Abstract
Aotus nancymaae, the owl monkey, provides a useful laboratory model for research to develop drugs and vaccines against human falciparum malaria; however, many Plasmodium falciparum parasites are unable to invade A. nancymaae erythrocytes, rendering the parasites noninfective to the monkeys. In previous work, we identified a key polymorphism that determined the inheritance of erythrocyte invasion in a genetic cross of two P. falciparum clones that were virulent (GB4) or noninfective (7G8) to A. nancymaae. This polymorphism, an isoleucine-to-lysine polymorphism at position 204 (I204K) of the GB4 erythrocyte binding protein PfRH5, was nevertheless not found in several other P. falciparum lines that could also invade A. nancymaae erythrocytes. Alternative PfRH5 polymorphisms occur at different positions in these virulent parasites, and additional polymorphisms are found in P. falciparum parasites that cannot infect A. nancymaae. By allelic replacement methods, we have introduced the polymorphisms of these A. nancymaae-virulent or noninfective parasites at codons 204, 347, 358, 362, 410, and 429 of the endogenous PfRH5 gene in the noninfective 7G8 line. 7G8 transformants expressing the polymorphisms of the A. nancymaae-virulent parasites show neuraminidase-sensitive (sialic acid-dependent) invasion into the monkey erythrocytes, whereas 7G8 transformants expressing the PfRH5 alleles of noninfective parasites show little or no invasion of these erythrocytes. Parasites harboring PfRH5 polymorphisms 204K or 204R are also able to invade rat erythrocytes and are differentially sensitive to the removal of surface sialic acids by neuraminidase. These studies offer insights into the PfRH5 receptor-binding domain and interactions that support the invasion of various primate and rodent erythrocytes by P. falciparum.
Keywords: Malaria, Virulence, Aotus nancymaae, Sialic acid, Rattus norvegicus, Mus musculus
1. Introduction
Invasion of erythrocytes by malaria parasites is a multistep, complex process that involves a number of diverse and perhaps functionally redundant receptor-ligand interactions [1, 2]. Invasion is initiated when a merozoite attaches to an erythrocyte [3–5]. The parasite repositions so the apical end is in direct contact with the erythrocyte surface and then secretes the contents of the rhoptries and micronemes, specialized apical organelles that store and release the molecules necessary for host cell invasion [6]. Parasite ligands in this released material bind to erythrocyte molecules, forming a tight junction between the host and parasite membrane surfaces. The tight junction develops into an annulus that supports entry of the parasite into the erythrocyte as the annulus is translocated by an actin-myosin motor from the apical to posterior ends of the merozoite [7]. After entry of the merozoite, the erythrocyte membrane seals, completing the process of invasion.
In Plasmodium falciparum, a number of important erythrocyte binding ligands occur in two families, the erythrocyte binding-like (EBL) molecules and the reticulocyte binding-like (RBL) molecules [1, 2, 8]. Members of the EBL family are orthologs of the Duffy binding protein of Plasmodium vivax, appear to be stored in the micronemes, and include erythrocyte binding antigen 175 (EBA-175), EBA-140 (also known as BAEBL), EBA-181 (or JESEBL) and EBL-1 [9–16]. P. falciparum members of the RBL family include five proteins of the rhoptries: PfRH1 (PfNBP1), PfRH2a, PfRH2b, PfRH4 and PfRH5 [17–25]. These proteins were originally identified by their homology to RBL members of other Plasmodium species, including the P. vivax reticulocyte binding proteins PvRBP1 and PvRBP [26] and Py235 proteins of the rodent malaria parasite Plasmodium yoelii that mediate erythrocyte selection and virulence [27].
Despite knowledge of several known ligand-erythrocyte receptor interactions, much about these interactions in erythrocyte invasion pathways remains to be understood. In P. falciparum, many interactions appear to be redundant and individually dispensable, offering an advantage that may promote parasite survival when particular ligands or receptors are compromised, for example by host immune defenses or polymorphisms of the erythrocytes [28–30]. In some cases, members of the EBL and RBL families may function in cooperation [31]. Some but not all erythrocyte receptors for P. falciparum ligands have been identified: in the EBL family, EBA-175 binds sialic acid residues on the integral membrane protein glycophorin A [9, 32, 33], EBA-140 binds sialic acids on glycophorin C [12, 34, 35], and EBL-1 binds sialic acids on glycophorin B [16]. In studies of the RBL family, complement receptor 1 (CR1) and an isoform of basigin (BSG-S, the Ok blood group antigen, CD147) have been identified as human erythrocyte receptors for PfRH4 and PfRH5, respectively [36, 37], and the binding domains of PfRH1, PfRH4 and PfRH5 have been mapped to N-terminal regions of their sequences [23–25, 38, 39].
