Abstract
Transferrin is an abundant serum metal-binding protein best known for its role in iron delivery. The human disease congenital atransferrinemia and animal models of this disease highlight the essential role of transferrin in erythropoiesis and iron metabolism. Patients and mice deficient in transferrin exhibit anemia and a paradoxical iron overload attributed to deficiency in hepcidin, a peptide hormone synthesized largely by the liver that inhibits dietary iron absorption and macrophage iron efflux. Studies of inherited human disease and model organisms indicate that transferrin is an essential regulator of hepcidin expression. In this paper, we review current literature on transferrin deficiency and present our recent findings, including potential overlaps between transferrin, iron and manganese in the regulation of hepcidin expression.
Keywords: Transferrin, iron, manganese, hepcidin, hypotransferrinemia, atransferrinemia, anemia
Transferrin deficiency in human biology
Primary deficiency
The role of transferrin (TF) in human biology is best exhibited by the primary TF deficiency observed in congenital atransferrinemia/hypotransferrinemia. In this rare disease, autosomal recessive mutations in the TF gene lead to TF deficiency. The resulting microcytic, hypochromic anemia, responsive to infusions of TF or plasma, demonstrates the essential role of TF in iron delivery for erythropoiesis. Intriguingly, although few cases have been reported, this condition is not always diagnosed in early life (Beutler et al. 2000; Asada-Senju et al. 2002; Knisely et al. 2004; Aslan et al. 2007; Chen et al. 2009), suggesting that TF deficiency can have a late onset or is not severe enough in all instances to adversely affect embryonic or postnatal development and viability. Paradoxically, patients also develop iron (Fe) overload in multiple tissues, indicating that TF is not essential for Fe delivery to all organs. This Fe overload is attributed to deficiency in the iron regulatory hormone hepcidin, the role of which will be discussed below.
Secondary deficiency
Given that TF levels decrease in conditions such as inflammation and Fe overload (Morton and Tavill 1977; Ritchie et al. 1999), it is not surprising that TF deficiency has been reported in clinical situations other than congenital atransferrinemia. Multiple examples exist:
Negative correlation between levels of the inflammatory cytokine IL-6 and plasma TF levels in depressed individuals suggests that hypotransferrinemia may reflect an inflammatory state in depression (Maes et al. 1992, 1993).
Hypotransferrinemia observed in patients with chronic alcoholism may reflect inflammation and/or liver damage caused by alcohol or alcohol-related nutritional deficiencies (Borini and Guimarães 1999).
Studies on patients prior to or undergoing chronic hemodialysis revealed a high rate of hypotransferrinemia which was attributed to chronic inflammation or to administration of intravenous Fe for treatment of anemia prior to the onset of hemodialysis (Kirschbaum 1999; Descombes and Fellay 2000).
Hypotransferrinemia can also result from excessive urinary protein losses in nephrotic syndrome, a condition characterized in part by urinary excretion of albumin and other plasma proteins and associated with several different diseases (Warshaw et al. 1984; Prinsen et al. 2001; Vaziri 2001).
Postnatal hypotransferrinemia has been noted in preterm newborn infants where serum TF levels correlated with postconceptional rather than postnatal age (Galet et al. 1976).
A study in critically ill patients documented that decreased TF levels were associated with increased pulmonary vascular permeability (Aman et al. 2011).
Hypotransferrinemia is also a noted characteristic of GRACILE syndrome, an autosomal recessive metabolic disorder characterized by multiple abnormalities including fetal growth retardation and early death and attributed to mutations in BCS1L, a gene required for activity of complex III of the respiratory chain (Fellman 2002).
While not a state of TF deficiency per se, aberrant levels of TF glycosylation are used as indicators of chronic alcohol consumption (Golka and Wiese 2004) and congenital disorders of glycosylation, a group of inherited diseases characterized by defective glycan biosynthesis (Jaeken 2010); while an absence of glycosylation does not impair binding of TF to TF receptor or Fe, it does impair cellular Fe uptake in vitro and in vivo (Hu et al. 1991; Mason et al. 1993; Hoefkens et al. 1997).
Iron metabolism in models of transferrin deficiency
A mouse model of congenital atransferrinemia arose spontaneously in a BALB/cJ colony several decades ago (Bernstein 1987). Originally referred to as hypotransferrinemic or hpx mice, this strain harbors a splicing defect in the mouse TF gene that results in minimal serum TF levels (Huggenvik et al. 1989; Trenor et al. 2000). As in patients with the analogous human disease, hpx mice develop an anemia responsive to injections of exogenous TF, further highlighting the essential role of TF in erythropoiesis, and a paradoxical Fe overload (Craven et al. 1987; Dickinson et al. 1996; Raja et al. 1999), again indicating the non-essential role of TF in delivery to organs other than bone marrow. While the role of TF in brain Fe homeostasis is still unclear (Dickinson and Connor 1995; Dickinson et al. 1996; Takeda et al. 1998, 2001; Malecki et al. 1999; Beard et al. 2005), Fe overload in liver and other tissues is attributed to gastrointestinal Fe hyperabsorption which can be suppressed by transfusion with washed red blood cells or exogenous TF (Buys et al. 1991; Raja et al. 1999).
