Abstract
The roles of Wnts in neural development, synaptogenesis, and cancer are generally well characterized. Nonetheless, evidence exists that interactions between the immune and nervous systems control major brain regenerative processes ranging from physiological or pathological (reparative) regeneration to neurogenesis and synaptic plasticity. Recent studies describe deregulated Wnt-Fzd signaling in degenerative and inflammatory central nervous system (CNS) disorders, and the expression of Wnt signaling components in the immune system, and in immune-like cells of the mammalian CNS. This would suggest a likely involvement of Wnts in inflammation-driven brain damage and inflammation-directed brain repair. Here, we review how Wnts modulate neuroimmune interactions and offer a perspective on the most challenging therapeutic opportunities for those CNS diseases where injury-reactive Wnt-flavored inflammation precedes secondary neurodegeneration.
Keywords: Wnt, inflammation, neurodegeneration, neuroimmune interactions, neurogenesis, neural repair
Wnt, the immune system, and neuronal health
The Wnt family of secreted glycoproteins are cell- and tissue-specific ligands that orchestrate a wide range of processes in the developing and adult brain, including neural induction and patterning, cell proliferation, cell fate specification, cell polarization and migration, axon guidance, synaptogenesis, adult neurogenesis, and neuron maintenance and regeneration [1-3]. The continuous presence of Wnt proteins in the adult brain, their central role in maintaining cell integrity, and the emerging recognition that Wnt signaling is deregulated in degenerative and inflammatory CNS disorders [4,5], clearly anticipates that Wnt signaling may play critical roles in maintaining and/or protecting neuronal functions in both healthy and diseased states.
Increasing evidence suggests that neuroimmune interactions control several regenerative processes in the brain, including neuronal homeostasis and the promotion of neuronal restoration, neurogenesis, and synaptic plasticity in response to injury [6]. The expression of Wnt ligands and Wnt signaling components has only recently been identified in cells of the immune system [7] and immune-like cells of the mammalian CNS – including macrophages/microglia and astrocytes [8,9] – suggesting that this signaling pathway might play a key role in inflammation-driven brain damage or inflammation-directed brain repair.
New evidence prompts us to propose a novel ‘intrinsic Wnt brain repair hypothesis’ inspired by available data as well as some redundancy of Wnt signaling in neuroimmune interactions after CNS injuries [10-16]. This redundancy holds promise for targeting the key actors of this pathway to ‘switch on’ endogenous brain repair capabilities.
Wnt signaling has a multifaceted role in CNS diseases, in which inflammation and oxidative stress, the key hall-marks of the aged diseased brain, modulate Wnt signaling cascades via interactions between macrophage/microglia and astrocytes [8,9,15,17,18]. Reciprocally, sustained Wnt/β-catenin activation restrains inflammation, incites neuroprotection, and promotes neurogenesis [8,10,15-17].
Central to this Wnt/neuroimmune dialog are interactions between glia and neurons or glia and neural stem/progenitor cells (NPCs) brought about by crosstalk through glycogen synthase kinase-3β (GSK-3β) and β-catenin, two principal Wnt signaling molecules that aim to overcome inflammatory neural tissue damage while promoting tissue repair or restoration.
We envision translating the knowledge of basal Wnt-driven neuroimmune interactions (as opposed to injury-reactive responses) into innovative therapeutics with great clinical potential for CNS diseases where injury-reactive inflammation drives secondary neurodegeneration, such as traumatic injuries, Parkinson’s disease (PD), and stroke. Building our hypothesis on solid emerging evidence, in this opinion article we synthesize available experimental information to produce a testable model to inspire future research endeavors.
The Wnt landscape
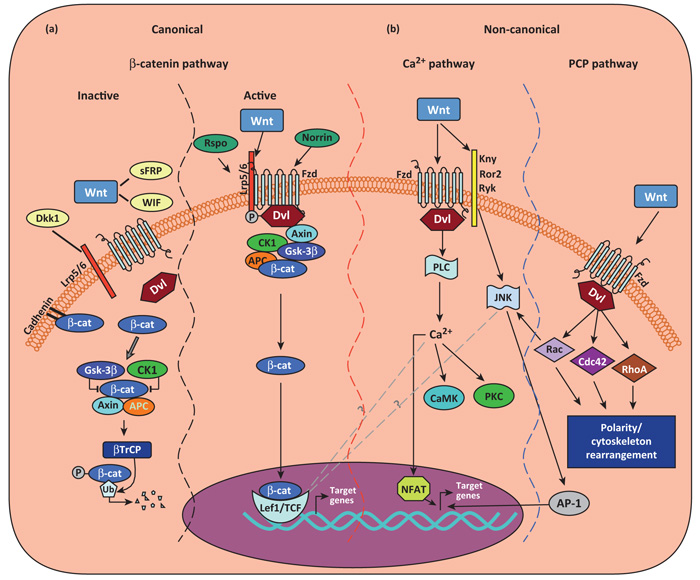
Initially identified as a key signaling system of Drosophila development, the Wnt (Wingless-related MMTV integration site) family of secreted, lipid-modified glycoproteins [19] has emerged as a multifunctional signaling cascade (see Glossary). Members of the Wnt family control the proliferation and/or differentiation and survival of a variety of cell types, including progenitor stem cells, postmitotic neurons, and glial cells [20-22]. A total of 19 Wnt proteins have been identified in mammals (see ‘The Wnt homepage’ at http://www.stanford.edu/group/nusselab/cgi-bin/wnt/), which are classified into functional groups according to their ability to induce a secondary body axis in Xenopus embryos and activate specific signaling cascades. Briefly, these classes are the Wnt1 subtype (Wnt2, Wnt3, Wnt3a, and Wnt8a) and the Wnt5a type (Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11). Generally, the Wnt1 class works via the canonical Wnt/β-catenin signaling pathway, whereas the Wnt5a class operates via the non-canonical Wnt/planar cell polarity (PCP) or Wnt/Ca2+ pathways; nevertheless, a functional Wnt classification is an oversimplification as there are contexts in which the same Wnt protein will activate different pathways depending on the specific cell type and the receptor present [23]. Common to all three pathways is the binding of a Wnt ligand to the seven-pass transmembrane receptors of the Frizzled (Fzd) family, although Wnt can also bind to other single-pass transmembrane receptors [24,25]. The specific activation of any one of these three signaling pathways appears to depend on the specific complement of Fzd receptors and coreceptors of the low-density lipoprotein receptor-related protein (Lrp) family on the cell surface and the Wnt ligand activating these receptors (Figure 1). Several key molecules have central roles in Wnt pathways and understanding their functions is crucial for understanding the roles of Wnt in disease.
Figure 1.
Canonical or Wnt/β-catenin–TCF/LEF signaling. Three Wnt-dependent pathways have been proposed: canonical Wnt/β-catenin pathway and non-canonical Wnt/PCP and Wnt/Ca2+ pathways. In the canonical Wnt/β-catenin pathway (a), in the absence of Wnt ligands (or in the presence of Wnt inhibitor WIF, sFRPs, or Dkk1), cytosolic β-catenin (β-cat) is targeted to proteolytic degradation through phosphorylation by adenomatous poliposis coli (APC)–Axin–glycogen synthase kinase-3β (GSK-3β)–casein kinase 1 (CK1) destruction complex. Phosphorylated β-cat becomes ubiquitinated through action of β-transducin repeat containing protein–(β-TrCP)-dependent E3 ubiquitin ligase complex and degraded in the proteasome. In the active state, Wnt ligand binding to Fzd receptors and their coreceptors Lrp5/6 leads to the activation of the cytoplasmic protein dishevelled (Dvl), and subsequent recruitment of Axin complex to the Lrp coreceptor, resulting in inhibition of the β-catenin destruction complex. Consequently, hypophosphorylated β-cat accumulate in the cytoplasm and enter the nucleus, where it regulates target gene expression through partnerships with the TCR/LEF1 family of transcription factors, resulting in changes in gene transcription. Two types of proteins, Norrin and R-spondins (Rspo), which are unrelated to Wnt, act as Wnt agonists. In the non-canonical Wnt–Ca2+ signaling pathway (b), the binding of Wnt promotes Fzd-mediated activation of pertussis Toxin-sensitive heterotrimeric guanine nucleotide-binding proteins (G proteins). This, in turn, stimulates the release of Ca2+ from intracellular stores, which leads to the activation of Ca2+-dependent effector molecules. Several Ca2+-sensitive targets – protein kinase C (PKC), Ca2+–calmodulin-dependent protein kinase II (CamKII), and the Ca2+–calmodulin-sensitive protein phosphatase calcineurin – have been identified downstream of the Wnt–Ca2+ pathway. Targets of the Wnt–Ca2+ pathway appear to interact with the Wnt–β-catenin pathway at multiple points. Additionally, Fzd receptors in association with Kny, Ror2, or Ryk receptors can activate JNK, promoting target gene expression through AP-1. In the non-canonical Wnt/PCP pathway, the binding of Wnts activates RhoA/B, Cdc42, or Rac1. Dsh activates Rac1 and Rac1 can also activate JNK, resulting in the NFAT pathway. Abbreviations: Dkk1, Dickkopf 1; Fzd, Frizzled; sFRPs, secreted Frizzled-related proteins; Lrp, low-density lipoprotein receptor-related protein; PCP, planar cell polarity; TCF/LEF, T cell factor/lymphoid enhancer factor; WIF, Wnt inhibitory protein; Wnt, Wingless-related MMTV integration site.
