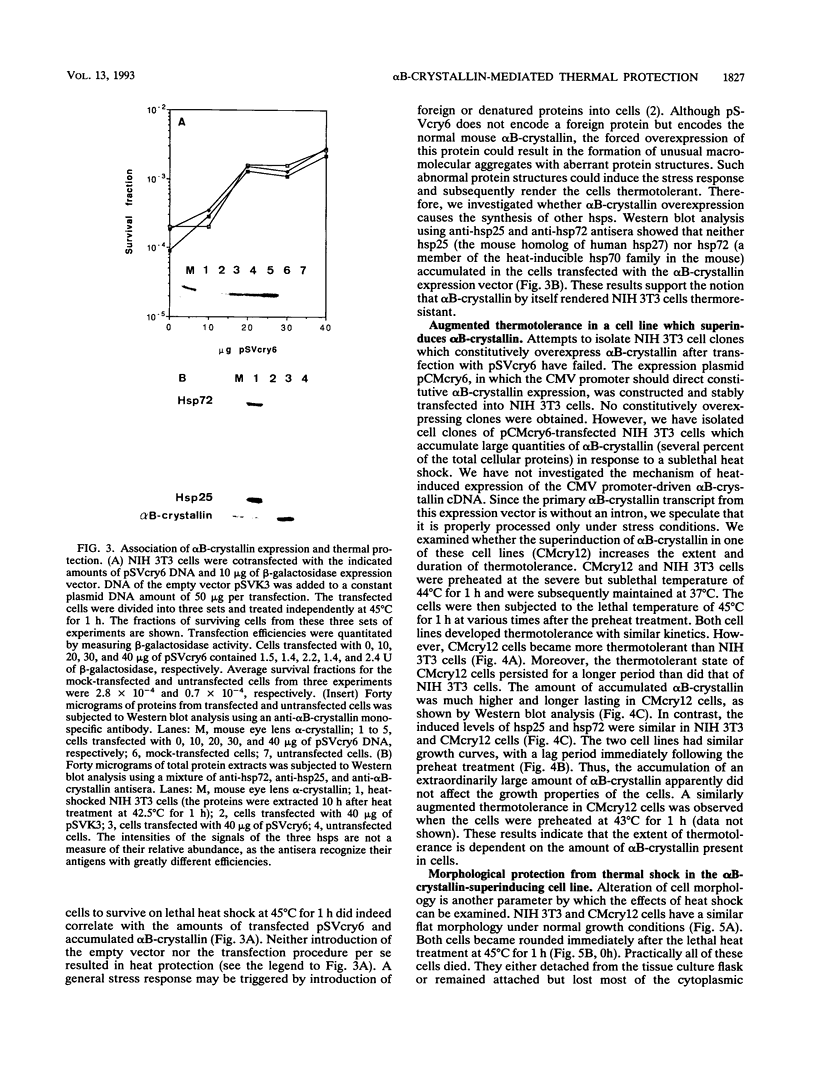
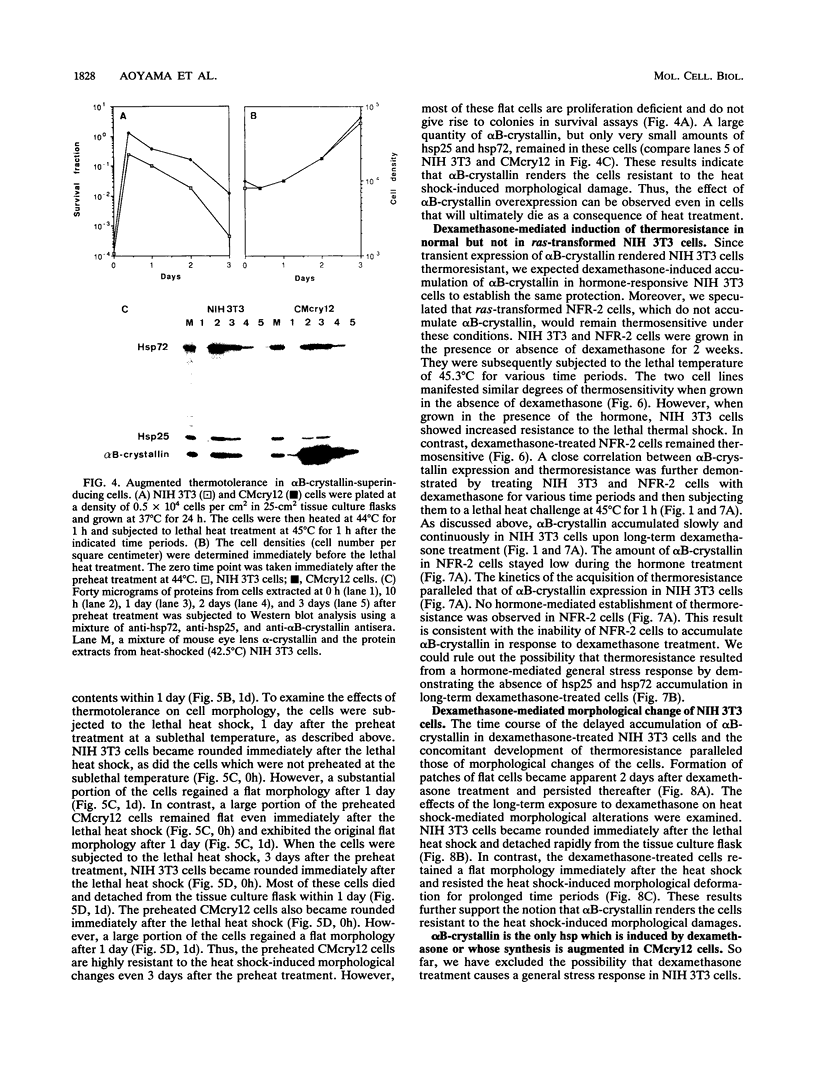
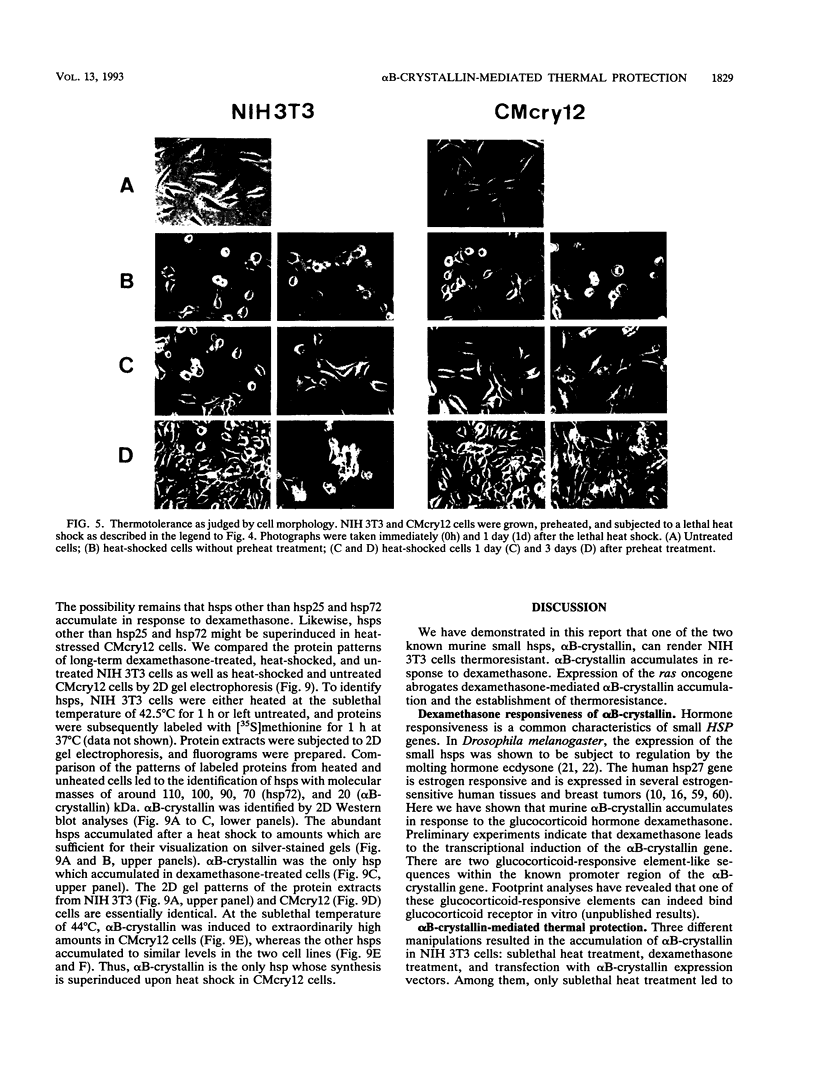
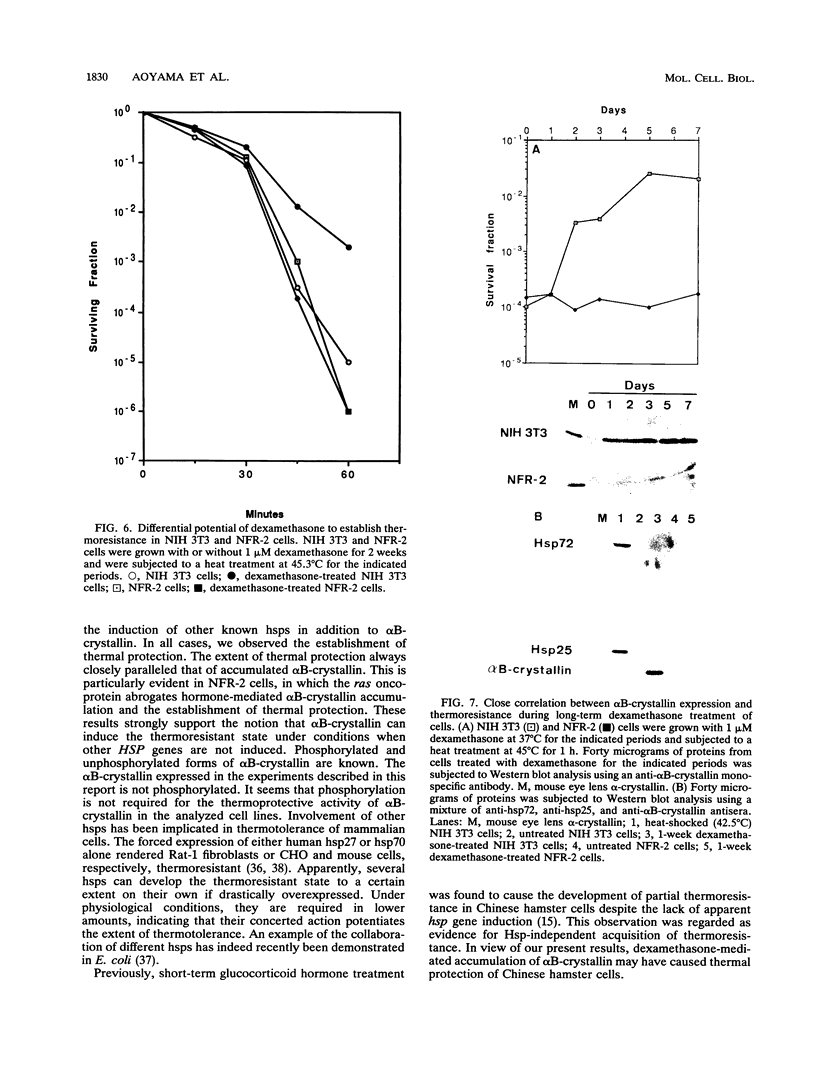
Abstract
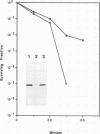
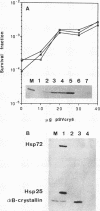
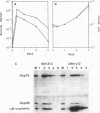
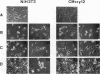
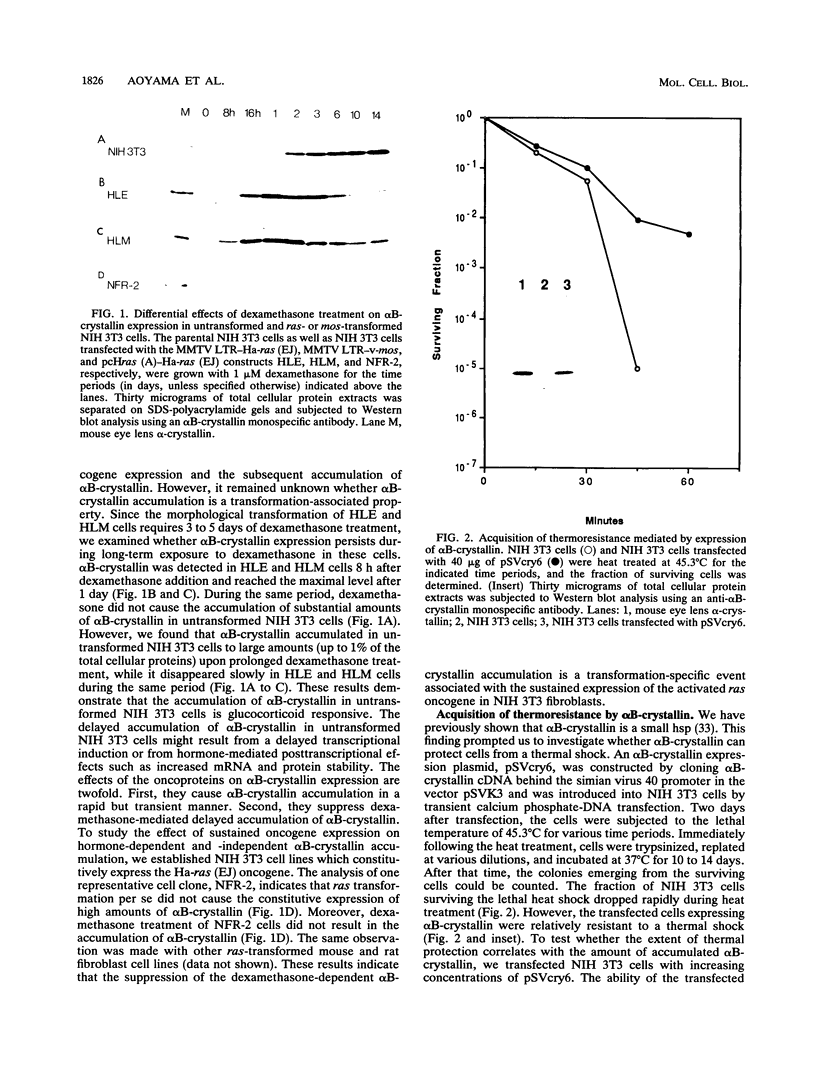
alpha B-crystallin, a major soluble protein of vertebrate eye lenses, is a small heat shock protein which transiently accumulates in response to heat shock and other kinds of stress in mouse NIH 3T3 fibroblasts. Ectopic expression of an alpha B-crystallin cDNA clone renders NIH 3T3 cells thermoresistant. alpha B-crystallin accumulates in response to the synthetic glucocorticoid hormone dexamethasone. Dexamethasone-treated NIH 3T3 cells become thermoresistant to the same extent as they accumulate alpha B-crystallin. A cell clone in which alpha B-crystallin is superinduced upon heat shock acquires augmented thermotolerance. Expression of the ras oncogene causes a rapid but transient accumulation of alpha B-crystallin within 1 day. Later, sustained ras oncogene expression suppresses the dexamethasone-mediated alpha B-crystallin accumulation. Thus, oncogenic transformation triggered by the ras oncogene interferes with hormone-mediated accumulation of alpha B-crystallin and concomitant acquisition of thermoresistance. Other known heat shock proteins do not accumulate in response to ectopic alpha B-crystallin expression or to dexamethasone treatment. These results indicate that alpha B-crystallin can protect NIH 3T3 fibroblasts from thermal shock.