While hpx mice exhibit increased duodenal protein levels of divalent metal transporter 1 (Dmt1), a cellular Fe importer essential for gastrointestinal Fe absorption (Canonne-Hergaux et al. 2001), the impressive Fe overload in hpx mice is primarily attributed to deficiency in hepcidin, a peptide hormone synthesized largely by the liver. By binding to and stimulating the internalization and degradation of the only known cellular iron exporter ferroportin, hepcidin serves to inhibit gastrointestinal Fe absorption and macrophage Fe efflux (Ganz and Nemeth 2011). Prior to our recent studies in hpx mice, there was ample experimental evidence that TF regulates hepcidin expression: hepcidin levels and TF saturation correlate in humans and mice (Gehrke et al. 2003; Wilkins et al. 2006); diferric TF regulates hepcidin expression in primary hepatocytes and immortalized cell lines (Lin et al. 2007; Gao et al. 2009; Ramey et al. 2009); TF-deficient zebrafish are anemic and hepcidin-deficient (Fraenkel et al. 2009); plasma transfusion increases urinary hepcidin levels in congenital atransferrinemic patients (Trombini et al. 2007); TF treatment increases hepcidin levels in a mouse model of β-thalassemia intermedia, an anemia associated with hepcidin deficiency and Fe overload (Li et al. 2010). Therefore, it was not surprising that treatment of hpx mice with exogenous TF restores hepcidin levels; furthermore, treatment of mice with the chemotherapeutic agent doxorubicin—thereby ablating bone marrow and other sites of erythropoiesis—followed by treatment with TF also leads to increased hepcidin levels, suggesting that TF can regulate hepcidin expression independently of its role in erythropoiesis (Bartnikas et al. 2011).
In contrast, our recent finding that TF regulates hepcidin expression in an erythropoiesis-dependent manner (Bartnikas et al. 2011) was unexpected, given that TF-restricted erythropoiesis is not a form of erythropoiesis traditionally associated with suppression of hepcidin expression. Transfusion of hpx mice with wild-type red blood cells not only corrects the inherent anemia but also increases hepcidin levels, suggesting that erythropoiesis in hpx mice actively inhibits hepcidin expression. To further explore the mechanism of TF-dependent hepcidin regulation, we also intercrossed hpx mice to mice deficient in hemojuvelin, a bone morphogenetic protein (BMP) co-receptor essential for hepcidin expression. Treatment of TF- and hemojuvelin-deficient progeny with TF or red blood cell transfusions fails to increase hepcidin levels, suggesting that hemojuvelin is essential for TF-dependent and–independent hepcidin expression (Bartnikas and Fleming 2011). Given that TF-restricted erythropoiesis inhibits hepcidin levels, this latter finding suggests that erythropoietic inhibition of hepcidin expression acts on the hemojuvelin-hepcidin axis. Although the decreased liver protein levels of TF receptor 2 (Tfr2), a membrane protein required for hepcidin expression (Robb and Wessling-Resnick 2004), most likely contributes to hepcidin deficiency in hpx mice, the precise role of Tfr2 in hpx mice has yet to be elucidated, particularly given the recent finding that Tfr2 is required for biosynthesis and function of erythropoietin receptor, a factor required for erythroid progenitor differentiation (Forejtnikovà et al. 2010).
Regulation of hepatocyte hepcidin expression by manganese
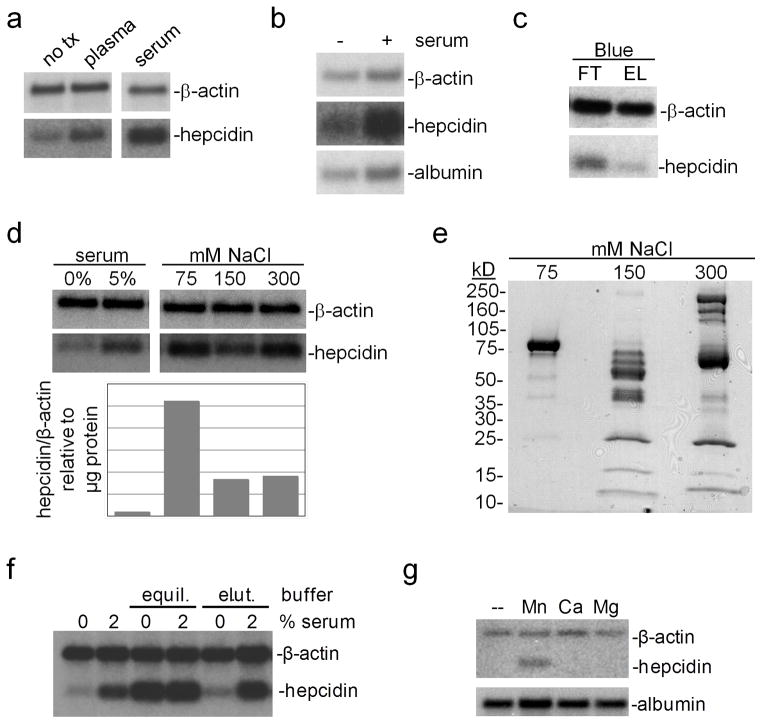
To identify serum factors involved in the regulation of hepcidin expression, we employed a primary hepatocyte culture-based method. We first treated mouse primary hepatocytes with or without human serum or plasma, both of which led to increased hepcidin levels and lesser changes in albumin levels relative to β-actin levels (Fig. 1a, b). As greater hepcidin levels were observed with serum rather than plasma treatment, we focused on serum for the remainder of our analysis. We next depleted serum of albumin using a GE HiTrap Blue column and treated primary hepatocytes with the resulting column flow-through and eluate fractions; the flow-through fraction had a more pronounced effect on hepcidin expression than did the column eluate (Fig. 1c), suggesting that albumin and other proteins with affinity for the Blue column had minimal effects on hepcidin levels in the primary hepatocyte assay. We next applied the Blue column flow-through to a GE HiTrap Q anion exchange column and eluted bound proteins with increasing NaCl concentrations. Treatment of hepatocytes with desalted fractions revealed that the 75 mM NaCl fraction had the most pronounced effect on hepcidin levels, when hepcidin levels were expressed relative to μg of protein per fraction (Fig. 1d). SDS-PAGE and Commassie staining demonstrated that the 75 mM NaCl fraction consisted largely of a roughly 75 kD protein (Fig. 1e). Mass spectrometric analysis of this band indicated that the most abundant protein in this sample was TF (data not shown).