Dishevelled (Dvl), a multifunctional cytoplasmic phosphoprotein, is a key transducer of Wnt signaling in all three Wnt–Fzd signaling cascades and acts at either the plasma membrane or in the cytoplasm [26]. GSK-3β holds a pivotal position in the canonical Wnt pathway, where it phosphorylates β-catenin in association with casein kinase (CK) 1, the adenomatous polyposis coli (APC) tumor suppressor protein, and the scaffolding protein axin. Activation of canonical Wnt signaling initially leads to the formation of a complex involving Dvl, axin, and GSK-3β. As a consequence, GSK-3β phosphorylation of β-catenin is downregulated, and β-catenin is allowed to accumulate in the cytoplasm [27]. The stabilized β-catenin then enters the nucleus to interact with T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to regulate the transcription of target genes involved in the cell cycle, survival, and differentiation (Figure 1a).
Wnt proteins are also implicated in the activation of other intracellular messengers, via non-canonical Wnt signaling (Figure 1b). Wnt can induce the release of calcium from intracellular stores, possibly via α heterotrimeric G proteins, leading to the activation of Ca2+-dependent effector molecules, including protein kinase C (PKC) or Ca2+/cal-modulin-dependent protein kinase (CamKII) [25,27,28] (Figure 1b). Wnt also stimulates the planar cell polarity pathway by activating the small GTPases Rho and Rac, leading to cytoskeletal rearrangements (Figure 1b). Another, although less well understood, Wnt-activated mechanism involves the tyrosine kinase receptor Ror and the related to tyrosine kinase protein Ryk, which control the activities of the JNK and Src kinases, respectively [25,28].
As a reflection of the importance of Wnt signaling, there are several levels of regulation at different steps of the Wnt cascade. Among these regulators, the secreted Dickkopf (Dkk) proteins function as antagonists of canonical Wnt signaling [27], whereas the secreted Frizzled-related proteins (sFRPs) and Wnt inhibitory protein (WIF) can both bind Wnt, thereby inhibiting interactions between Wnt and Wnt receptors. However, in certain tissues, specific sFRPs family members positively modulate canonical Wnt signaling [29], whereas in other cells they activate the Wnt/planar cell polarity/Rac1 pathway [30], suggesting that Wnt signaling outcome may depend, among other things, on the specific tissue and distribution of sFRPs. In addition, two types of proteins unrelated to Wnt, Norrin and the R-spondins (Rspo) [31], can act as Wnt agonists (Figure 1a).
As a whole, complex interregulation exists between the canonical and non-canonical pathways, so that activation of β-catenin suppresses the non-canonical Wnt/Ca2+ pathway; similarly, downregulation of β-catenin levels by Wnt antagonists may activate the non-canonical Wnt/Ca2+ pathway [23,25,28-30].
Intuitively, the deregulation of such a multifaceted signaling pathway would have a real impact on the pathophysiology of complex inflammatory CNS diseases. Under such deregulation, neural and non-neural cellular sources of Wnt, and responders to Wnt, could accumulate in the microenvironment and likely contribute to the development of a paradoxical intrinsic response to brain damage based upon both paracrine and autocrine loops.
Responses to Wnts in the mature immune system
Several important studies have addressed a potential role for Wnt signaling in regulating different functions in the immune system, both during development at the level of the thymus and bone marrow [7], as well as in mature peripheral immune cells [32]. Here, we first focus on the regulation of mature immune cell responses by the Wnt signaling pathway – especially those occurring in T cells, dendritic cells (DCs), and monocytes/macrophages – and then consider the likely consequences of this in the context of brain injuries or disease.
Circulating (peripheral) T cells dynamically express high levels of the Wnt pathway transcription factors TCF/LEF, levels which change in response to the T cell activation state [7]. In CD4+ T cells, TCF1 expression is regulated by TCR and cytokine signaling. Early exposure to interleukin (IL)-4 prepares CD4+ helper T (Th) cells for Th2 differentiation, whereby the Wnt–TCF–β-catenin axis positively regulates the Th2 initiation via induction of early GATA-3 expression in a TCR-dependent – but IL-4 receptor-independent – manner. This effect is likely direct, as Stat6 binds to several putative binding sites in the TCF7 locus in an IL-4-dependent manner [33]. Like TCF1, LEF1 is also downregulated in Th2 cells and its forced expression significantly reduces Th2 (anti)inflammatory cytokine secretion [34]. TCF1 also negatively regulates Th1/Th17 initiation, by suppressing the expression of interferon (IFN) γ [35]. Interestingly, these effects appear to be independent from β-catenin, but rather are likely due to direct suppression of Il17 gene transcription via direct binding to intronic TCF1 consensus motifs [36].
Less clear is the role of TCF1 or β-catenin in regulatory T (Treg) cells. However, recent evidence using either retroviral-mediated introduction of stabilized β-catenin [37] or systemic administration of a GSK-3β inhibitor [38] suggest that Wnt signaling pathways may enhance the expression of the transcription factor FoxP3, thus leading to increased survival and overall (tolerogenic) activity of CD4+/CD25+ Treg cells in vivo.
Wnt signaling is also important for DC differentiation. Whereas the Wnt–TCF/LEF–β-catenin axis cooperates with Notch signaling to promote the differentiation of DCs (but not macrophages) from hematopoietic progenitor cells cultured with granulocyte macrophage colony-stimulating factor (GM-CSF), Wnt5a-dependent non-canonical Wnt signaling inhibits DC differentiation [39].
Toll-like receptor agonists in macrophages induce the proinflammatory Wnt5a that works through autocrine and paracrine non-canonical Wnt signaling pathways via Fzd5. By contrast, the Wnt3a/Fzd1-dependent canonical Wnt signaling pathway is anti-inflammatory [40].
Thus, depending on the sources, mechanisms of signaling, and crosstalk with other pathways, the response to Wnt ligands in immune cells may have both pro- and anti-inflammatory effects (Table 1).
Table 1. Wnt signaling in neuroimmune interactions.
| Protein | Source | Role in the immune/neural systems | Signaling | Refs |
|---|---|---|---|---|
| Wnt1 | Activated astrocytes (VM and striatal); activated endothelial cells; hippocampal neurons (HNs) |
Promotes survival and neuroprotection of midbrain DA neurons; rescues SVZ stem/progenitor cells; induces extravasation of activated T cells; promotes astrocyte–neuron and astrocyte–microglia crosstalk; protects HNs against inflammatory/oxidative insult, in vivo/in vitro |
Canonical/β-catenin | [8,10,17,63,79,80] |
| Wnt2 | Mouse macrophages | ND | Canonical/β-catenin | [59] |
| Wnt2b | Activated endothelial cells | Promotes extravasation of activated T cells | ND | [63] |
| Wnt3a | Activated macrophages; neural stem/precursor cells; hippocampus, HNs |
Anti-inflammatory in macrophages; promotes self-renewal, clonal expansion, and neurogenesis of SVZ and SGZ stem/progenitor cells; protects hippocampus, HNs, and adult hippocampal stem/precursors (in vivo/in vitro) |
Canonical/β-catenin | [4,5,40,42,46,47, 51,53,71,73,86] |
| Wnt4 | Activated endothelial cells | Promotes extravasation of activated T cells | Canonical/β-catenin | [63] |
| Wnt5a | Activated CD4+ T cells and human macrophages; activated endothelial cells, astrocytes, and microglia |
Stimulates chemokine-directed T cell migration; inhibits DC differentiation; tolerogenic on DCs; proinflammatory in macrophages and microglia; promotes autocrine endothelial inflammation; promotes proinflammatory response in microglia |
Non-canonical/β-catenin [9,18,40,58,60, 62-64] |
|
| Wnt7a | ND | Promotes self-renewal, clonal expansion, and neurogenesis of SGZ stem/progenitor cells |
TLX/catenin and Wnt–Ca2+ | [46,48] |
| Wnt7b | Mouse macrophages | Promotes programmed cell death of vascular endothelial cells and tissue healing |
Wnt–Ca2+ | [59,61] |
| Wnt8b | Activated endothelial cells | Promotes extravasation of activated T cells | Canonical/β-catenin | [63] |
| Wnt10b | Mouse macrophages | ND | Canonical/β-catenin | [59] |
Responses to Wnt in the adult brain
The established function of Wnt signaling in regulating neurogenesis and synaptogenesis during the development of the neural tube [41], along with the expression of Wnt ligands and Wnt signaling components and in the adult mammalian CNS [4,42], suggests that Wnts may also play important roles in maintaining and protecting the brain throughout life, both under physiological conditions as well as upon injury.