Full text
PDF

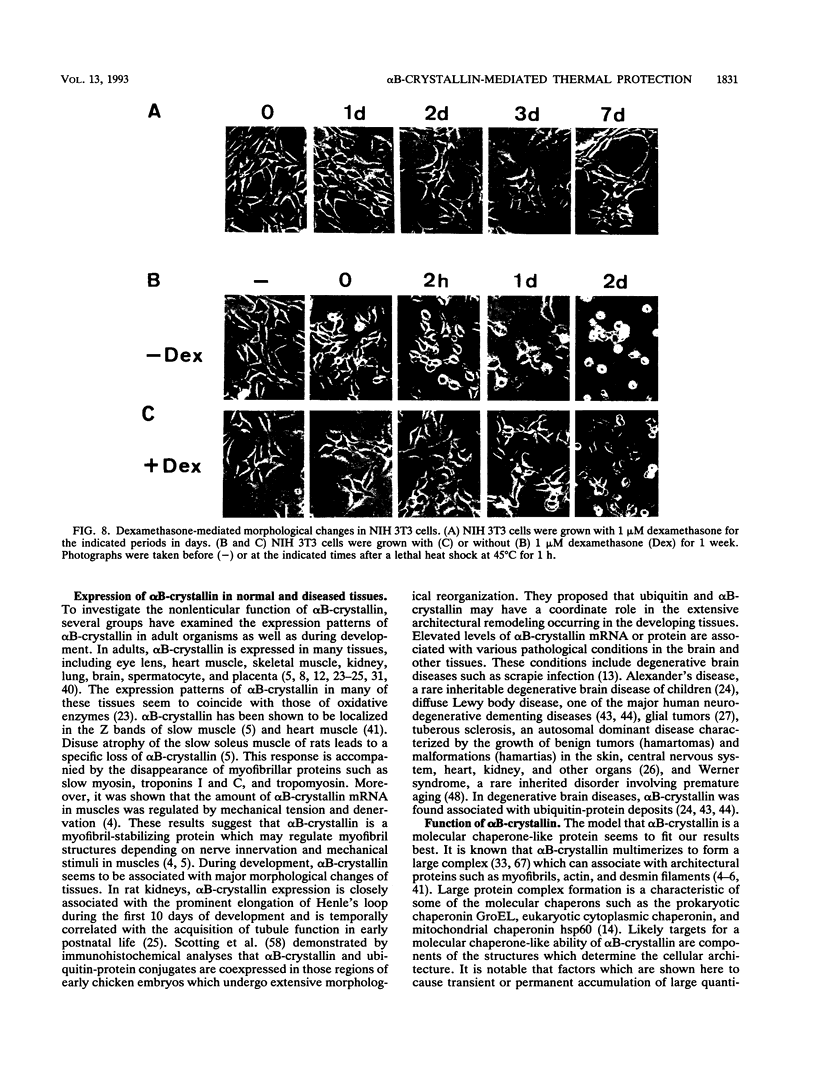
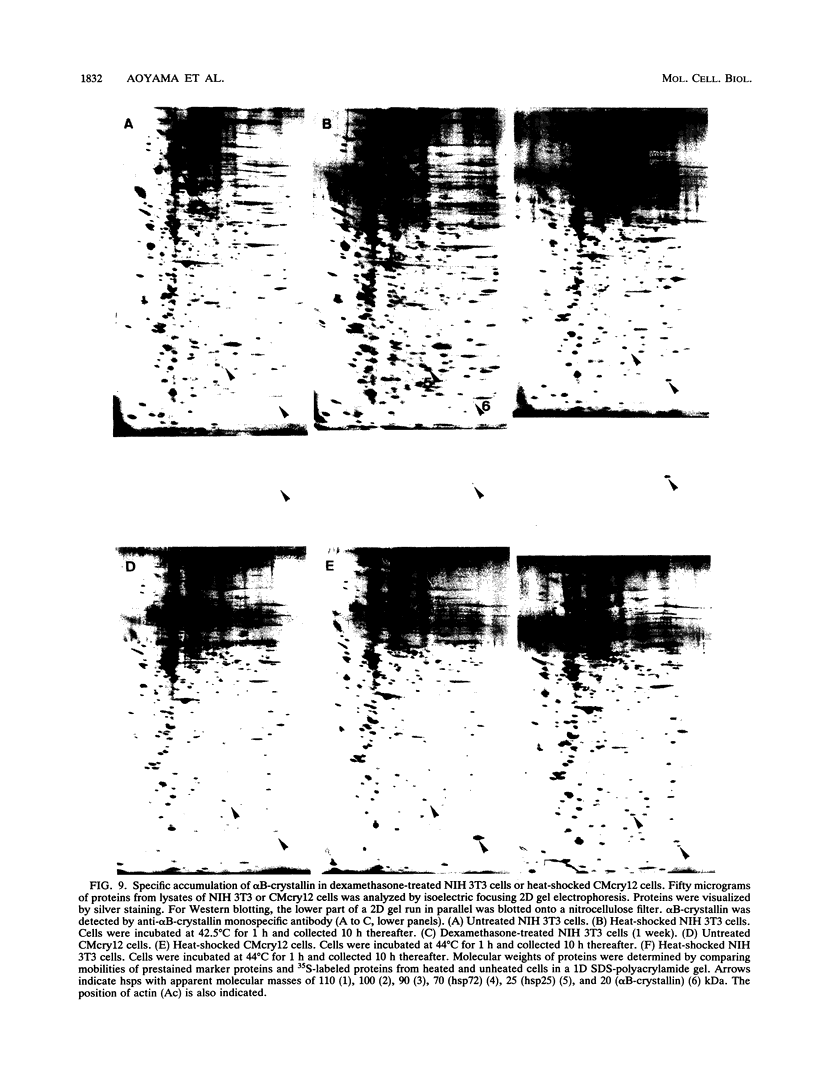



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Aoyama A., Klemenz R. Oncogene-mediated effects on cellular gene expression. Crit Rev Oncog. 1993;4(1):53–94. [PubMed] [Google Scholar]
- Atomi Y., Yamada S., Nishida T. Early changes of alpha B-crystallin mRNA in rat skeletal muscle to mechanical tension and denervation. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1323–1330. doi: 10.1016/0006-291x(91)92083-v. [DOI] [PubMed] [Google Scholar]
- Atomi Y., Yamada S., Strohman R., Nonomura Y. Alpha B-crystallin in skeletal muscle: purification and localization. J Biochem. 1991 Nov;110(5):812–822. doi: 10.1093/oxfordjournals.jbchem.a123665. [DOI] [PubMed] [Google Scholar]
- Bennardini F., Wrzosek A., Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992 Aug;71(2):288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- Berger E. M., Woodward M. P. Small heat shock proteins in Drosophila may confer thermal tolerance. Exp Cell Res. 1983 Sep;147(2):437–442. doi: 10.1016/0014-4827(83)90225-2. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Chrétien P., Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988 Oct;137(1):157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Ciocca D. R., Adams D. J., Edwards D. P., Bjercke R. J., McGuire W. L. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983 Mar;43(3):1204–1210. [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Fisher G. A., Anderson R. L., Hahn G. M. Glucocorticoid-induced heat resistance in mammalian cells. J Cell Physiol. 1986 Jul;128(1):127–132. doi: 10.1002/jcp.1041280119. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Blum-Salingaros M., McGuire W. L. Induction of the estrogen-regulated "24K" protein by heat shock. Cancer Res. 1989 Aug 1;49(15):4126–4129. [PubMed] [Google Scholar]
- Gaestel M., Gross B., Benndorf R., Strauss M., Schunk W. H., Kraft R., Otto A., Böhm H., Stahl J., Drabsch H. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem. 1989 Jan 15;179(1):209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J., Emmons T., Takemoto L. The ability of lens alpha crystallin to protect against heat-induced aggregation is age-dependent. Curr Eye Res. 1992 Aug;11(8):817–822. doi: 10.3109/02713689209000754. [DOI] [PubMed] [Google Scholar]