Various P. falciparum lines are able to invade erythrocytes that lack receptors for specific PfEBL- or PfRH-proteins (because of mutations or after enzyme treatment in the laboratory). Conversely, knock-out parasites that no longer express individual PfEBL or PfRH proteins generally retain their ability to invade erythrocytes [2]. An important exception, however, has been the unsuccessful attempt in at least two laboratories to knock out the gene encoding PfRH5 [2, 23]. Together with more recent evidence that invasion of human erythrocytes can be inhibited by a soluble pentamerized form of basigin, by anti-basigin monoclonal antibodies, or by reduction of basigin levels on the erythrocyte surface [37], these findings suggest that P. falciparum depends upon the PfRH5-basigin interaction for effective invasion of human erythrocytes. Unlike other members of the PfRH family, PfRH5 lacks a transmembrane domain; it instead interacts with a processed EGF-like protein (PfRipr) to form a complex that associates with an unidentified partner on the merozoite membrane (attempts to disrupt the PfRipr gene were also unsuccessful) [40].
The New World monkey Aotus nancymaae (owl monkey) is one of a limited number of primates susceptible to infection with human malaria parasites and available as a model for vaccine and drug development research. However, only some P. falciparum parasite lines are able to invade the erythrocytes of these monkeys and produce infections in vivo. Recently, using a laboratory genetic cross between the GB4 and 7G8 P. falciparum clones from Ghana and Brazil, we identified an I→K substitution at position 204 (I204K) of PfRH5 as an important GB4 determinant for the invasion of A. nancymaae red blood cells [23]. Further, in contrast to the finding that engineered absence of all glycans from human basigin did not alter PfRH5 binding [37], removal (by neuraminidase) of sialic acids from the Aotus erythrocytes abrogated the binding of I204K-containing PfRH5 as well as invasion of the GB4 parent and I204K-containing progeny [23]. Certain other P. falciparum parasites that carry PfRH5 polymorphisms different from I204K are also able to infect A. nancymaae [23–25, 41]. Here, we report on investigations of these PfRH5 polymorphisms and interactions that support the invasion of various primate and rodent erythrocytes by P. falciparum.
2. Materials and methods
2.1. Parasites
The 7G8 P. falciparum clone, 7G8 × GB4 progeny clone LC12, and their transformants containing substitutions at PfRH5 positions 204 and 408, specifically 7G8204I/407I (7G8II control transformant), 7G8204K/407V (7G8KV), 7G8204I/407V (7G8IV), LC12204K/407V (LC12KV control transformant), LC12204I/407I (LC12II) and LC12204K/407I (LC12KI) have been previously described [23]. The original 7G8 clone [42] does not invade A. nancymaae erythrocytes and is not infective to A. nancymaae owl monkeys, whereas the LC12 parasite progeny clone invades A. nancymaae erythrocytes and is highly virulent to owl monkeys [23]. P. falciparum parasites were cultivated using standard techniques [43, 44]. Donor O+ human blood was obtained from the Interstate Blood Bank (Memphis, TN).
2.2. Plasmid constructs
Using strategies similar to those for generation of the 7G8204I/407I, 7G8204K/407V, 7G8204I/407V, LC12204K/407V, LC12204I/407I, LC12204K/407I transformed lines [23], six constructs were designed to generate additional codon changes in the endogenous PfRH5 gene of the 7G8 clone (Table 1). For this purpose, 1396-bp fragments of PfRH5 were amplified by PCR from the genomic DNA of P. falciparum Dd2, Malayan Camp (MalCamp), Santa Lucia, and FVO parasites. Primers 5′ (TGCGGCCGCATGAAGACTATAAAAATGTGG) and 3′(ACTGCAGATGCTTTGTCTAATTAGAG) provided a NotI or PstI site, respectively, for cloning into the pHD22Y vector [45]. The amplified fragments were incorporated into plasmids pHD22Y-rh5Dd2, pHD22Y-rh5MalCamp, pHD22Y-rh5SantaLucia, and pHD22Y-rh5FVO. A fifth construct, pHD22Y-rh5429N, was generated using the site-directed mutagenesis kit QuikChange II, according to the manufacturer’s instructions (Stratagene, La Jolla, CA).
Table 1.