Neurogenesis continues at the level of at least two main discrete neurogenic germinal-like regions of the postnatal mammalian brain, the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus [6]. In the SVZ, new neurons (type A cells) are continuously born from transit-proliferating type C progenitor cells that derive from slow-cycling stem cells (type B1 cells). The SVZ neuroblasts form chains of migration along the rostral migratory stream (RMS) to reach the olfactory bulb, where they terminally differentiate into olfactory bulb interneurons. New neurons are also formed at the level of the SGZ of the DG, where radial astrocytes (type 1 cells) function as the primary precursors of the new granular neurons, either directly or through the generation of intermediate progenitor cells (type 2 cells) [43]. These germinal area astrocytes work as real pacemakers of adult neurogenesis, as they receive internal and external inputs from their main shaft, which is close to the cell bodies of other cells (including microglial cells and neurons), as well as from the end feet of their radial processes that contact endothelial cells in the SVZ [44], or embed into the molecular layer in the SGZ [45]. Adult neurogenesis is therefore regulated by both intrinsic (cell autonomous) and extrinsic (cell non-autonomous) factors, which may include paracrine factors, neurotransmitters, seizures, enriched environmental conditions, hormones, exercise, inflammation, and antidepressants [45].
Hippocampal stem/progenitor cells express the receptors and the signaling components for Wnt proteins [42], including Wnt3a and Wnt7a, and the Wnt/β-catenin pathway is active at the level of the SGZ [46]. Interestingly, both Wnt3a (via Fzd) and Wnt7a (via nuclear receptor TLX that activates β-catenin) promote self-renewal and clonal expansion [47,48], as well as neurogenesis from adult hippocampal stem/progenitor cells in vitro and in vivo.
More recent work has revealed that hippocampal stem/progenitor cells produce Wnt to self-stimulate low levels of canonical Wnt signaling, and identified the prospero-related homeodomain transcription factor Prospero homeobox protein 1 (Prox 1) as a novel target of β-catenin–TCF/LEF signaling in vivo [49], thus providing additional indication for an active autocrine Wnt signaling loop within the SGZ niche [50]. Importantly, the transcriptional activation of NeuroD1, a proneural basic helix–loop–helix (bHLH) transcription factor essential for the development of the CNS, and in particular, the generation of granule cells in the hippocampus and cerebellum, is triggered by canonical Wnt/β-catenin activation in neural progenitors [51].
β-Catenin signaling also plays a role in the proliferation of stem/progenitor cells in the SVZ of the adult mouse brain. Sustained β-catenin signaling (via GSK-3β inhibition) increases the numbers of new integrated neurons in the olfactory bulb in vivo [52], whereas Wnt-3a and Wnt-5a promote self-renewal and neurogenesis in vitro [53]. Two recent studies have identified novel interactors of Wnt signaling/β-catenin in the adult SVZ niche. Marinaro et al. showed that the homeodomain interacting protein kinase-1 gene (Hipk1) specifically interacts with β-catenin to regulate the Wnt-dependent differentiation (but not the proliferation) of neural stem/progenitor cells in the adult SVZ [54]; and Zhang et al. showed that the bHLH transcription factors Mash1, Id2, and Hes1 that promote the differentiation of neural stem/progenitor cells in the adult SVZ have opposite effects on β-catenin: Id2 and Hes1 act as stimulators, and Mash1 inhibits the expression of β-catenin and GSK-3β [55].
Outside the SVZ and SGZ, Wnts – through both the canonical and non-canonical signaling pathways – are also key regulators of proliferation and differentiation of dopaminergic (DAergic) neuronal progenitor cells during ventral midbrain (VM) neurogenesis [56]. Although it remains to be clarified whether the generation of new DAergic neurons continues in the adult midbrain, some compelling evidence is accumulating to define a role for β-catenin in the survival and protection of adult midbrain DA neurons both in response to Wnt1/Fzd1 signaling [17], as well as upon negative regulation by the PD-related protein parkin [57].
The innate and (mal)adaptive Wnt response to brain injuries
Wnt ligands are the most potent, naturally occurring stimulatory factors to activate Wnt signaling. In mammals, likely sources of Wnt ligands include both immune cells and neural cells (Table 1). Activated CD4+ T cells produce Wnt5a in response to chemokine signaling, and this autocrine Wnt signaling augments chemokine-directed T cell migration independently from β-catenin [58]. Macrophages are also a source of Wnts. Cultured macrophages from mice express Wnt2 and Wnt10b, and use Wnt7b as a short-range paracrine signal to induce programmed cell death in vascular endothelial cells of the developing eye in vivo [59]. In humans, DCs as well as CD14+ monocyte-derived classically activated macrophages significantly upregulate Wnt5a in response to pathogens (e.g., parasites) [60], whereas tissue-healing macrophages produce Wnt7b to stimulate organ repair and regeneration by inducing the canonical Wnt pathway in epithelial cells [61]. Furthermore, this increase in Wnt5a signaling during monocyte differentiation activates non-canonical Ca2+/calmodulin-dependent protein kinase II/nuclear factor (NF)-κB signaling that confers tolerogenic capabilities to Wnt5a-expressing DCs [62]. Activated astrocytes upregulate expression of Wnt1 [8] and Wnt5a [18], and microglia increase β-catenin levels in response to inflammatory astroglial communication [18], both in vitro and in vivo. Endothelial cells express multiple Wnt transcripts, including Wnt1, Wnt2b, Wnt4, Wnt5a, and Wnt8b. This suggests a role for paracrine Wnt signaling in T cell extravasation [63] and/or autocrine Wnt signaling – via Wnt5a and then cyclooxygenase-2 – in endothelial inflammation [64].
Given that Wnt signaling modulates a wide range of physiological responses in both the immune system and neurons, a great challenge is to understand how Wnt signaling affects disease conditions where critical interactions between the brain and the immune system orchestrate the response to injury and affect the progression of damage, repair, or regeneration.
After brain stroke, stem/progenitor cells in the SVZ shift from asymmetric to symmetric cell division, and migrate towards the ischemic boundary region where they attempt to integrate and replace damaged neurons [65]. Wnt/β-catenin signaling is upregulated in the stroke-damaged brain, where it orchestrates the symmetry of division of SVZ stem/progenitor cells, limits the extent of ischemic neuronal damage [5], and controls postischemia neurogenesis [66].
After traumatic brain injury (TBI; but not spinal cord damage) acute β-catenin signaling occurs in proliferating NG2+ progenitor cells of the cortex, which spreads 4 to 7 days later to reactive cortical astrocytes [67], and significant expression of Wnt5a/Fzd2 is observed in the ipsilateral hippocampus [68].
In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), the selective disruption of the E-cadherin-mediated DC-DC adhesions partly triggered by Wnt/β-catenin signaling induces tolerogenic DCs [69]. Furthermore, Tcf7−/− EAE mice develop a significantly more severe disease (compared to littermates) that is associated with an increase in Th17 cell numbers in secondary lymphoid organs [36].
In the mouse, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced injury to the basal ganglia recapitulates several pathogenetic processes operative in PD, and genes downstream of the canonical Wnt/β-catenin signaling pathway, including Axin2, are downregulated within the SVZ niche, whereas GSK-3β protein levels are upregulated [10]. Some of these findings are found in parallel with chronic dopamine depletion, and are associated with a non-cell autonomous decrease in NPC proliferation and neurogenesis in the SVZ of rodents and non-human primate models of PD, as well as in postmortem brains of individuals with PD [10,70].
Conversely, activating Wnt/β-catenin signaling by either systemic or intracerebroventricular (i.c.v.) infusion of GSK-3β antagonists promotes NPC proliferation and neurogenesis in the SVZ of both healthy [10,52] and MPTP-treated mice [10]. Interestingly, a substantial nigrostriatal DA reinnervation is observed in MPTP mice upon treatment with a GSK-3β antagonist [8,10,17].
Together, these data anticipate an active and concerted role for Wnt/β-catenin signaling cascades in response to major acute, chronic, neuroinflammatory, and neurodegenerative injuries.