- Ireland R. C., Berger E., Sirotkin K., Yund M. A., Osterbur D., Fristrom J. Ecdysterone induces the transcription of four heat-shock genes in Drosophila S3 cells and imaginal discs. Dev Biol. 1982 Oct;93(2):498–507. doi: 10.1016/0012-1606(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Iwaki A., Liem R. K., Goldman J. E. Expression of alpha B-crystallin in the developing rat kidney. Kidney Int. 1991 Jul;40(1):52–56. doi: 10.1038/ki.1991.178. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Goldman J. E. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990 Jan;38(1):31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Tateishi J. Immunohistochemical demonstration of alphaB-crystallin in hamartomas of tuberous sclerosis. Am J Pathol. 1991 Dec;139(6):1303–1308. [PMC free article] [PubMed] [Google Scholar]
- Iwaki T., Wisniewski T., Iwaki A., Corbin E., Tomokane N., Tateishi J., Goldman J. E. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am J Pathol. 1992 Feb;140(2):345–356. [PMC free article] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kato K., Shinohara H., Kurobe N., Inaguma Y., Shimizu K., Ohshima K. Tissue distribution and developmental profiles of immunoreactive alpha B crystallin in the rat determined with a sensitive immunoassay system. Biochim Biophys Acta. 1991 May 24;1074(1):201–208. doi: 10.1016/0304-4165(91)90062-l. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Aoyama A., Hoffmann S., Simpson R. J., Moritz R. L., Schäfer R. Alpha B crystallin accumulation is a specific response to Ha-ras and v-mos oncogene expression in mouse NIH 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):803–812. doi: 10.1128/mcb.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Jaggi R., Werenskiold A. K. The v-mos and c-Ha-ras oncoproteins exert similar effects on the pattern of protein synthesis. Oncogene. 1989 Jun;4(6):799–803. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Longoni S., James P., Chiesi M. Cardiac alpha-crystallin. I. Isolation and identification. Mol Cell Biochem. 1990 Dec 3;99(1):113–120. [PubMed] [Google Scholar]
- Longoni S., Lattonen S., Bullock G., Chiesi M. Cardiac alpha-crystallin. II. Intracellular localization. Mol Cell Biochem. 1990 Sep 21;97(2):121–128. doi: 10.1007/BF00221053. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Lowe J., Landon M., Pike I., Spendlove I., McDermott H., Mayer R. J. Dementia with beta-amyloid deposition: involvement of alpha B-crystallin supports two main diseases. Lancet. 1990 Aug 25;336(8713):515–516. doi: 10.1016/0140-6736(90)92075-s. [DOI] [PubMed] [Google Scholar]
