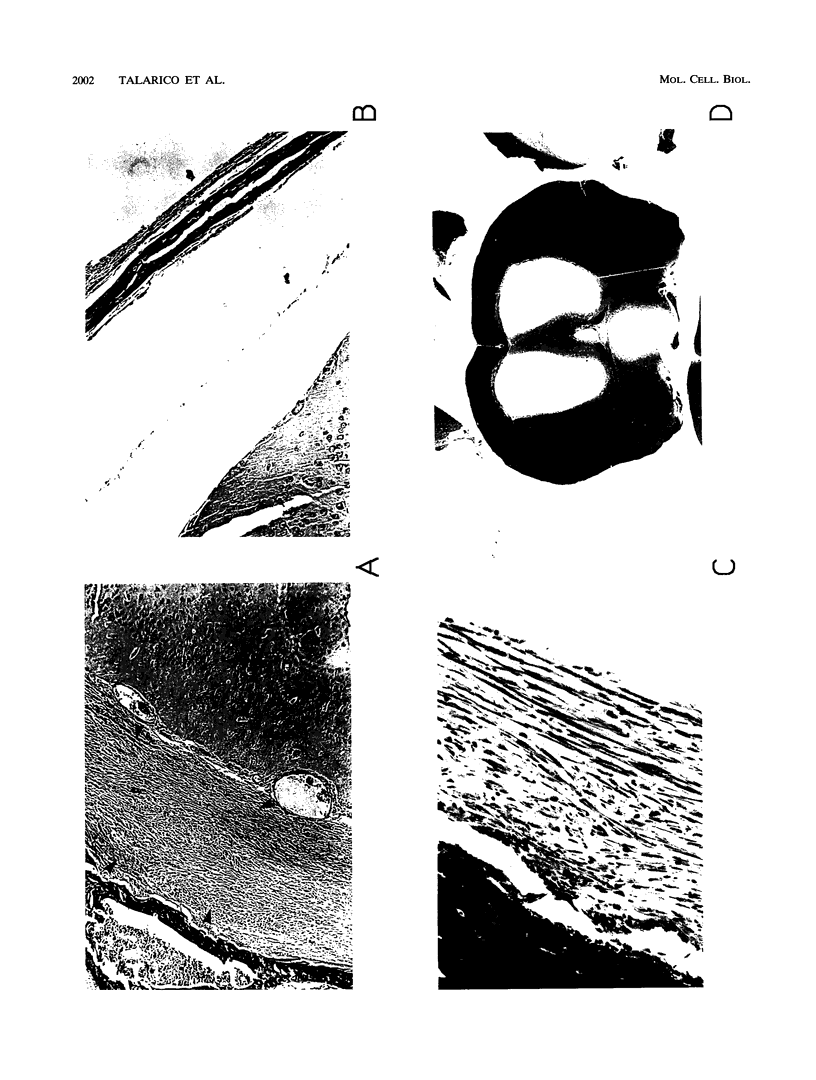
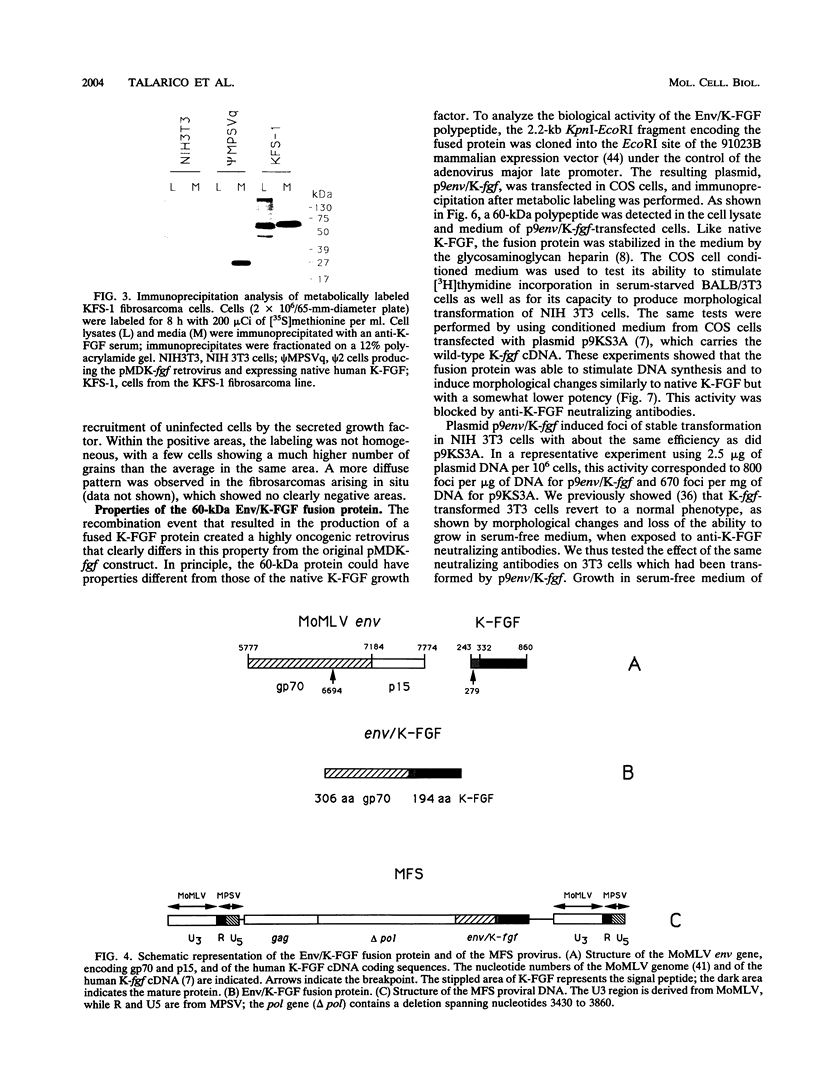
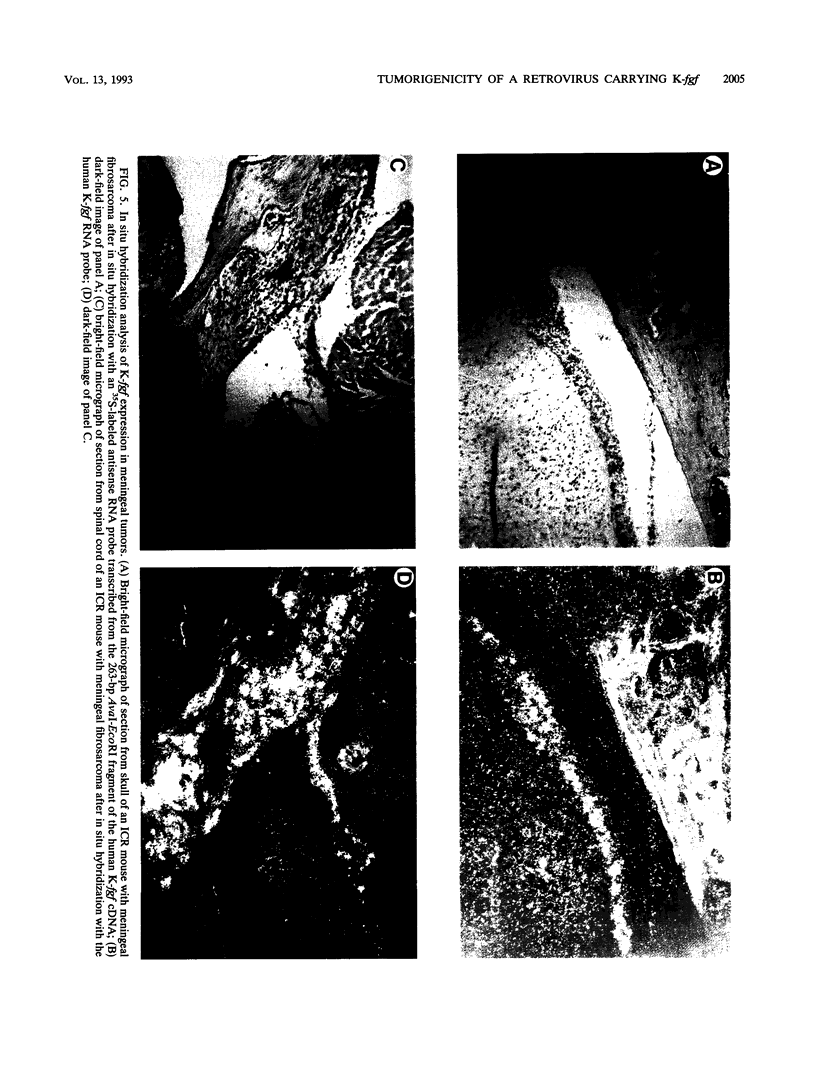
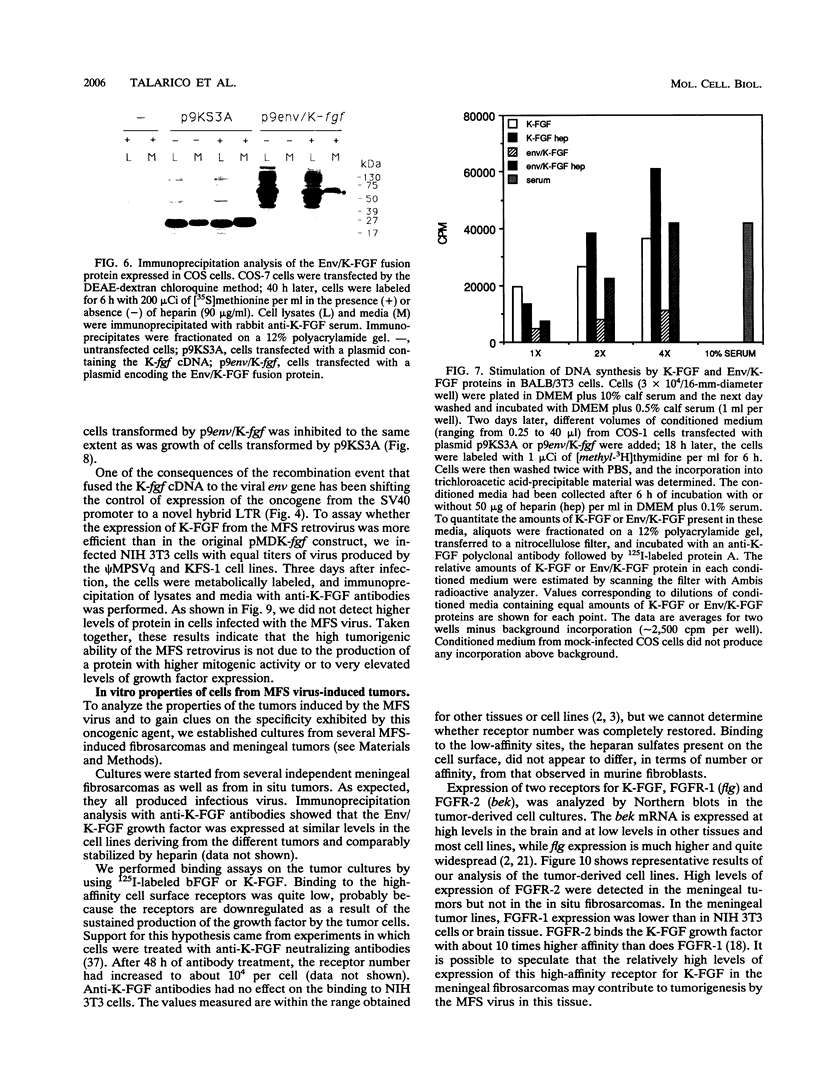
Abstract
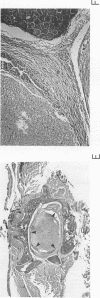
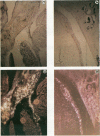
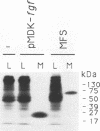
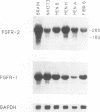
The K-fgf/hst oncogene encodes a growth factor of the fibroblast growth factor (FGF) family and transforms cells through an autocrine mechanism which requires extracellular activation of its receptor(s). To identify the cell and tissue targets of K-fgf oncogenic potential in vivo, we constructed a recombinant retrovirus carrying the human K-fgf cDNA and injected it, together with helper Moloney murine leukemia virus, into immunocompetent as well as nude mice. The original construct was highly transforming in tissue culture but produced no detectable pathologies in vivo with the exception of a single fibrosarcoma which arose after a long latency. The virus produced by this tumor appears to have undergone a complex series of recombination events involving the helper Moloney murine leukemia virus. It encodes an Env/K-FGF fusion protein whose expression is under the control of a hybrid long terminal repeat. This virus (designated MFS, for meningeal fibrosarcoma) induces tumors in mice with high frequency and short latency. These neoplasms consist of aggressive fibrosarcomas of soft tissue as well as diffuse meningeal tumors originating from the dura mater that surround the whole central nervous system and cause severe hydrocephalus. The Env/K-FGF fusion protein expressed by the MFS virus has retained all of the biological properties of native K-FGF, including secretion, mitogenic activity, heparin binding, and neutralization by anti-K-FGF antibodies. These and other results indicate that the tumors induced by the MFS virus result from the oncogenic potential of K-FGF.