PfRH5 polymorphisms and invasion of native and transformed P. falciparum 7G8 and LC12 lines
| PfRH5 polymorphisms | Erythrocyte invasion rate (% ± S.E.)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasite lineb | Transfection plasmid | 204 | 347 | 358 | 362 | 407 | 410 | 429 | A. nancymaae | Rat | Mouse |
| 7G8 | — | I | N | Y | E | I | I | K | 0.4 ± 0.2c | 0.2 ± 0.2 | N.D.d |
| LC12 | — | K | N | Y | E | V | I | K | 55.4 ± 4.2c | 25.7 ± 2.9 | N.D. |
| 7G8204I/407I | pHD22Y-rh57G8 | I | N | Y | E | I | I | K | 2.1 ± 0.4 | 0.2 ± 0.2 | 58.4 ± 29.4 |
| 7G8204K/407V | pHD22Y-rh5LC12 | K | N | Y | E | V | I | K | 38.7 ± 7.6 | 23.5 ± 2.3 | 57.8 ± 9.9 |
| 7G8204I/407V | pHD22Y-rh5LC12 | I | N | Y | E | V | I | K | 4.9 ± 3.0 | 0.0 ± 0.0 | N.D. |
| LC12204K/407V | pHD22Y-rh5LC12 | K | N | Y | E | V | I | K | 43.2 ± 7.3 | 20.5 ± 3.6 | 52.0 ± 2.9 |
| LC12204I/407I | pHD22Y-rh57G8 | I | N | Y | E | I | I | K | 4.6 ± 1.0 | 0.1 ± 2.9 | 48.5 ± 4.5 |
| LC12204K/407I | pHD22Y-rh57G8 | K | N | Y | E | I | I | K | 34.6 ± 5.4 | 20.4 ± 2.7 | N.D. |
| 7G8204R | pHD22Y-rh5PaloAlto | R | N | Y | E | I | I | K | 29.4 ± 6.6 | 26.7 ± 4.0 | N.D. |
| 7G8347D | pHD22Y-rh5SantaLucia | I | D | Y | E | I | I | K | 16.8 ± 2.6 | 0.0 ± 0.0 | N.D. |
| 7G8347Y/429N | pHD22Y-rh5MalCamp | I | Y | Y | E | I | I | N | 26.8 ± 2.0 | 0.5 ± 0.5 | 53.8 ± 10.3 |
| 7G8358F/362D | pHD22Y-rh5FVO | I | N | F | D | I | I | K | 71.6 ± 5.7 | 0.0 ± 0.0 | 22.8 ± 0.5 |
| 7G8410M | pHD22Y-rh5Dd2 | I | N | Y | E | I | M | K | 2.0 ± 0.2 | 0.0 ± 0.0 | N.D. |
| 7G8429N | pHD22Y-rh5N429 | I | N | Y | E | I | I | N | 2.2 ± 1.4 | 0.0 ± 0.0 | 41.9 ± 5.3 |
Relative to rates of invasion into human erythrocytes.
Parasite transfectants 7G8204I/407I, 7G8204K/407V, 7G8204I/407V, LC12204K/407V, LC12204I/407I and LC12204K/407I previously reported as 7G8II, 7G8KV, 7G8IV, LC12KV, LC12II and LC12KI, respectively [23].
As reported in [23].
N.D., not determined.
During the course of our experiments, we noted that some plasmids consistently integrated into the target chromosome by single crossover events toward the 3′ of the 7G8 RH5 gene, so that transfectants expressing all of the desired codon changes were difficult to obtain. This proved to be a particular problem for the case of the Palo Alto I204R polymorphism [23]. We therefore designed a sixth construct to express the amino acid sequence of the Palo Alto parasite but with codon adjustments, so that all codons downstream of I204R were with the nucleotide preferences of Escherichia coli instead of P. falciparum (pHD22Y-rh5PaloAlto; GENEART, Toronto, Canada). Because the AT-rich PfRH5 3′ flanking sequence in the original design proved difficult to synthesize, this region of pHD22Y-rh5PaloAlto sequence was also modified so that it contains the 3′ flanking region of Plasmodium berghei dihydrofolate reductase-thymidilate synthase [46]. The resulting synthetic I204R PfRH5 sequence, along with the dihydrofolate reductase-thymidilate synthase terminator, provided a 1.7-kb fragment that was cloned into pHD22Y using the NotI and PstI sites. All other constructs in this study utilized the PfRH5 endogenous 3′ flanking region. The plasmid constructs were confirmed by DNA sequencing.
2.3. Parasite transfections
Uninfected human erythrocytes were loaded with purified plasmid DNA by electroporation and mixed with mature-stage 7G8 parasites as described [47]. After 2 days, 5 nM WR99210 was applied to the cultures to select parasites carrying the transfected plasmids. After ~60 days of WR99210 pressure, homologous integration into endogenous PfRH5 was detected by PCR. The transformed parasites were cryopreserved, confirmed by Southern blotting, and the presence of the desired codon polymorphisms in the allelic exchanges was verified by polymerase chain reaction (PCR) amplification and DNA sequencing.