The Wnt strategy for self-defense against injuries
Acute and chronic brain injuries activate both innate and adaptive self-protective responses in the brain. Reactive astrocytes, microglial cells, and CNS infiltrating monocyte-derived macrophages are the key actors playing both detrimental and neuroprotective roles via an array of trophic factors, inflammatory cytokines, and chemokines, as well as the induction of neurogenic transcription factors. Such a wide range of innate and adaptive immune mediators also contributes to the extrinsic regulation of adult neurogenesis within the SVZ and SGZ niches [6,65].
Within this context, several questions arise as to what extent this ‘intrinsic Wnt/neuroinflammatory response’ may be set into motion after brain injury, which cell-to-cell interactions (and signaling mechanisms) might arise, and whether deregulation of the intrinsic Wnt/neuroinflammatory response might act as a primum movens in the glial and neuronal failure that ultimately occurs. The functional consequences at the neuronal level are diverse, depending on the type of injury and inherent self-repair capacities available (Table 2).
Table 2. Wnt/neuroimmune connections in stroke, TBI, and PD.
| Disease | Hallmarks/risk factors | Wnt signaling in rodent models | Neuroimmune interactions |
|---|---|---|---|
| Stroke | Acute brain injury leading to long-term disability. Few therapeutic options available Male gender, atherosclerosis, autoimmune disease, infection, and inflammation increase the risk [98,99] High circulating Dkk1 levels in patients after acute ischemic stroke [100] |
|
|
|
Traumatic
brain injury (TBI) |
Acute brain injury resulting in delayed cell death and altered neuronal architecture Gender differences in immune, inflammatory, and cell death responses [102] Unbalanced pro/anti-inflammatory cytokines/chemokines [103] |
|
|
|
Parkinson’s
disease (PD) |
Chronic degeneration of DA pigmented neurons in the SNpca, Lewy bodies, depletion of striatal DA, and astrogliosis. No cure available Parkin [57], LRRK2 [14,106], GSK-3β [107], Nurr1 [108], gene mutations, aging, male gender, GR-deficiencya, E2-deficiency, and environmental toxins augment the risk [87] |
|
Abbreviations: GR, glucocorticoid receptor; HIF-1α, hypoxia-inducible factor (HIF)-1α; SNpc, substantia nigra pars compacta; Str, striatum.
Several key studies support a neuroprotective role for endogenous and exogenous Wnt/β-catenin signaling activation after brain injury [4,5,42,71-74]. Here, we discuss the Wnt/neuroimmune connections in the context of an acute (i.e., ischemic or traumatic) brain insult, and in the course of PD progression (Table 2).
In a rodent model of brain stroke, expression of the Wnt antagonist Dkk1 in the ischemic area is required to promote neuronal death [5], and GSK-3β is involved in TBI [75]. Conversely, activation of Wnt/β-catenin signaling through GSK-3β inhibition is neuroprotective and anti-inflammatory [5,75-78] (Table 2). In keeping with these findings, during transient middle cerebral artery occlusion (MCAo) in rats, the blockade of Wnt1 signaling with a Wnt1 antibody or Dkk1 significantly enhances cerebral infarction and the accumulation of neurological deficits, suggesting that endogenous neuronal Wnt1 provides an important level of intrinsic neuroprotection in the context of oxidative stress [79]. Reciprocally, the transient over-expression of Wnt1 or the application of exogenous recombinant Wnt1 reduces cerebral infarction, and markedly improves neurological recovery [79]. Importantly, Wnt1 also represses inflammatory microglial activation [80], very much likely through astrocyte–microglia and glia–neuron crosstalk (Figure 2). Intrinsic Wnt1/Fzd1/β-catenin signaling and astrocyte–neuron crosstalk are required to maintain a normal complement of DAergic neurons [17] and may also participate in an early, global self-protective response in experimental PD (Table 2 and Figure 2) in which exposure to MPTP stimulates the astrocyte–microglia crosstalk via Wnt1 (Figure 2) [8,10,17].
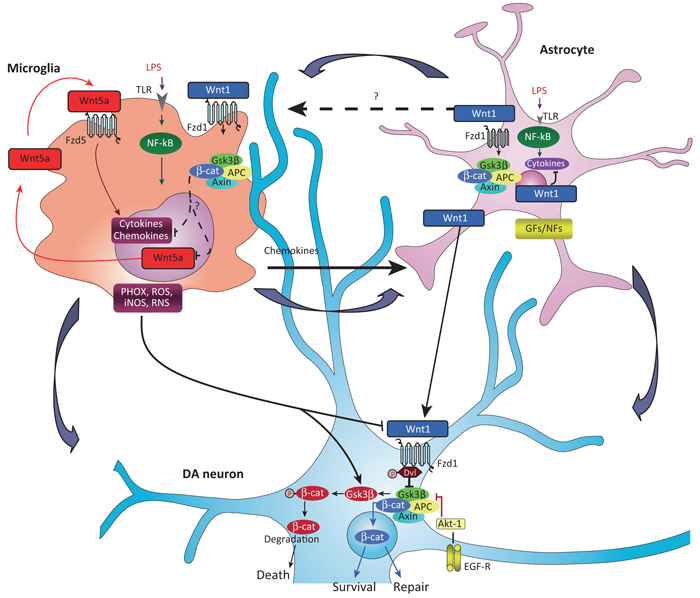
Figure 2.
Wnt tripartite regulation in the brain. Macrophage/microglia harbor Fzd5 and Fzd1 subtype receptors and can respond to both non-canonical Wnt5a- and canonical Wnt1-type ligands [9,18,40]. Upon inflammatory challenge with LPS or brain injury, TLRs and IFN-γ-mediated activation, macrophage/microglia produce a panel of proinflammatory cytokines and chemokines, of which Wnt5a constitutes one part of a self-perpetrating cycle, via autocrine Wnt5A/CamKII activation and paracrine stimulation of Th-1 cytokines [40]. Upregulation of microglial PHOX-derived ROS, iNOS-derived NO, and GSK-3β, a known regulator of NF-κB-dependent gene transcription, further exacerbate microglia reaction [8,10]. Chemokine-activated astrocytes respond to microglia by inducing the expression and release of Wnt1-type ligands [8,10,17]. It is proposed that astrocyte-derived Wnt1-type ligands may restrain inflammation, via stimulation of microglia Fzd1 receptors (in analogy to what is observed in macrophages [40]), possibly resulting in the attenuation of cytokines and Wnt5a overexpression (broken arrows). At the neuronal level, MPTP-induced microglial PHOX and RNS upregulate GSK-3β in DA neurons [8,10,17] leading to β-catenin phosphorylation and proteosomal degradation, which may increase DA neuron vulnerability and further incite cell death [8,17]. However, depending on the severity of brain insult, the degree of inflammation and specific vulnerability factors (age, gender, concomitant presence of stressors and gene mutations [87]), astrocyte–neuron crosstalk, via Wnt1 may serve a protective role, via stabilization of β-catenin in the cytoplasm, its nuclear translocation, followed by transcription of prosurvival genes, that may lead to neuroprotection and/or neurorepair [8,17]. Chemokine-activated astrocytes can also promote neurogenesis from adult VM progenitors, in vitro, via Wnt/β-catenin signaling activation [8]. In stark contrast, aging-induced loss of astrocyte-derived Wnt1 response [8,15], or Wnt/β-catenin signaling antagonism with Dkk1 [17], may result in DA neuron failure to repair. Potential crosstalk between glial GFs/NFs and Wnt/β-catenin signaling via Akt/GSK-3β/β-catenin cascades are also illustrated (see [17]). Abbreviations: CamKII, Ca2+–calmodulin-dependent protein kinase II; DA, dopamine; Dkk1, Dickkopf 1; EGF, epidermal growth factor; Fzd, Frizzled; GFs/NFs, glial-derived growth/neurotrophic factors; IFN-γ, interferon-γ; LPS, lipopolysaccharide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB, nuclear factor (NF)-κB; PHOX, phagocyte oxidase; RNS, reactive nitrite species; ROS, reactive oxygen species; Th-1, T helper 1 cells; TLR, Toll-like receptor; VM, ventral midbrain; Wnt, Wingless-related MMTV integration site.
With this perspective in mind, it is plausible that astrocyte-dictated canonical Wnt/β-catenin signaling in microglial cells have a number of beneficial consequences. First, the inflammation-dependent upregulation of canonical Wnt1-like ligands mitigates inflammation and oxidative stress (Figure 2), thereby switching the (inflammation-associated) harmful microglia phenotype to a protective (neurogenic) functional status [10,81]. Furthermore, astrocytes (or microglial cells) can signal onto insulted neurons via β-catenin and (re)program an antiapoptotic/prosurvival gene response that promotes neurorestoration [8,17] (Figure 2). Finally, inflammation-dependent canonical Wnt crosstalk between astrocyte and microglia can rescue neurogenesis within and outside the injured niches[8,10,15,17] (Figures 2 and 3).