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Basilico C. Isolation of a rearranged human transforming gene following transfection of Kaposi sarcoma DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5660–5664. doi: 10.1073/pnas.84.16.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolberg D. S., Hollingsworth R., Hertle M., Bissell M. J. Wounding and its role in RSV-mediated tumor formation. Science. 1985 Nov 8;230(4726):676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Moellmann G., Ghosh S., Edwards M., Halaban R. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J Cell Biol. 1989 Dec;109(6 Pt 1):3115–3128. doi: 10.1083/jcb.109.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Fan H., Croker B. Infection of preimplantation mouse embryos and of newborn mice with leukemia virus: tissue distribution of viral DNA and RNA and leukemogenesis in the adult animal. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4008–4012. doi: 10.1073/pnas.72.10.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan M., DiSorbo D., Hou J. Z., Hoshi H., Mansson P. E., McKeehan W. L. High and low affinity binding of heparin-binding growth factor to a 130-kDa receptor correlates with stimulation and inhibition of growth of a differentiated human hepatoma cell. J Biol Chem. 1988 Aug 15;263(23):11306–11313. [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mansukhani A., Dell'Era P., Moscatelli D., Kornbluth S., Hanafusa H., Basilico C. Characterization of the murine BEK fibroblast growth factor (FGF) receptor: activation by three members of the FGF family and requirement for heparin. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3305–3309. doi: 10.1073/pnas.89.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Moscatelli D., Talarico D., Levytska V., Basilico C. A murine fibroblast growth factor (FGF) receptor expressed in CHO cells is activated by basic FGF and Kaposi FGF. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Gazit A., Arnstein P., Aaronson S. A. Generation of fibrosarcomas in vivo by a retrovirus that expresses the normal B chain of platelet-derived growth factor and mimics the alternative splice pattern of the v-sis oncogene. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2693–2697. doi: 10.1073/pnas.86.8.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Placzek M., Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5678–5682. doi: 10.1073/pnas.86.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Mori M., Taira M., Yoshida T., Matsukawa S., Shimizu K., Sekiguchi M., Terada M., Sugimura T. Transforming gene from human stomach cancers and a noncancerous portion of stomach mucosa. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatier P., Morgenstern J., Beddington R. S. Permissiveness to murine leukemia, virus expression during preimplantation and early postimplantation mouse development. Development. 1990 Jul;109(3):655–665. doi: 10.1242/dev.109.3.655. [DOI] [PubMed] [Google Scholar]
- Souyri M., Koehne C. F., O'Donnell P. V., Aldrich T. H., Furth M. E., Fleissner E. Biological effects of a murine retrovirus carrying an activated N-ras gene of human origin. Virology. 1987 May;158(1):69–78. doi: 10.1016/0042-6822(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Souyri M., Vigon I., Charon M., Tambourin P. Oncogenicity of human N-ras oncogene and proto-oncogene introduced into retroviral vectors. J Virol. 