Fig. 1.
Response of hepatocyte hepcidin levels to serum: (a–b) Primary hepatocytes were treated in serum-free media (Optimem) with 0 or 5% human serum or plasma overnight, then harvested and analyzed by Northern blot for hepcidin, albumin and β-actin RNA levels. (c) 1 mL human serum, diluted four-fold with 20 mM Tris pH 8.0, was fractionated with a 5 mL column volume (CV) GE HiTrap Blue column with high affinity for albumin; flowthrough (FT) and eluate (EL) fractions were collected and concentrated and desalted to equal volumes with 5 kD molecular weight cut-off centrifugal filtration devices. Hepatocytes were treated overnight with equal volumes of FT and EL fractions; RNA was isolated and analyzed for β-actin and hepcidin mRNA levels. (d) 1 mL human serum was diluted and chromatographed with GE HiTrap Blue (5 mL CV) and Q anion exchange (1 mL CV) columns. Fractions were eluted from the Q column with 75, 150 and 300 mM NaCl and concentrated and desalted using centrifugal filtration devices. Hepatocytes were treated overnight with 0 or 5% serum or equal volumes of anion exchange fractions. RNA was isolated and analyzed by Northern blot. Band intensity was quantitated by NIH Image software and expressed as a ratio of hepcidin to β-actin mRNA band intensity per μg of fraction protein. (e) Fractions from (d) were subjected to denaturing, reducing SDS PAGE and Coomassie staining. (f) Hepatocytes were treated in serum-free media with 0 or 2% human serum and 0 or 10% concanavalin A equilibration (20 mM Tris pH 7.4/0.5 M NaCl/5 mM MgCl2, MnCl2, CaCl2) or elution buffer (500 mM methyl-α-D-glucopyranoside) overnight, then analyzed for hepcidin and β-actin expression levels by Northern blot. (g) Hepatocytes were incubated in serum-free media with or without 50 μM MnCl2, CaCl2 or MgCl2 overnight, then analyzed as in (f). Results in all panels are representative of three independent experiments
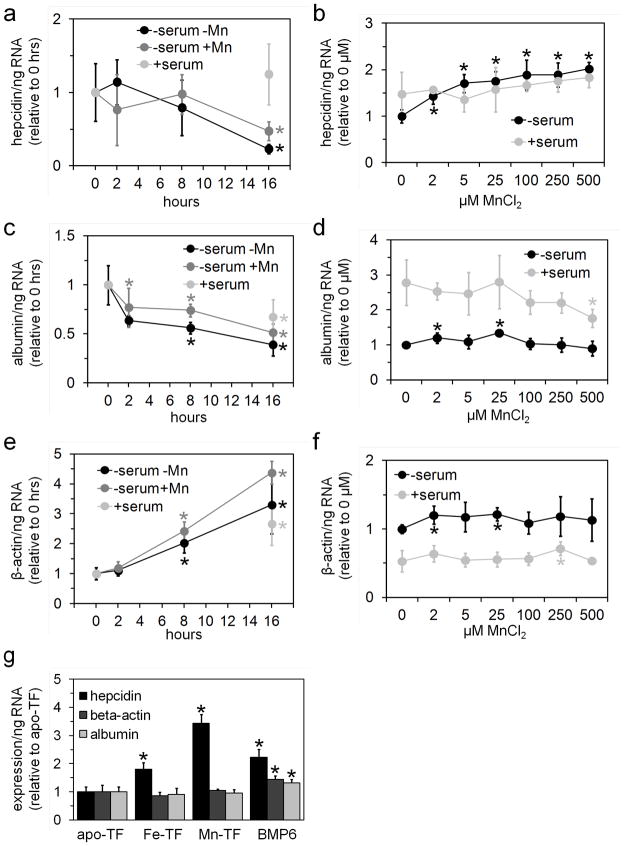
While performing control experiments analyzing the effect of column equilibration and elution buffers on hepatocyte hepcidin levels, we noted that treatment of hepatocytes with the equilibration buffer for a concanavalin A column led to increased hepcidin levels (Fig. 1f). Individual testing of the salts present in this buffer revealed that treatment of hepatocytes with MnCl2 increased hepcidin levels (Fig. 1g). We next examined the time-dependence of the Mn effect by incubating freshly isolated hepatocytes in serum-replete media or in serum-free media with or without 100 μM MnCl2 for 0–16 hours (Fig. 2a,c,e); we also examined the dose-dependence of the Mn effect by incubating hepatocytes in serum-relete or –free media with 0–500 μM MnCl2 for 16 hours (Fig. 2b,d,f). For this and all following experiments, we used fetal bovine serum, not human serum, as our source of serum. Hepcidin RNA levels decreased in hepatocytes incubated in serum-free media with or without MnCl2 by 16 hours but not in hepatocytes incubated in serum-replete media (Fig. 2a); Mn treatment had a dose-dependent effect on hepcidin levels but only in hepatocytes treated in serum-free media (Fig. 2b). Albumin RNA levels decreased in hepatocytes with time irrespective of treatment conditions (Fig. 2c) and did not exhibit any consistent dose-dependence relative to Mn treatment levels (Fig. 2d). Notably, the decrease in albumin levels in hepatocytes treated here with serum differs from the increase noted in hepatocytes treated with human serum (Fig. 1b); this difference may reflect the type of serum used. β-actin RNA levels increased in hepatocytes with time irrespective of treatment conditions (Fig. 2e) and did not exhibit any consistent dose-dependence relative to Mn treatment levels (Fig. 2f).