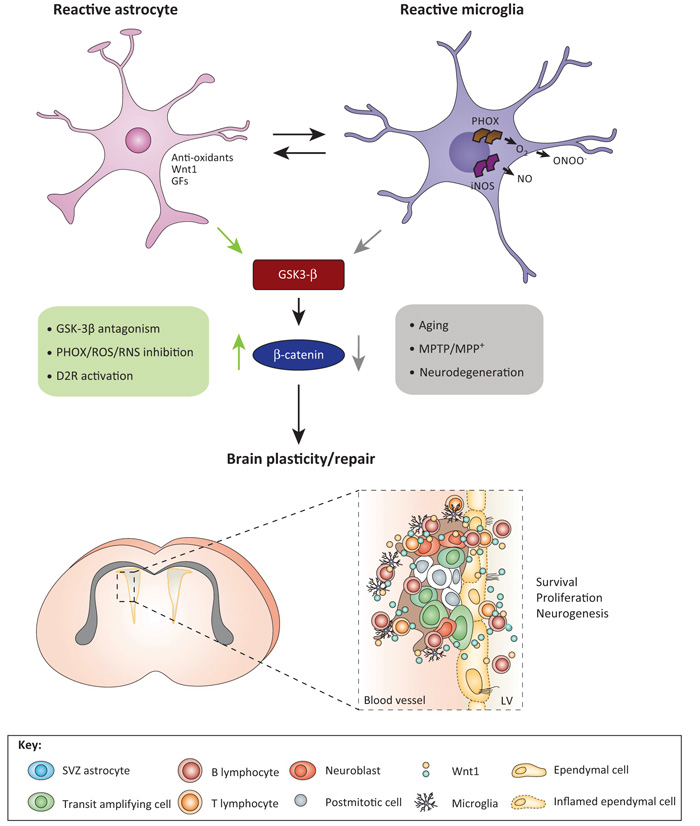
Figure 3.
Crosstalk between Wnt and local inflammatory signaling pathways for the control of SVZ niche homeostasis. During the early degeneration phase of MPTP toxicity, hyperactivated microglia contribute to the impairment of SVZ neurogenesis at different levels. By increasing oxidative and nitrosative stress and in synergy with MPTP/MPP+ direct toxicity, microglial-derived mediators (PHOX-derived ROS and iNOS-derived NO and peroxynitrite) may act as a molecular switch for cell signaling pathways critically involved in the physiological control of NPC homeostasis, with harmful consequences for NPC physiology, at least in part through GSK-3β activation, followed by phosphorylation and consequent degradation of β-catenin [10,15]. By contrast, pharmacological mitigation of inflammation and oxidative stress with Apo, L-Nil, or HCT1026 upregulate β-catenin and successfully rescue NPC proliferation and neuroblast formation, a process associated with striatal DAergic neuroprotection, with further positive modulation of SVZ proliferation via D2-R-activated mechanisms [10]. The mutual role of astrocyte–microglial interactions in the plasticity of SVZ response to MPTP is exemplified by the astrocyte’s ability to overcome microglial inhibitory effects, through, besides others, Wnt1-mediated activation Wnt/β-catenin signaling [10]. Different upstream and downstream signaling cascades may converge in finely tuning β-catenin transcriptional activity. For example, NO can inhibit EGF-R and the phosphoinositide 3-kinases (PI3K)/AKT survival pathway, and GSK-3β is a downstream target of Akt; RNS-induced nitration regulates the p85 subunit of PI3 kinase; and a variety of molecules including growth factors and neurotransmitter, including DA via D2 receptor can signal through (PI3K)/AKT/GSK-3β and Wnt signaling activation, thus, crosstalk among Wnt/β-catenin and prominent intracellular pathways may be envisioned to fine tune the SVZ neurogenic potential/plasticity observed herein [10]. Crosstalk between SVZ astrocytes (blue), transit-amplifying cells (red), neuroblasts (orange), ependymal (yellow) cells, microglia (violet), and lymphocytes via Wnt in SVZ niche are schematically illustrated. Abbreviations: Apo, apocynin, a ROS antagonist; DA, dopamine; D2-R, dopaminergic receptor subtype 2; HCT1026, [2-fluoro-α-methyl(1,1′-biphenyl)-4-acetic-4-(nitrooxy)butyl ester], a mixed cyclooxygenase (COX1/COX2) inhibitor NO-donating non-steroidal anti-inflammatory drug (NSAID) endowed with additional anti-inflammatory activity and reduced side effects; L-Nil, L-N6-(1-iminoethyl)-lysine, a specific inhibitor of inducible nitric oxide synthase (iNOS); MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NPC, neural stem/progenitor cell; PHOX, phagocyte oxidase; RNS, reactive nitrogen species; ROS, reactive oxygen species; SVZ, subventricular zone. Adapted from [111].
Hence, the evidence that microglia-derived inflammatory chemokines – including monocyte chemotactic protein (MCP)-1/CCL2 and macrophage inflammatory protein (MIP)1α/CCL3 – also induce the expression of Wnt1 in astrocytes of the VM further suggests that the chemically defined inflammatory milieu critically operates bidirectionally in maintaining the intrinsic Wnt/neuroinflammatory response [8,17]. Under specific, controlled conditions, adult VM astrocytes may also re-express region-specific factors – such as Wnt1 [8,82] – and participate in the regulation of diverse aspects of DA neuron homeostasis in the injured VM, thus mitigating DA neuron death and/or enhancing survival, expansion, and differentiation from DA progenitors [8,17,82].
By contrast, the astrocyte–macrophage/microglia cross-talk might turn into a dangerous liaison if the non-canonical Wnt/Ca2+ pathway prevails, for example, as a result of exacerbated inflammation encouraged by upregulated Wnt inhibitors or non-canonical Wnt ligands (Figure 2), as well described in the SVZ of MPTP-treated parkinsonian mice (Figure 3) [10]. Here, microglia overactivation leading to exaggerated generation of proinflammatory mediators including Reactive oxygen and nitrogen species (ROS and RNS) within the SVZ microenvironment was temporally related to Wnt/β-catenin signaling inhibition and NPC impairment [10]. This inhibition was facilitated by upregulating active GSK-3β, which favors degradation of β-catenin, resulting in impaired NPC homeostasis and decreased neurogenic potential [10]. Importantly, dysfunctional or aberrant astrocyte–microglia crosstalk may also act as key driver of SVZ neurogenic decline observed with age via dysregulated Wnt/β-catenin signaling [15].
Conversely, dialog between astrocytes and NPCs results in highly beneficial upregulation of Wnt1-type ligands, promoting substantial rescue of neurogenic impairment of SVZ–NPCs. Likewise, exogenous activation of Wnt/β-catenin signaling by inhibition of GSK-3β, Wnt1 exposure, or ROS/RNS antagonism overrided MPTP-induced impaired neurogenesis associated with DA recovery [10]. Together, these results implicate an active and concerted role of reactive astrocytes and microglia in the remodeling of the injured SVZ niche via a crosstalk between inflammation and Wnt/β-catenin signaling cascades [10,15] (Figure 3), with potential therapeutic implications for SVZ manipulation to incite neuronal repair (see [15,16,83,84] and next section).
Importantly, a specific hormonal background, which includes glucocorticoids [85] and estrogens [86], may take part in the ‘intrinsic Wnt/neuroinflammatory response’, thus foreshadowing gender effects in neuroinflammation and neuroprotection, effects that are already observed in the incidence of and response to stroke, traumatic brain injuries, and PD (Table 2).
Finally, owing to its essential role in most fundamental developmental processes, the ‘intrinsic Wnt/neuroinflammatory response’ might contribute to the host’s ability to respond to future brain attacks, by participating in the programming of glia–neuron crosstalk during prenatal life, for example, when exposure to a panel of genetic and environmental influences may predispose to major inflammation- and/or environment-dependent insults [87]. Likewise, a dysfunctional ‘intrinsic Wnt/neuroinflammatory response’ may underlie certain increased neuronal vulnerability to stroke, traumatic brain injuries, and PD with aging, upon the withdrawal of estrogens at menopause in female gender, or under conditions affecting (hypo vs hyper) the stress axis (Table 2).
Targeting Wnt to foster innate brain repair potential
The undoubted significance that Wnt/β-catenin signaling holds as a target against neurological human diseases has prompted investigations of the therapeutic potential of interfering with such a crucial cell signaling machinery by targeting one of its major checkpoints, GSK-3β [88]. Evidence from rodent models of different neurodegenerative diseases including stroke, TBI, and PD suggest that neuronal and/or behavioral symptoms can be ameliorated and inflammatory reactions mitigated after systemic treatment with either nonspecific (e.g., lithium) or specific GSK-3β inhibitors (Table 2). However, given that perturbed Wnt/β-catenin signaling likely plays a key role in tumorigenesis, the therapeutic value of this enzyme as a drug target remains clouded by uncertainty over the potential of antagonists to promote tumorigenesis [88,89]. It may be possible to overcome some of these limitations by combining new technologies that allow for specific cell targeting and currently available neurosurgical, oral, or systemic interventions.