1989 Sep;63(9):3944–3953. doi: 10.1128/jvi.63.9.3944-3953.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Arbuthnott C., Kollek R., Coggins L., Ostertag W. Comparison of myeloproliferative sarcoma virus with Moloney murine sarcoma virus variants by nucleotide sequencing and heteroduplex analysis. J Virol. 1984 Jun;50(3):725–732. doi: 10.1128/jvi.50.3.725-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Weinberg R. A. Analysis of viral and somatic activations of the cHa-ras gene. J Virol. 1985 Jan;53(1):260–265. doi: 10.1128/jvi.53.1.260-265.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico D., Basilico C. The K-fgf/hst oncogene induces transformation through an autocrine mechanism that requires extracellular stimulation of the mitogenic pathway. Mol Cell Biol. 1991 Feb;11(2):1138–1145. doi: 10.1128/mcb.11.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico D., Ittmann M., Balsari A., Delli-Bovi P., Basch R. S., Basilico C. Protection of mice against tumor growth by immunization with an oncogene-encoded growth factor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4222–4225. doi: 10.1073/pnas.87.11.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackym P. A., Popper P., Ward P. H., Micevych P. E. In situ hybridization for the study of gene expression in neuro-otologic research. Otolaryngol Head Neck Surg. 1990 Oct;103(4):519–526. doi: 10.1177/019459989010300402. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Courtneidge S. A., Wagner E. F. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell. 1988 Jan 15;52(1):121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]