Fig. 2.
Effect of manganese on hepatocyte hepcidin levels: (a, c, e) Primary hepatocytes were treated in serum-free media (Optimem) without (black circles) or with 100 μM MnCl2 (dark gray circles) or in serum–replete media (containing 10% fetal calf serum) without added MnCl2 (light gray circles) for 0–16 hours then harvested and analyzed by QPCR for hepcidin (a), albumin (c) and β-actin (e) RNA levels. Values are normalized to 0 hour value; asterisks indicate value differs significantly (t-test P<0.05) from 0 hour value. (b, d, f) Primary hepatocytes were treated in serum-free (dark circles) or –eplete media (light gray circles) with 0–500 μM MnCl2 for 16 hours then harvested and analyzed by QPCR for hepcidin (b), albumin (d) and β-actin RNA levels (f). Values are normalized to 0 μM MnCl2 serum-free value; asterisks indicate value differs significantly (t-test P<0.05) from 0 μM MnCl2, same serum treatment. (g) Primary hepatocytes were treated in serum-free media with or without 3 mg/mL apo-TF, 100% saturated Fe-TF or 50% saturated Mn-TF or 50 ng/mL BMP6 overnight, then analyzed for expression levels as in (a–f). Asterisks indicate value differs significantly (t-test P<0.05) from apo-TF value. For all panels, gene RNA levels were expressed relative to total ng RNA used for QPCR; bars indicate standard deviation; each value represents the average of 4–6 biological replicates
We next focused on the effect of Mn-replete TF (Mn-TF) on hepcidin expression in primary hepatocytes, given that TF is essential for the regulation of hepcidin expression in vivo and is the major Mn-binding species in blood (Vincent and Love 2011). Mn and Fe occupy the same binding sites in TF and both require oxidation to the trivalent state for binding to TF (Vincent and Love 2011). We first loaded apo-TF with MnCl2 or CuCl2 using a protocol adapted from Aisen et al. that has been shown to result in trivalent metal binding to TF (1969). We decided to prepare copper (Cu)-loaded TF (Cu-TF) as a negative control as Cu is not known to bind TF in vivo. Both Mn-TF and Cu-TF exhibited absorbance maxima similar to those previously published (Lehrer 1969; Aisen et al. 1969). Measurement of metal concentration in the TF preparations revealed roughly 1 mole Mn per mole Mn-TF and 2.5 mole Cu per mole Cu-TF; loading of Cu onto fully Fe-saturated TF (Fe-TF) resulted in 1.5 mol Fe and 1 mole Cu per mol TF, suggesting that Cu can displace Fe in our loading protocol and also binds non-specifically to TF. Notably, measurement of TF saturation in commercially obtained Fe-TF and our preparations of Mn-TF and Cu-TF detected near 100% saturation for Fe-TF and <10% saturation for Mn- and Cu-TF, possibly reflecting lability of Mn and Cu binding to TF during saturation measurement (data not shown). Treatment of primary hepatocytes with or without apo-TF or Fe-TF led to increased hepcidin levels only in hepatocytes treated with Fe-TF, as expected (data not shown). Treatment of primary hepatocytes with Fe-TF, Mn-TF but not apo-TF led to increased hepcidin levels, with bone morphogenetic protein 6 (BMP6) included as a positive control (Fig. 2g). Surprisingly, treatment of primary hepatocytes with 0.05–3 mg/mL Cu-TF or 5–50 μM CuCl2 in serum-free media led to cell death (data not shown).