- Lowe J., McDermott H., Pike I., Spendlove I., Landon M., Mayer R. J. alpha B crystallin expression in non-lenticular tissues and selective presence in ubiquitinated inclusion bodies in human disease. J Pathol. 1992 Jan;166(1):61–68. doi: 10.1002/path.1711660110. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Sanchez Y., Stitzel J. D., Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991 Sep 19;353(6341):270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins and their genes: diversity and tissue-specific expression. FASEB J. 1989 Jun;3(8):1933–1940. doi: 10.1096/fasebj.3.8.2656357. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992 Mar 5;267(7):4277–4280. [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Santos E., Pulciani S., Barbacid M. Characterization of a human transforming gene isolated from T24 bladder carcinoma cells. Fed Proc. 1984 May 15;43(8):2280–2286. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Scotting P., McDermott H., Mayer R. J. Ubiquitin-protein conjugates and alpha B crystallin are selectively present in cells undergoing major cytomorphological reorganisation in early chicken embryos. FEBS Lett. 1991 Jul 8;285(1):75–79. doi: 10.1016/0014-5793(91)80728-l. [DOI] [PubMed] [Google Scholar]
- Seymour L., Bezwoda W. R., Meyer K., Behr C. Detection of P24 protein in human breast cancer: influence of receptor status and oestrogen exposure. Br J Cancer. 1990 Jun;61(6):886–890. doi: 10.1038/bjc.1990.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor A., Benz C., Moore D., 2nd, Goldman E., Edgerton S., Landry J., Schwartz L., Mayall B., Hickey E., Weber L. A. Stress response protein (srp-27) determination in primary human breast carcinomas: clinical, histologic, and prognostic correlations. J Natl Cancer Inst. 1991 Feb 6;83(3):170–178. doi: 10.1093/jnci/83.3.170. [DOI] [PubMed] [Google Scholar]
- Touray M., Ryan F., Saurer S., Martin F., Jaggi R. mos-induced inhibition of glucocorticoid receptor function is mediated by Fos. Oncogene. 1991 Feb;6(2):211–217. [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Domain structure and evolution in alpha-crystallins and small heat-shock proteins. FEBS Lett. 1985 Feb 11;181(1):1–6. doi: 10.1016/0014-5793(85)81102-9. [DOI] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990 Feb;30(2):140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]