For example, recombinant Wnts or Wnt mimetics (e.g., circular peptides, small molecule compounds, or RNA aptamers) [90], either alone or in combination with other signaling molecules such as Notch mimetics, fibroblast growth factor, and bone morphogenetic proteins, may open new windows to tissue engineering for regenerative medicine in neuroscience [90]. Hence, large numbers of midbrain DA neurons based on expanding and differentiating neural stem/progenitor cells present in the human VM tissue have been obtained with Wnt5a, which promoted DA differentiation of expanded cells resulting in improved morphological maturation, midbrain DA marker expression, DA release, and electrophysiological properties [91], with potential clinical implications.
Recently, lentiviral-mediated Wnt3a (LV-Wnt3a-HA) injection in the striatum or the SVZ was used as an approach to increase Wnt/β-catenin signaling in situ, in the endothelin-1 focal ischemia rodent model [16]. Gene delivery of Wnt3a into the striatum significantly enhanced functional recovery after ischemic injury and increased the number of BrdU-positive cells that differentiated into mature neurons in the ischemic striatum and SVZ, which was associated with reduced neuronal injury [16], prompting further studies to assess the feasibility of this approach in other neurodegenerative settings.
In parallel to (or in combination with) nanotechnologies, there is also the (more) immediate chance to harness the inflammatory response through targeted modulation of the innate immune response via crosstalk between inflammatory mediators and Wnt/β-catenin signaling using oral/systemic therapeutic approaches. In PD, the oral administration of the nitric oxide (NO)-donating non-steroidal anti-inflammatory drug flurbiprofen (NO-flurbiprofen) activates Wnt/β-catenin signaling, resulting in GSK-3β downregulation with beneficial effects for neuronal outcome [10], illuminating alternative options for inhibitory control of GSK-3β. Additionally, this NO-flurbiprofen-induced mitigation of the inflammatory SVZ microenvironment protects NPCs against mitochondrial impairment and cell death, and promotes proliferation and neurogenesis in the SVZ, that is associated to a substantial striatal DA reinnervation both in young and aged mice [10,15].
Given that a putative dysfunction of SVZ output has been proposed as a pathological substrate for non-motor symptoms (e.g., hyposmia) or as a factor aggravating neurodegeneration via a limited capacity of the brain to repair itself via neurogenesis (see [83,84]), this inflammation-dependent modulation of the SVZ niche (via Wnt/β-catenin signaling modulation) may suggest a potential window of opportunity for therapeutic strategies aimed at mitigating the inflammatory microenvironment, upregulating endogenous neurogenesis, and/or favoring the integration or survival of new neurons, to incite neurorepair [10,15,92-94].
Interestingly, significant recovery of the lost paracrine Wnt signaling in the aged brain [95], or mitigation of the microglia inflammatory phenotype impacting on Wnt/β-catenin signaling in the young brain [96], can also be achieved by the very same exercise or environmental (enrichment) conditions that have been shown to recreate a cellular and humoral youthful brain repair-enhancing environment after CNS injuries [97] (Table 2).
Concluding remarks and future perspectives
Overall, the evidence shows that the intrinsic Wnt/neuroinflammatory response plays a central role in regulating the function(s) of stem cells and progenitor cells, postmitotic neurons and glia, as well as peripheral and central immune cells. Together, these results indicate that different possibilities exist for next-generation therapies for treating CNS diseases where injury-reactive inflammation drives secondary neurodegeneration, such as traumatic injuries, PD, and stroke. Understanding the intracellular signaling networks that dictate the intrinsic Wnt/neuroinflammatory response is critical for identifying new potential therapeutic targets. Harnessing Wnt signaling as an intrinsic neuroimmune molecular mechanism may emerge as a powerful tool to regulate immune homeostasis within and outside of the brain.
Acknowledgments
The authors thank Jayden A. Smith for critically reviewing the article, and acknowledge the contribution of past and present members of the Marchetti and Pluchino laboratories, who have contributed to (or inspired) this manuscript. This work has received support from the Italian Ministry of Health (Con. no. 82; Ps-CARDIO ex 56 and PS-NEURO ex 56 to B.M.); the Italian Ministry of Research (grant CR/2008-12/to B.M.); the Italian Ministry of Research and University (MIUR, to B.M.); the OASI (IRCCS) Institution for Research and Care on Mental Retardation and Brain Aging Troina (EN) Italy (to B.M.); the National Multiple Sclerosis Society (NMSS, partial grants RG-4001-A1 to S.P.); the Italian Multiple Sclerosis Association (AISM, grant 2010/R/31 to S.P.); the Italian Ministry of Health (GR08-7 to S.P.), Wings for Life (grant XBAG/163 to S.P.); Banca Agricola Popolare di Ragusa (BAPR, unrestricted grant to S.P.); the European Research Council (ERC) under the ERC-2010-StG Grant agreement no. 260511-SEM_SEM and the European Community (EC) 7th Framework Program (FP7/2007-2013) under Grant Agreement no. 280772-iONE.
Glossary
- aPKC signaling
regulates epithelial and neuronal polarity and migration. Wnt/Frizzled (Fzd) signaling via Dishevelled (Dvl) induces aPKC activation, leading to phosphorylation (inhibition) of Par1/MARK2 kinase, and regulation of microtubules
- Axin2
integral part of a negative feedback loop that acts to restrain or desensitize Wnt signaling
- Canonical Wnt signaling pathway
is activated when a Wnt ligand binds to a seven-pass transmembrane Frizzled (Fzd) receptor and its coreceptor, low-density lipoprotein receptor-related protein (Lrp) 5/6. In cells not exposed to Wnt, β-catenin levels are kept low through interactions with a multiprotein β-catenin destruction complex formed by the protein kinases GSK-3β and CK1, the adenomatous polyposis coli (APC) tumor suppressor protein, and the scaffolding protein axin. β-Catenin is degraded after phosphorylation by GSK-3β and CK1 through the ubiquitin pathway, which involves interactions with β-transducin repeat containing protein (β-TrCP). The continual elimination of β-catenin prevents β-catenin from reaching the nucleus, and Wnt target genes are thereby repressed by the DNA-bound T cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins. Wnt ligand stimulation of Fzd receptor and its coreceptors Lrp5/6 leads to the activation of the cytoplasmic protein dishevelled (Dvl) and subsequent recruitment of Axin complex to the Lrp coreceptor. These events lead to inhibition of β-catenin phosphorylation and the stabilization of β-catenin, which then accumulates and translocates to the nucleus to form complexes with TCF/LEF. Binding of β-catenin to TCF/LEF removes transcriptional repressors such as Groucho and initiates the transcription of TCF/LEF target genes (see Figure 1a in main text). In cells, β-catenin is normally associated with adherens junctions and can also be free in the cytoplasm. Several important effects of the canonical Wnt pathway include cell proliferation and differentiation/maturation, body axis specification, and morphogenic signaling
- Casein kinase 1 (CK1)
family of monomeric serine–threonine protein kinases (α, γ, δ, ε) that have been suggested to play a role in the phosphorylation of Dvl in the Wnt/β-catenin signaling pathway. Wnt binding to Lrp causes a rapid increase in phosphorylation of the cytoplasmic domain of Lrp by CK1γ, which ultimately promotes binding of Axin to Lrp and activation of the Wnt signaling pathway. Different CK1 proteins have different functions. CK1α phosphorylates β-catenin for its degradation; CK1ε or δ phosphorylates Dvl, although functional significance is less clear
- CD4+ T cells
subset of T cells that play an important role in the adaptive immune system. After many cell generations, mature CD4+ T cells differentiate into effector, memory, and regulatory T cells
- CD8+ T cells
cytotoxic T cells that have the ability to kill cancer cells and infected (e.g., by viruses or bacteria) or damaged/dying cells. CD8+ T cells recognize antigens via the surface T cell receptor (TCR); if the TCR is specific for the antigen, it binds to the Class I major histocompatibility complex (MHC) molecule–antigen pair and the CD8+ T destroys the cell. CD8+ T cells also have the capability to secrete inflammatory cytokines
- Dendritic cells (DCs)
are critical elements of adaptive immunity responsible for acquiring and presenting antigens, as well as for positively and negatively regulating immune responses to various physiological and pathological stimuli
- Dickkopf (Dkk) proteins
extracellular antagonists of Wnt signaling. Four members (1–4) have been identified in mammals, but only Dkk1, 2, and 4 have been documented to function as endogenous antagonists of canonical Wnt signaling. Wnt antagonism by Dkk requires the binding of the C-terminal cysteine-rich domain of Dkk to the Wnt coreceptor, Lrp5/6 (see [27]). In addition to Lrp5/6, Dkk molecules can also bind to another cell surface protein called Kremen, but its role is much less critical. Dkks are crucial for embryonic cell fate and bone formation, and abnormal Dkk function has been implicated in cancers, bone diseases, and neurodegenerative disorders including Alzheimer’ disease and stroke (see [4,5,27] and references therein)
- Dishevelled (Dvl)
a key component of the membrane-associated Wnt receptor complex. Dvl is a cytoplasmic phosphoprotein that acts directly downstream of Fzd receptors. Dvl proteins carry an N-terminal DIX (Dishevelled/Axin) domain, a central PDZ (PSD-95; DLG, ZO1) domain, and a DEP (Dishevelled, EGL-10, Pleckstrin) domain, each with a variety of interaction partners. Depending on Wnt stimulation and receptor context, Dvl can inhibit the β-catenin destruction complex, thereby stabilizing β-catenin and activating canonical Wnt signaling, or Dvl can regulate some of the non-canonical branches of Wnt signaling
- Effector T cells
a subset of antigen-activated T cells, which differentiate into one of several subtypes including Th1, Th2, Th3, and Th17 cells, and secrete different cytokines to facilitate a different type of immune response. Signaling from the antigen-presenting cell directs the T cells to differentiate into a particular subtype