Speculations on Recent Findings
While our recent in vivo experiments suggest that TF modulates hepcidin expression in erythropoiesis-dependent and–independent mechanisms and hemojuvelin is essential for TF-dependent hepcidin expression, the mechanism by which erythropoiesis in hpx mice inhibits hepcidin expression is poorly understood. Candidates for factors that mediate this inhibition include BMP signaling antagonists Gdf15 (Tanno et al. 2007), Twsg1 (Tanno et al. 2009) and Bmper (Patel et al. 2011) and hypoxia inducible factors (Peyssonnaux et al. 2007). Roles for these factors in the regulation of hepcidin expression in hypotransferrinemia have yet to be tested in vivo. Our in vitro experiments also demonstrate that TF regulates hepcidin expression in primary hepatocytes, as previously described by several other groups; however, our finding that Mn modulates hepatocyte hepcidin expression is novel. Whether or not this Mn phenomenon has physiologic relevance remains to be determined and requires much more detailed investigations than those presented here.
Mn is an essential trace element required for bone, fat, carbohydrate and nutrient metabolism (Bowman et al. 2011). Mn can be acquired through dietary intake and inhalation and, unlike Fe, undergoes hepatobiliary excretion. While few if any case reports of Mn deficiency exist, Mn excess typically results in neurological conditions, such as manganism, a Parkinson’s disease-like condition due to environmental Mn overexposure (Bowman et al. 2011), and encephalopathy in patients on total parenteral nutrition (Chalela et al. 2011) or with liver failure (Chetri and Choudhuri 2003). A recently published case report on manganism documented a roughly ten-fold increase in serum Mn levels relative to the 5.5–18.2 nM reference range (Brna et al. 2011); although these Mn levels are much lower than those shown above to influence hepatocyte hepcidin levels, it is difficult to directly compare patient data with in vitro assays.
Multiple overlaps in Mn and Fe metabolism are currently known (Roth and Garrick 2003; Fitsanakis et al. 2010). For example, Mn can be transported by DMT1 and ferroportin, cell membrane transporters essential for dietary Fe absorption (Au et al. 2008; Yin et al. 2010; Madejczyk and Ballatori 2011). As hepcidin binds to and stimulates the internalization and degradation of ferroportin. Mn-dependent regulation of hepcidin expression could serve as a negative feedback loop by which excess Mn leads to decreased dietary Mn absorption. Regulation of hepcidin by Mn may not always be adaptive, particularly in conditions of Fe deficiency where increased blood Mn levels have been noted in human population studies (Kim et al. 2005; Meltzer et al. 2010; Kim and Lee 2011). If Mn levels do impact hepcidin levels in vivo, increased Mn levels in conditions of Fe deficiency could increase hepcidin levels, thereby worsening Fe deficiency; alternatively, increased Mn levels in normal Fe states could increase hepcidin levels and lead to Fe deficiency.
Aberrant Mn levels have also been observed in hereditary hemochromatosis, a disease of hepcidin deficiency and Fe overload caused by mutations in HFE, hemojuvelin and other genes (Pietrangelo 2010). Mn and Fe content correlate significantly in livers from hemochromatosis patients (Altstatt et al. 1967). While one group observed increased blood Mn levels in hemochromatosis patients (Hesketh et al. 2008), another recently demonstrated decreased blood Mn levels in women carrying at least one disease-associated HFE allele and mice deficient in Hfe (Claus Henn et al. 2011). Mitochondria from Hfe-deficient mice also contain decreased levels of Mn and Mn superoxide dismutase activity and increased lipid peroxidation, all of which respond to intraperitoneal Mn injection (Jouihan et al. 2008). If Mn levels do impact hepcidin levels in vivo, increased or decreased Mn levels could respectively attenuate or exacerbate the hepcidin deficiency inherent to hereditary hemochromatosis.
Materials and methods
The protocol for hepatocyte isolation was approved by the Animal Care and Use Committee of Children’s Hospital Boston. Hepatocytes were isolated from eight-week old C57BL/6J female mice by catheterization of the vena cava and clipping of the portal vein followed by perfusion at 1.5 mL/min with 4.5 mL perfusion buffer (HBSS without calcium or magnesium/50 mM HEPES pH 8.0/0.5 mM EDTA) and 9 mL collagenase buffer (HBSS with calcium and magnesium/50 mM HEPES/1% bovine serum albumin/0.05% Sigma collagenase type IV). The portal vein was intermittently clamped during collagenase perfusion to increase yield. The liver was then dissected, placed in hepatocyte media (1:1 DMEM/F12/20 mM HEPES pH 8.0/1 mM sodium pyruvate/non-essential amino acids/10% fetal calf serum/penicillin/streptomycin/glutamine) and mechanically dissociated with forceps. The cell mixture was filtered through a 100 μm nylon strainer, centrifuged for 3 minutes at 50 g and resuspended in Percoll Plus (GE/Amersham) and PBS to a final concentration of 35% Percoll. The suspension was centrifuged for 10 minutes at 400 g and the pellet resuspended in hepatocyte media. Viable cell count was determined by trypan blue exclusion. 