be of interest to study the effects of isoforms other than PKCα on titin. For example, PKCβ is markedly upregulated in end-stage DCM and causes myofilament dysfunction61, but nothing is known as yet about whether and how it phosphorylates titin. Finally, as mentioned earlier it might be possible that diastolic stiffness can be reduced through reduction of oxidative stress-induced disulfide bonds.
Treating patients with titin truncation mutations will undoubtedly be challenging. In line with the observations that such mutations do not necessarily cause functional or morphological changes, it is possible that gene therapy or other approaches designed to increase production of non-mutated titin could delay or abolish the development of DCM. If the idea that stresses encountered later in life result in induction of DCM is correct56, perhaps patients at risk for DCM could be treated prophylactically with drugs such as angiotensin converting enzyme inhibitors or beta-blockers.
In summary, titin is a major determinant of myocardial and ventricular function and plays a key role in nuclear signaling in response to mechanical stress. Isoform switching and changes in post-translational modification, especially phosphorylation, are increasingly recognized as contributors to the pathophysiology of acquired heart disease. Most recently, as predicted based on its size and critical functions, mutations of titin have emerged as a major cause of DCM. The rapidly increasing in-depth knowledge of titin and how it is modified in disease provides novel avenues for developing molecular therapeutics.
Acknowledgments
Funding Sources: This work was supported by NIH grants R01 HL062881 (HG) and RO1 HL089944 (ML) and a generous gift from Allan and Alfie Norville to HLG.
Footnotes
Conflict of Interest Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106:1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowits R, Podolsky RJ. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987;105:2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor M, Graw S, Sinagra G, Barnes C, Slavov D, Brun F, Pinamonti B, Salcedo EE, Sauer W, Pyxaras S, Anderson B, Simon B, Bogomolovas J, Labeit S, Granzier H, Mestroni L. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 8.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 9.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 10.Greaser ML, Krzesinski PR, Warren CM, Kirkpatrick B, Campbell KS, Moss RL. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. J Muscle Res Cell Motil. 2005;26:325–332. doi: 10.1007/s10974-005-9039-0. [DOI] [PubMed] [Google Scholar]
- 11.Tskhovrebova L, Trinick J. Flexibility and extensibility in the titin molecule: analysis of electron microscope data. J Mol Biol. 2001;310:755–771. doi: 10.1006/jmbi.2001.4700. [DOI] [PubMed] [Google Scholar]
- 12.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, Granzier H. Molecular mechanics of cardiac titin’s PEVK and N2B spring elements. J Biol Chem. 2002;277:11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 14.Greaser ML, Berri M, Warren CM, Mozdziak PE. Species variations in cDNA sequence and exon splicing patterns in the extensible I-band region of cardiac titin: relation to passive tension. J Muscle Res Cell Motil. 2002;23:473–482. doi: 10.1023/a:1023410523184. [DOI] [PubMed] [Google Scholar]
- 15.Kellermayer MS, Smith SB, Bustamante C, Granzier HL. Complete unfolding of the titin molecule under external force. J Struct Biol. 1998;122:197–205. doi: 10.1006/jsbi.1998.3988. [DOI] [PubMed] [Google Scholar]
- 16.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79:3226–3234. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trombitas K, Wu Y, Labeit D, Labeit S, Granzier H. Cardiac titin isoforms are coexpressed in the half-sarcomere and extend independently. Am J Physiol Heart Circ Physiol. 2001;281:H1793–1799. doi: 10.1152/ajpheart.2001.281.4.H1793. [DOI] [PubMed] [Google Scholar]
- 18.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 20.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 21.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 22.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 23.Hudson B, Hidalgo C, Saripalli C, Granzier H. Hyperphosphorylation of mouse cardiac titin contributes to transverse aortic constriction-induced diastolic dysfunction. Circ Res. 2011;109:858–866. doi: 10.1161/CIRCRESAHA.111.246819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. J Mol Cell Cardiol. 2007;42:186–195. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Ozcelik C, Saar K, Hubner N, Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18:766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 27.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–638. 617, 638. doi: 10.1161/CIRCRESAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson BR, Bogomolovas J, Labeit S, Granzier H. The effects of PKCalpha phosphorylation on the extensibility of titin’s PEVK element. J Struct Biol. 2010;170:270–277. doi: 10.1016/j.jsb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson BD, Hidalgo CG, Gotthardt M, Granzier HL. Excision of titin’s cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol. 2010;48:972–978. doi: 10.1016/j.yjmcc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 34.Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F. A novel mechanism involving four-and-a-half LIM domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem. 2012;287:29273–29284. doi: 10.1074/jbc.M112.372839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, 2nd, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo CG, Methawasin M, Chung C, Bogomolavas J, Gasch A, Labeit S, Mattiazi A, Granzier H. The Multifunctional Calcium/Calmodulin-Dependent Protein Kinase II Delta (CaMKIIδ) Phosphorylates Titin N2B and PEVK Spring Elements. Biophys J. 2012;102:559a. [Google Scholar]