- Frizzled (Fzd)
a family of serpentine receptor proteins that serve as receptors in the Wnt signaling pathway. When activated, Fzd leads to activation of Dvl in the cytosol. Fzd is critical for nearly all Wnt signaling, and the N-terminal Fzd cysteine-rich domain (CRD) serves as the Wnt binding domain. Dysregulation of the Wnt/Fzd system is associated with a variety of human hereditary diseases, and modulation of Wnt signaling is actively targeted for cancer, regenerative medicine, stem cell therapy, bone growth, and wound healing (see [27])
- Glycogen synthase kinase (GSK)-3β
a serine/threonine protein kinase that is active in a number of central intracellular signaling pathways, including those that regulate cellular proliferation, migration, inflammation and immune responses, glucose regulation, and apoptosis. Within the canonical Wnt signaling pathway, GSK-3β phosphorylates β-catenin, targeting it for degradation. The modulation of GSK-3β by various protein kinases also affects the innate and adaptive immune response by modulating cytokine production and proliferation in naïve and memory CD4+ T cells and by regulating macrophage/microglial-stimulated expression of pro/anti-inflammatory cytokines
- GSK-3β-microtubule (MT) signaling
inhibits and activates phosphorylation of microtubule-associated protein (MAP)1B by GSK-3β and JNK, respectively, resulting in increased MT stability during axonogenesis and synaptogenesis
- Lipopolysaccharides (LPS)
bacterial endotoxins that are found in the outer membrane of Gram-negative bacteria. They elicit strong immune responses in animals, via the LPS-sensing Toll-like receptor (TLR)-4
- Low-density lipoprotein receptor-related protein (Lrp) 5/6
a transmembrane cell surface protein involved in receptor-mediated endocytosis of lipoproteins and protein ligands. Lrp5/6 functions as a receptor or coreceptor (with Fzd) for Wnt upstream of the canonical Wnt/β-catenin signaling cascade. Lrp5/6 is also a specific, high-affinity receptor for Dkk, which blocks Lrp6-mediated Wnt signaling
- Lymphoid enhancer binding factor (LEF)/TCF
a family of transcription factors that includes LEF1, TCF1, TCF3, and TCF4. After activation of the canonical Wnt/β-catenin pathway, active β-catenin binds to LEF/TCF, displacing their repressors. This TCF–β-catenin complex translocates to the nucleus where it binds to TCF/LEF cognate DNA sequences through a high mobility group (HMG) domain and regulates gene transcription. Without active β-catenin in the nucleus, TCF and LEF remain associated with their repressors on their cis elements and inhibit gene transcription
- Memory T cells
subset of T cells that have encountered antigen during a prior infection or vaccination. At a second encounter with the antigen, memory T cells reproduce to mount a faster and stronger immune response (expanding into large numbers of effector T cells) than the first response to antigen
- Monocytes/macrophages
professional phagocytes that function in both innate and adaptive immunity of vertebrate animals. Their role is to phagocytose cells, cellular debris, and pathogens and stimulate lymphocytes and other immune cells to respond to pathogens (via antigen presentation)
- MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)
a neurotoxin precursor used in rodent and non-human primate models to induce PD-like disease. It is converted in the brain into the toxin MPP+ (1-methyl-4-phenylpyridinium), which selectively destroys dopaminergic neurons
- Norrie
disease pseudoglioma protein (Nrp, Norrin) is structurally unrelated to Wnt and can act as Wnt agonist in the canonical pathway (see Figure 1a in main text)
- Receptor tyrosine kinase-like orphan receptor (Ror) ½
receptor tyrosine kinases required for establishing cell polarity, asymmetric cell division, and cell migration during key morphological processes, including neural tube formation, testes development, and heart and bone formation
- Regulatory T (Treg) cells
a subset of T cells that downregulate the immune system and maintain tolerance to self-antigens
- R-spondins (Rspo) proteins
are structurally unrelated to Wnt and act as Wnt agonists (see Figure 1a in main text). A small family of seven-pass transmembrane receptors, the Lgr5 family, was shown to mediate Rspo input into the canonical Wnt pathway. The Lgr receptors bind Rspo with high affinity and are essential for signal enhancement of low-dose Wnt [31]
- Secreted Fzd-related proteins (sFRP) 1–5
classically defined as negative regulators of Wnt signaling, sFRPs can directly bind to the Wnt ligands, they can also antagonize one another’s activity, bind to Fzd receptors, and provide axon-guidance information. Importantly, sFRPs can interact with different proteins, thereby interfering with molecular cascades other than those activated by Wnt. Recently, sFRP1 and sFRP2 were shown to be required for the activation of canonical Wnt signaling implicated in the specification of the mouse eye periphery [29]. By contrast, in developing DA cells, sFRP1 and sFRP2 were recently shown to activate the Wnt/planar cell polarity/Rac1 pathway [30]. The expression of sFRPs is altered in different pathological states including cancers, bone pathologies, and retinal degeneration, which indicates that their activity is fundamental for tissue homeostasis [29]
- T cell receptor (TCR)
molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to MHC molecules. When the TCR engages with antigen and MHC, the T lymphocyte is activated through a series of biochemical events mediated by associated enzymes, coreceptors, specialized accessory molecules, and activated or released transcription factors
- T lymphocytes
white blood cells that mature in the thymus and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes by the presence of a TCR on the cell surface
- Toll-like receptor (TLR)
a class of receptor proteins that recognize structurally conserved molecules derived from microbes to activate immune cell responses
- Type 1 cells
GFAP+/Nestin+/BLBP+ radial glia-like stem cells of the SGZ of the dentate gyrus of the mammalian hippocampus
- Type 2 cells
GFAP−/Nestin+/Sox2+ non-radial transit amplifying progenitor cells of the SGZ of the dentate gyrus of the mammalian hippocampus
- Type B1 cells
GFAP+/Nestin+ radial glia-like stem cells of the SVZ of the mammalian forebrain that are exposed to the ventricle and contact blood vessels via the apical cilia and basal ends, respectively
- Type B2 cells
GFAP+ parenchymal astrocytes in the SVZ of the mammalian forebrain
- Type C cells
GFAP−/Nestin+/Sox2+/Dxc+ rapidly dividing progenitor cells of the SVZ of the mammalian forebrain
- Type E cells
ependymal cells of the adult mammalian SVZ niche
References
- 1.Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol. 2012;4:a008003. doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willert K, Nusse R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison-Uy SJ, Pleasure SJ. Wnt signaling and forebrain development. Cold Spring Harb. Perspect. Biol. 2012;4:a008094. doi: 10.1101/cshperspect.a008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toledo EM, et al. Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Mastroiacovo F, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 6.Martino G, et al. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staal FJ, et al. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 8.L’Episcopo F, et al. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol. Dis. 2011;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halleskog C, et al. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L’Episcopo F, et al. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J. Neurosci. 2012;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depboylu C, et al. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 2012;238:183–191. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Maiese K, et al. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin. Ther. Targets. 2012;16:1203–1214. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi H, et al. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS ONE. 2012;7:e36560. doi: 10.1371/journal.pone.0036560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berwick DC, Harvey K. LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6. Hum. Mol. Genet. 2012;21:4966–4979. doi: 10.1093/hmg/dds342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L’Episcopo F, et al. Aging-induced Nrf2–ARE pathway disruption in the subventricular zone (SVZ) drives neurogenic impairment in parkinsonian mice via PI3K–Wnt/β-catenin dysregulation. J. Neurosci. 2013 doi: 10.1523/JNEUROSCI.3206-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shruster A, et al. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS ONE. 2012;7:e40843. doi: 10.1371/journal.pone.0040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L’Episcopo F, et al. A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron–astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol. Neurodegener. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halleskog C, et al. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J. Neuroinflammation. 2012;9:111. doi: 10.1186/1742-2094-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolich BD, et al. Wnt family proteins are secreted and associated with the cell surface. Mol. Biol. Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 21.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 23.van Amerongen R, et al. Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 24.Janda CY, et al. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 26.Huang T, et al. Nuclear factor of activated T cells (NFAT) proteins repress canonical Wnt signaling via its interaction with Dishevelled (Dvl) protein and participate in regulating neural progenitor cell proliferation and differentiation. J. Biol. Chem. 2011;286:37399–37405. doi: 10.1074/jbc.M111.251165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 28.van Amerongen R. Alternative wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012;4:a007914. doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteve P, et al. Secreted frizzled-related proteins are required for Wnt/β-catenin signalling activation in the vertebrate optic cup. Development. 2011;138:4179–4184. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- 30.Kele J, et al. SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells. 2012;30:865–875. doi: 10.1002/stem.