100 000 viable cells per well were allowed to adhere to collagen-coated six-well plates for 4–6 hours then washed with PBS. Hepatocytes were treated overnight in 1 mL Optimem or hepatocyte media in a 37 °C/5% CO2 incubator. Serum fractionation was performed on a Pharmacia FPLC system using GE HiTrap columns. Serum, diluted to 25% with 20 mM Tris pH 8.0, was applied at 1 mL/min to HiTrap Blue HP (5 mL column volume) and Q HP (1 mL) columns in series; after extensive washing of columns, proteins were eluted from Blue and Q columns respectively with 20 mM Tris pH 8.0/2 M NaCl and 20 mM Tris pH 8.0/75–1000 mM NaCl step gradient. Eluates were concentrated and desalted with Millipore Amicon Ultra 5 kD molecular weight cut-off centrifugal filtration devices. RNA was harvested from hepatocytes using QIAGEN RNeasy kit. For Northern blots, mRNA levels were measured by denaturing formaldehyde agarose gel electrophoresis of at least 2 μg of each RNA sample, with equal ug of RNA loaded per sample in each gel, and Northern blotting using Strategene’s Quikhyb reagent and radiolabelled gene-specific cDNA probes. For quantitative polymerase chain reaction (QPCR), mRNA levels were measured as previously described (Bartnikas et al. 2011). Human apo- and holo (Fe)-TF were purchased from Sigma. Apo-TF was first dissolved in 0.1 M KCl/0.05 M Tris pH 7.5 then subjected to buffer exchange with the same buffer using Millipore Centrifugal Filter Units to remove any phosphate present in the original TF preparation. Apo-TF was loaded with Mn or Cu by incubating 0.25 mM apo-TF with 2.5 mM Mn(II) or Cu(II)-citrate pH 5.0 and 40 mM NaHCO3 overnight at 4 °C with agitation followed by a buffer exchange to PBS using Millipore Centrifugal Filter Units; Mn(II) and Cu(II)-citrate complexes were prepared by mixing 10 mM MnCl2 or CuCl2 with 10 mM citric acid then adjusting pH to 5.0. Copper concentrations in TF preparations were measured using Quantichrom Copper Assay Kit (Bioassay); Mn concentrations in TF preparations were measured using 4-(2-pyridylazo)resorcinol as previously described (Högbom et al. 2005). TF saturations were measured using the Iron/UIBC kit (Thermo Fisher).
Acknowledgments
This work was supported by NIH K99DK084122 and a grant from the Cooley’s Anemia Foundation. I would like to thank Mark Fleming, Nancy Andrews and members of their laboratories for support, assistance and advice.
Footnotes
There are no known conflicts of interest.
References
- Aisen P, Aasa R, Redfield AG. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969;244:4628–4633. [PubMed] [Google Scholar]
- Altstatt LB, Pollack S, Feldman MH, Reba RC, Crosby WH. Liver manganese in hemochromatosis. Proc Soc Exp Biol Med. 1967;124:353–355. doi: 10.3181/00379727-124-31741. [DOI] [PubMed] [Google Scholar]
- Aman J, van der Heijden M, van Lingen A, Girbes ARJ, van Nieuw Amerongen GP, van Hinsbergh VWM, Groeneveld ABJ. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:89–97. doi: 10.1097/CCM.0b013e3181feb46a. [DOI] [PubMed] [Google Scholar]
- Asada-Senju M, Maeda T, Sakata T, Hayashi A, Suzuki T. Molecular analysis of the transferrin gene in a patient with hereditary hypotransferrinemia. J Hum Genet. 2002;47:355–359. doi: 10.1007/s100380200049. [DOI] [PubMed] [Google Scholar]
- Aslan D, Crain K, Beutler E. A new case of human atransferrinemia with a previously undescribed mutation in the transferrin gene. Acta Haematol. 2007;118:244–247. doi: 10.1159/000112726. [DOI] [PubMed] [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnikas TB, Fleming MD. Hemojuvelin is essential for transferrin-dependent and -independent hepcidin expression in mice. Haematologica. 2011 doi: 10.3324/haematol.2011.054031. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117:630–637. doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Li N, Connor JR. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res. 2005;79:254–261. doi: 10.1002/jnr.20324. [DOI] [PubMed] [Google Scholar]
- Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med. 1987;110:690–705. [PubMed] [Google Scholar]
- Beutler E, Gelbart T, Lee P, Trevino R, Fernandez MA, Fairbanks VF. Molecular characterization of a case of atransferrinemia. Blood. 2000;96:4071–4074. [PubMed] [Google Scholar]
- Borini P, Guimarães RC. Liver synthesis function in chronic asymptomatic or oligosymptomatic alcoholics: correlation with other liver tests. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:97–102. doi: 10.1590/s0041-87811999000300006. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brna P, Gordon K, Dooley JM, Price V. Manganese toxicity in a child with iron deficiency and polycythemia. J Child Neurol. 2011;26:891–894. doi: 10.1177/0883073810393962. [DOI] [PubMed] [Google Scholar]
- Buys SS, Martin CB, Eldridge M, Kushner JP, Kaplan J. Iron absorption in hypotransferrinemic mice. Blood. 1991;78:3288–3290. [PubMed] [Google Scholar]
- Canonne-Hergaux F, Levy JE, Fleming MD, Montross LK, Andrews NC, Gros P. Expression of the DMT1 (NRAMP2/DCT1) iron transporter in mice with genetic iron overload disorders. Blood. 2001;97:1138–1140. doi: 10.1182/blood.v97.4.1138. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Bonillha L, Neyens R, Hays A. Manganese encephalopathy: an under-recognized condition in the intensive care unit. Neurocrit Care. 2011;14:456–458. doi: 10.1007/s12028-010-9476-5. [DOI] [PubMed] [Google Scholar]