- 38.Hidalgo C, Chung CS, Saripalli C, Methawasin M, Hutchinson K, Tsaprailis G, Labeit S, Mattiazzi A, Granzier H. The multifunctional Ca2+/calmodulin-dependent protein kinase II delta (CaMKIIδ) phosphorylates cardiac titin’s spring elements. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.11.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 40.Grutzner A, Garcia-Manyes S, Kotter S, Badilla CL, Fernandez JM, Linke WA. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J. 2009;97:825–834. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedrud J, Labeit S, Gotthardt M, Granzier H. Mechanics on myocardium deficient in the N2B region of titin: the cardiac-unique spring element improves efficiency of the cardiac cycle. Biophys J. 2011;101:1385–1392. doi: 10.1016/j.bpj.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grieve DJ, Shah AM. Oxidative stress in heart failure. More than just damage. Eur Heart J. 2003;24:2161–2163. doi: 10.1016/j.ehj.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem Biophys Res Commun. 1999;262:411–417. doi: 10.1006/bbrc.1999.1221. [DOI] [PubMed] [Google Scholar]
- 44.Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T, Takahashi M, Hohda S, Ueda K, Nouchi T, Hiroe M, Marumo F, Imaizumi T, Yasunami M, Kimura A. Titin mutations as the molecular basis for dilated cardiomyopathy. Biochem Biophys Res Commun. 2002;291:385–393. doi: 10.1006/bbrc.2002.6448. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto Y, Hayashi T, Inagaki N, Takahashi M, Hiroi S, Nakamura T, Arimura T, Nakamura K, Ashizawa N, Yasunami M, Ohe T, Yano K, Kimura A. Functional analysis of titin/connectin N2-B mutations found in cardiomyopathy. J Muscle Res Cell Motil. 2005;26:367–374. doi: 10.1007/s10974-005-9018-5. [DOI] [PubMed] [Google Scholar]
- 46.Gerull B, Atherton J, Geupel A, Sasse-Klaassen S, Heuser A, Frenneaux M, McNabb M, Granzier H, Labeit S, Thierfelder L. Identification of a novel frameshift mutation in the giant muscle filament titin in a large Australian family with dilated cardiomyopathy. J Mol Med. 2006;84:478–483. doi: 10.1007/s00109-006-0060-6. [DOI] [PubMed] [Google Scholar]
- 47.Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 48.Yoskovitz G, Peled Y, Gramlich M, Lahat H, Resnik-Wolf H, Feinberg MS, Afek A, Pras E, Arad M, Gerull B, Freimark D. A novel titin mutation in adult-onset familial dilated cardiomyopathy. Am J Cardiol. 2012;109:1644–1650. doi: 10.1016/j.amjcard.2012.01.392. [DOI] [PubMed] [Google Scholar]
- 49.Siegert R, Perrot A, Keller S, Behlke J, Michalewska-Wludarczyk A, Wycisk A, Tendera M, Morano I, Ozcelik C. A myomesin mutation associated with hypertrophic cardiomyopathy deteriorates dimerisation properties. Biochem Biophys Res Commun. 2011;405:473–479. doi: 10.1016/j.bbrc.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 50.Duboscq-Bidot L, Charron P, Ruppert V, Fauchier L, Richter A, Tavazzi L, Arbustini E, Wichter T, Maisch B, Komajda M, Isnard R, Villard E. Mutations in the ANKRD1 gene encoding CARP are responsible for human dilated cardiomyopathy. Eur Heart J. 2009;30:2128–2136. doi: 10.1093/eurheartj/ehp225. [DOI] [PubMed] [Google Scholar]
- 51.Arimura T, Bos JM, Sato A, Kubo T, Okamoto H, Nishi H, Harada H, Koga Y, Moulik M, Doi YL, Towbin JA, Ackerman MJ, Kimura A. Cardiac ankyrin repeat protein gene (ANKRD1) mutations in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:334–342. doi: 10.1016/j.jacc.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 52.Arimura T, Matsumoto Y, Okazaki O, Hayashi T, Takahashi M, Inagaki N, Hinohara K, Ashizawa N, Yano K, Kimura A. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2007;362:281–287. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- 53.Hershberger RE, Parks SB, Kushner JD, Li D, Ludwigsen S, Jakobs P, Nauman D, Burgess D, Partain J, Litt M. Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy. Clin Transl Sci. 2008;1:21–26. doi: 10.1111/j.1752-8062.2008.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leinwand LA, Tardiff JC, Gregorio CC. Mutations in the sensitive giant titin result in a broken heart. Circ Res. 2012;111:158–161. doi: 10.1161/RES.0b013e3182635ca2. [DOI] [PubMed] [Google Scholar]
- 55.Gotthardt M, Hammer RE, Hubner N, Monti J, Witt CC, McNabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem. 2003;278:6059–6065. doi: 10.1074/jbc.M211723200. [DOI] [PubMed] [Google Scholar]
- 56.Gramlich M, Michely B, Krohne C, Heuser A, Erdmann B, Klaassen S, Hudson B, Magarin M, Kirchner F, Todiras M, Granzier H, Labeit S, Thierfelder L, Gerull B. Stress-induced dilated cardiomyopathy in a knock-in mouse model mimicking human titin-based disease. J Mol Cell Cardiol. 2009;47:352–358. doi: 10.1016/j.yjmcc.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM. Population-based variation in cardiomyopathy genes. Circ Cardiovasc Genet. 2012;5:391–399. doi: 10.1161/CIRCGENETICS.112.962928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E, For the Heart Failure Clinical Research N PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) Trial: Rationale and Design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr., Linke WA, Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–2891. doi: 10.1161/CIRCULATIONAHA.111.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68:195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004;110:982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]