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glinka A, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue HH, Zhao DM. Regulation of mature T cell responses by the Wnt signaling pathway. Ann. N. Y. Acad. Sci. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- 33.Maier E, et al. Inhibition of suppressive T cell factor 1 (TCF-1) isoforms in naive CD4+ T cells is mediated by IL-4/STAT6 signaling. J. Biol. Chem. 2011;286:919–928. doi: 10.1074/jbc.M110.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossain MB, et al. Lymphoid enhancer factor interacts with GATA-3 and controls its function in T helper type 2 cells. Immunology. 2008;125:377–386. doi: 10.1111/j.1365-2567.2008.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Q, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat. Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Q, et al. T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. J. Immunol. 2011;186:3946–3952. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Y, et al. β-Catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 38.Graham JA, et al. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3β inhibition. J. Biol. Chem. 2010;285:32852–32859. doi: 10.1074/jbc.M110.150904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, et al. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30:845–859. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaale K, et al. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur. J. Cell Biol. 2011;90:553–559. doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 43.Seri B, et al. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 44.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 47.Kalani MY, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu Q, et al. Orphan nuclear receptor TLX activates Wnt/β-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup. pp. 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karalay O, et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wexler EM, et al. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adachi K, et al. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 53.Yu JM, et al. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- 54.Marinaro C, et al. Wnt signaling has opposing roles in the developing and the adult brain that are modulated by Hipk1. Cereb. Cortex. 2012;22:2415–2427. doi: 10.1093/cercor/bhr320. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, et al. The modulatory effects of bHLH transcription factors with the Wnt/β-catenin pathway on differentiation of neural progenitor cells derived from neonatal mouse anterior subventricular zone. Brain Res. 2010;1315:1–10. doi: 10.1016/j.brainres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Castelo-Branco G, Arenas E. Function of Wnts in dopaminergic neuron development. Neurodegener. Dis. 2006;3:5–11. doi: 10.1159/000092086. [DOI] [PubMed] [Google Scholar]
- 57.Rawal N, et al. Parkin protects dopaminergic neurons from excessive Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2009;388:473–478. doi: 10.1016/j.bbrc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh MC, et al. Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood. 2009;114:1366–1373. doi: 10.1182/blood-2008-08-175869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lobov IB, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaussabel D, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 61.Lin SL, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valencia J, et al. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J. Immunol. 2011;187:4129–4139. doi: 10.4049/jimmunol.1101243. [DOI] [PubMed] [Google Scholar]
- 63.Wu B, et al. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J, et al. Wnt5a induces endothelial inflammation via β-catenin-independent signaling. J. Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 65.Ekdahl CT, et al. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 66.Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29:528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- 67.White BD, et al. β-Catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu LJ, et al. Suppression of Frizzled-2-mediated Wnt/Ca2+ signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience. 2012;213:19–28. doi: 10.1016/j.neuroscience.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 69.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 71.Inestrosa NC, Toledo EM. The role of Wnt signalling in neuronal dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim H, et al. Downregulation of Wnt/β-catenin signalling causes degeneration of hippocampal neurons in vivo. Neurobiol. Aging. 2011;32:2316.e1–e15. doi: 10.1016/j.neurobiolaging.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Shruster A, et al. Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid β-peptide. J. Neurochem. 2011;116:522–529. doi: 10.1111/j.1471-4159.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- 74.Purro SA, et al. The secreted Wnt antagonist Dickkopf-1 is required for amyloid β-mediated synaptic loss. J. Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dash PK, et al. Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS ONE. 2011;6:e24648. doi: 10.1371/journal.pone.0024648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chuang DM, et al. GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic stroke. Front. Mol. Neurosci. 2011;4:15. doi: 10.3389/fnmol.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu F, et al. Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J. Neurotrauma. 2012;29:362–374. doi: 10.1089/neu.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou X, et al. GSK-3β inhibitors suppressed neuroinflammation in rat cortex by activating autophagy in ischemic brain injury. Biochem. Biophys. Res. Commun. 2011;411:271–275. doi: 10.1016/j.bbrc.2011.06.117. [DOI] [PubMed] [Google Scholar]
- 79.Chong ZZ, et al. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid. Med. Cell. Longev. 2010;3:153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chong ZZ, et al. Cellular demise and inflammatory microglial activation during β-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell. Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butovsky O, et al. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Castelo-Branco G, et al. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol. Cell. Neurosci. 2006;31:251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Winner B, et al. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 84.Hoglinger GU, et al. Quantitative evaluation of the human subventricular zone. Brain. 2012;135:e221. doi: 10.1093/brain/aws087. 1–4. author reply e222, 1–6. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti B, et al. Glucocorticoid receptor–nitric oxide crosstalk and vulnerability to experimental parkinsonism: pivotal role for glia–neuron interactions. Brain Res. Brain Res. Rev. 2005;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 86.Zhang QG, et al. Role of Dickkopf-1,an antagonist of the Wnt/β-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marchetti B, Abbracchio MP. To be or not to be (inflamed) – is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol. Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Phukan S, et al. GSK3β: role in therapeutic landscape and development of modulators. Br. J. Pharmacol. 2010;160:1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel S, Woodgett J. Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 2008;14:351–353. doi: 10.1016/j.ccr.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr. Pharm. Biotechnol. 2011;12:160–170. doi: 10.2174/138920111794295710. [DOI] [PubMed] [Google Scholar]
- 91.Ribeiro D, et al. Efficient expansion and dopaminergic differentiation of human fetal ventral midbrain neural stem cells by midbrain morphogens. Neurobiol. Dis. 2012;49C:118–127. doi: 10.1016/j.nbd.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Rueger MA, et al. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience. 2012;215:174–183. doi: 10.1016/j.neuroscience.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 93.Sakata H, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallenquist U, et al. Ibuprofen attenuates the inflammatory response and allows formation of migratory neuroblasts from grafted stem cells after traumatic brain injury. Restor. Neurol. Neurosci. 2012;30:9–19. doi: 10.3233/RNN-2011-0606. [DOI] [PubMed] [Google Scholar]
- 95.Okamoto M, et al. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- 96.Ehninger D, et al. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian Y, et al. Effects of gender on gene expression in the blood of ischemic stroke patients. J. Cereb. Blood Flow Metab. 2012;32:780–791. doi: 10.1038/jcbfm.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seifert-Held T, et al. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 2011;218:233–237. doi: 10.1016/j.atherosclerosis.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Cunningham LA, et al. Roles for HIF-1α in neural stem cell function and the regenerative response to stroke. Bhav. Brain Res. 2012;227:410–417. doi: 10.1016/j.bbr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leitgeb J, et al. Effects of gender on outcomes after traumatic brain injury. J. Trauma. 2011;71:1620–1626. doi: 10.1097/TA.0b013e318226ea0e. [DOI] [PubMed] [Google Scholar]
- 103.Hellewell SC, Morganti-Kossmann MC. Guilty molecules, guilty minds? The conflicting roles of the innate immune response to traumatic brain injury. Mediators Inflamm. 2012;2012:356494. doi: 10.1155/2012/356494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dash PK, et al. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. Neurosci. Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 105.Kovesdi E, et al. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sancho RM, et al. Mutations in the LRRK2 Roc-COR tandem domain link Parkinson’s disease to Wnt signalling pathways. Hum. Mol. Genet. 2009;18:3955–3968. doi: 10.1093/hmg/ddp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duka T, et al. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kitagawa H, et al. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signaling. Mol. Cell. Biol. 2007;27:7486–7496. doi: 10.1128/MCB.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Wang W, et al. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 110.Petit-Paitel A, et al. Involment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. Plos One. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]