- Chen C, Wen S, Tan X. Molecular analysis of a novel case of congenital atransferrinemia. Acta Haematol. 2009;122:27–28. doi: 10.1159/000235614. [DOI] [PubMed] [Google Scholar]
- Chetri K, Choudhuri G. Role of trace elements in hepatic encephalopathy: zinc and manganese. Indian J Gastroenterol. 2003;22(Suppl 2):S28–30. [PubMed] [Google Scholar]
- Claus Henn B, Kim J, Wessling-Resnick M, Tellez-Rojo MM, Jayawardene I, Ettinger AS, Hernandez-Avila M, Schwartz J, Christiani DC, Hu H, Wright RO. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ Health. 2011;10:97. doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci U S A. 1987;84:3457–3461. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes E, Fellay G. Hypotransferrinemia in chronic hemodialyzed (HD) patients. Artif Organs. 2000;24:988–989. doi: 10.1046/j.1525-1594.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- Dickinson TK, Connor JR. Cellular distribution of iron, transferrin, and ferritin in the hypotransferrinemic (Hp) mouse brain. J Comp Neurol. 1995;355:67–80. doi: 10.1002/cne.903550109. [DOI] [PubMed] [Google Scholar]
- Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J Lab Clin Med. 1996;128:270–278. doi: 10.1016/s0022-2143(96)90028-1. [DOI] [PubMed] [Google Scholar]
- Fellman V. The GRACILE syndrome, a neonatal lethal metabolic disorder with iron overload. Blood Cells Mol Dis. 2002;29:444–450. doi: 10.1006/bcmd.2002.0582. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Garcia S, Aschner M. Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox Res. 2010;18:124–131. doi: 10.1007/s12640-009-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forejtnikovà H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, Gaudry M, Camaschella C, Lacombe C, Roetto A, Mayeux P, Verdier F. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010;116:5357–5367. doi: 10.1182/blood-2010-04-281360. [DOI] [PubMed] [Google Scholar]
- Fraenkel PG, Gibert Y, Holzheimer JL, Lattanzi VJ, Burnett SF, Dooley KA, Wingert RA, Zon LI. Transferrin-a modulates hepcidin expression in zebrafish embryos. Blood. 2009;113:2843–2850. doi: 10.1182/blood-2008-06-165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galet S, Schulman HM, Bard H. The postnatal hypotransferrinemia of early preterm newborn infants. Pediatr Res. 1976;10:118–120. doi: 10.1203/00006450-197602000-00010. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- Gao J, Chen J, Kramer M, Tsukamoto H, Zhang A-S, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke SG, Kulaksiz H, Herrmann T, Riedel H-D, Bents K, Veltkamp C, Stremmel W. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102:371–376. doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- Golka K, Wiese A. Carbohydrate-deficient transferrin (CDT)--a biomarker for long-term alcohol consumption. J Toxicol Environ Health B Crit Rev. 2004;7:319–337. doi: 10.1080/10937400490432400. [DOI] [PubMed] [Google Scholar]
- Hesketh S, Sassoon J, Knight R, Brown DR. Elevated manganese levels in blood and CNS in human prion disease. Mol Cell Neurosci. 2008;37:590–598. doi: 10.1016/j.mcn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Hoefkens P, Huijskes-Heins MI, de Jeu-Jaspars CM, van Noort WL, van Eijk HG. Influence of transferrin glycans on receptor binding and iron-donation. Glycoconj J. 1997;14:289–295. doi: 10.1023/a:1018510309524. [DOI] [PubMed] [Google Scholar]
- Högbom M, Ericsson UB, Lam R, Bakali HMA, Kuznetsova E, Nordlund P, Zamble DB. A high throughput method for the detection of metalloproteins on a microgram scale. Mol Cell Proteomics. 2005;4:827–834. doi: 10.1074/mcp.T400023-MCP200. [DOI] [PubMed] [Google Scholar]
- Hu WL, Chindemi PA, Regoeczi E. Reduced hepatic iron uptake from rat aglycotransferrin. Biol Met. 1991;4:90–94. doi: 10.1007/BF01135384. [DOI] [PubMed] [Google Scholar]
- Huggenvik JI, Craven CM, Idzerda RL, Bernstein S, Kaplan J, McKnight GS. A splicing defect in the mouse transferrin gene leads to congenital atransferrinemia. Blood. 1989;74:482–486. [PubMed] [Google Scholar]
- Jaeken J. Congenital disorders of glycosylation. Ann N Y Acad Sci. 2010;1214:190–198. doi: 10.1111/j.1749-6632.2010.05840.x. [DOI] [PubMed] [Google Scholar]
- Jouihan HA, Cobine PA, Cooksey RC, Hoagland EA, Boudina S, Abel ED, Winge DR, McClain DA. Iron–mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med. 2008;14:98–108. doi: 10.2119/2007-00114.Jouihan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lee B-K. Iron deficiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology. 2011;32:247–254. doi: 10.1016/j.neuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park JK, Choi Y, Yoo C-I, Lee CR, Lee H, Lee J-H, Kim S-R, Jeong T-H, Yoon CS, Park J-H. Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology. 2005;26:107–111. doi: 10.1016/j.neuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum B. Hypotransferrinemia of chronically hemodialyzed patients. Artif Organs. 1999;23:1047–1054. doi: 10.1046/j.1525-1594.1999.06328.x. [DOI] [PubMed] [Google Scholar]
- Knisely AS, Gelbart T, Beutler E. Molecular characterization of a third case of human atransferrinemia. Blood. 2004;104:2607. doi: 10.1182/blood-2004-05-1751. [DOI] [PubMed] [Google Scholar]
- Lehrer SS. Fluorescence and absorption studies of the binding of copper and iron to transferrin. J Biol Chem. 1969;244:3613–3617. [PubMed] [Google Scholar]
- Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, Cabantchik ZI, Bouhassira EE, Fabry ME, Ginzburg YZ. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochimica Et Biophysica Acta. 2011 doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Scharpe S, Bosmans E, Vandewoude M, Suy E, Uyttenbroeck W, Cooreman W, Vandervorst C, Raus J. Disturbances in acute phase plasma proteins during melancholia: additional evidence for the presence of an inflammatory process during that illness. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:501–515. doi: 10.1016/0278-5846(92)90056-k. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Cook BM, Devenyi AG, Beard JL, Connor JR. Transferrin is required for normal distribution of 59Fe and 54Mn in mouse brain. J Neurol Sci. 1999;170:112–118. doi: 10.1016/s0022-510x(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Mason AB, Miller MK, Funk WD, Banfield DK, Savage KJ, Oliver RW, Green BN, MacGillivray RT, Woodworth RC. Expression of glycosylated and nonglycosylated human transferrin in mammalian cells. Characterization of the recombinant proteins with comparison to three commercially available transferrins. Biochemistry. 1993;32:5472–5479. doi: 10.1021/bi00071a025. [DOI] [PubMed] [Google Scholar]
- Meltzer HM, Brantsaeter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, Stigum H, Ydersbond TA. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res. 2010;110:497–504. doi: 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Morton AG, Tavill AS. The role of iron in the regulation of hepatic transferrin synthesis. Br J Haematol. 1977;36:383–394. doi: 10.1111/j.1365-2141.1977.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Patel N, Masaratana P, Diaz-Castro J, Latunde-Dada O, Qureshi A, Lockyer P, Jacob M, Arno M, Matak P, Mitry R, Hughes R, Dhawan A, Patterson C, Simpson RJ, McKie AT. BMPER is a negative regulator of hepcidin and is up-regulated in hypotransferrinemic mice. The Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M111.310789. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408. 408.e1–2. doi: 10.1053/j.gastro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Prinsen BH, de Sain-van der Velden MG, Kaysen GA, Straver HW, van Rijn HJ, Stellaard F, Berger R, Rabelink TJ. Transferrin synthesis is increased in nephrotic patients insufficiently to replace urinary losses. J Am Soc Nephrol. 2001;12:1017–1025. doi: 10.1681/ASN.V1251017. [DOI] [PubMed] [Google Scholar]
- Raja KB, Pountney DJ, Simpson RJ, Peters TJ. Importance of anemia and transferrin levels in the regulation of intestinal iron absorption in hypotransferrinemic mice. Blood. 1999;94:3185–3192. [PubMed] [Google Scholar]
- Ramey G, Deschemin J-C, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94:765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104:4294–4299. doi: 10.1182/blood-2004-06-2481. [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Takeda A, Devenyi A, Connor JR. Evidence for non-transferrin-mediated uptake and release of iron and manganese in glial cell cultures from hypotransferrinemic mice. J Neurosci Res. 1998;51:454–462. doi: 10.1002/(SICI)1097-4547(19980215)51:4<454::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Takeda A, Takatsuka K, Connor JR, Oku N. Abnormal iron accumulation in the brain of neonatal hypotransferrinemic mice. Brain Res. 2001;912:154–161. doi: 10.1016/s0006-8993(01)02719-6. [DOI] [PubMed] [Google Scholar]
- Tanno T, Bhanu NV, Oneal PA, Goh S-H, Staker P, Lee YT, Moroney JW, Reed CH, Luban NLC, Wang R-H, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- Tanno T, Porayette P, Sripichai O, Noh S-J, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenor CC, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–1118. [PubMed] [Google Scholar]
- Trombini P, Coliva T, Nemeth E, Mariani R, Ganz T, Biondi A, Piperno A. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica. 2007;92:1407–1410. doi: 10.3324/haematol.11377. [DOI] [PubMed] [Google Scholar]
- Vaziri ND. Erythropoietin and transferrin metabolism in nephrotic syndrome. Am J Kidney Dis. 2001;38:1–8. doi: 10.1053/ajkd.2001.25174. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Love S. The binding and transport of alternative metals by transferrin. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.07.003. [in press] [DOI] [PubMed] [Google Scholar]
- Warshaw BL, Check IJ, Hymes LC, DiRusso SC. Decreased serum transferrin concentration in children with the nephrotic syndrome: effect on lymphocyte proliferation and correlation with serum immunoglobulin levels. Clin Immunol Immunopathol. 1984;33:210–219. doi: 10.1016/0090-1229(84)90076-x. [DOI] [PubMed] [Google Scholar]
- Wilkins SJ, Frazer DM, Millard KN, McLaren GD, Anderson GJ. Iron metabolism in the hemoglobin-deficit mouse: correlation of diferric transferrin with hepcidin expression. Blood. 2006;107:1659–1664. doi: 10.1182/blood-2005-07-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Lee E-SY, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]