2.4. Invasion assays
Mature-stage parasites were purified using the percoll/sorbitol method [48], washed three times, and adjusted to a final concentration of 1.0–1.5 × 107 parasitized erythrocytes/ml. Parasite suspension (100 µl) was added to 100 µl of target erythrocytes at 2 × 108 cells/ml in complete medium in a flat-bottomed 96-well plate, for an introduced parasitemia of approximately 5.0–7.5% [30]. The parasite suspension was also added to wells containing human or rhesus (Macaca mulatta) erythrocytes, which were included as positive and negative controls, respectively. Plates were incubated at 37°C for 18–24 h. The number of ring forms in at least 1,000 erythrocytes was counted for each determination. The invasion rate was calculated as a percentage from the observed frequency of ring-stage parasites in target cells relative to the observed frequency in control human erythrocytes. Any inoculation of erythrocytes from the purified parasite suspension was corrected by subtraction of the frequency of ring-stage parasites in control wells of rhesus erythrocytes, since rhesus erythrocytes are completely resistant to P. falciparum invasion and ring-stages can develop only in the inoculated cells [49, 50]. Each assay was performed in triplicate on at least three independent occasions. Blood from donor animals was obtained in compliance with National Institutes of Health guidelines under relevant Animal Care and Use Committee-approved protocols.
2.5. Sialic acid removal from erythrocytes by neuraminidase treatment
For the removal of erythrocyte surface sialic acids, 1 × 108 cells/ml were treated with 0.025 U/ml of Arthrobacter ureafaciens α2-3,6,8,9 neuraminidase (EMD Biosciences, Inc, San Diego, CA) in RPMI at 37°C for 1 h with gentle agitation and washed three times before use. For determination of sialic acid content, 1 × 108 erythrocytes were washed three times in phosphate buffered saline (PBS; 10 mM phosphate, 140 mM NaCl, pH 7.4), lysed in 400 µl of 5-mM KH2PO4 containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and incubated on ice for 10 min. After centrifugation at 10,000×g for 10 min at 4°C, the erythrocyte ghosts were gently removed and washed three times with PBS containing protease inhibitor cocktail. The ghost membranes were treated with 0.25 U/ml Ar. ureafaciens neuraminidase in PBS at 37°C for 1 h with gentle agitation. Supernatants containing cleaved sialic acids were collected, and residual sialic acid remaining on the ghost membranes was hydrolyzed by acid treatment (2 M acetic acid at 80°C for 3 h). The sialic acids released by neuraminidase treatment alone or by acid hydrolysis post neuraminidase treatment were derivatized with 1,2-diamino-4,5-methylene dioxybenzene (DMB) and analyzed by reverse-phase fluorometric high-performance liquid chromatography (HPLC) at the Glycotechnology Core Resource at the University of California, San Diego as described [51].
3. Results
3.1. Various PfRH5 polymorphisms can support sialic acid-dependent parasite invasion of A. nancymaae erythrocytes
PfRH5 codon changes introduced into the 7G8 and LC12 lines allowed us to explore the effects of various amino acid polymorphisms on the ability of P. falciparum to invade monkey and rodent erythrocytes (Table 1). The 7G8 and LC12 parasites from the 7G8×GB4 cross and six transformants encoding polymorphisms at PfRH5 positions 204 and 407 (7G8204I/407I, 7G8204K/407V, 7G8204I/407V, LC12204K/407V, LC12204I/407I, LC12204K/407I) have been described [23]. For the present study, we generated an additional six transformants of the 7G8 parasite by allelic exchange (Fig. 1). These included five transformants containing polymorphisms from four P. falciparum lines that efficiently invade A. nancymaae erythrocytes: PfRH5 204R from the Palo Alto line; 347D from the Santa Lucia line; 347Y and 429N from the Malayan Camp line; 358F and 362D from the FVO line; and 429N alone from the Malayan Camp line (transformants identified as 7G8204R, 7G8347D, 7G8347Y/429N, 7G8358F/362D, and 7G8429N in Table 1). A sixth transformant, 7G8410M, was also designed with the PfRH5 410M polymorphism of the Dd2 parasite that, like 7G8, is unable to invade Aotus erythrocytes [23]. All plasmids for these exchanges were constructed with the native PfRH5 nucleotide sequence except 7G8204R, which required a plasmid construct carrying a codon-adjusted open reading frame with reduced homology downstream of codon 204.
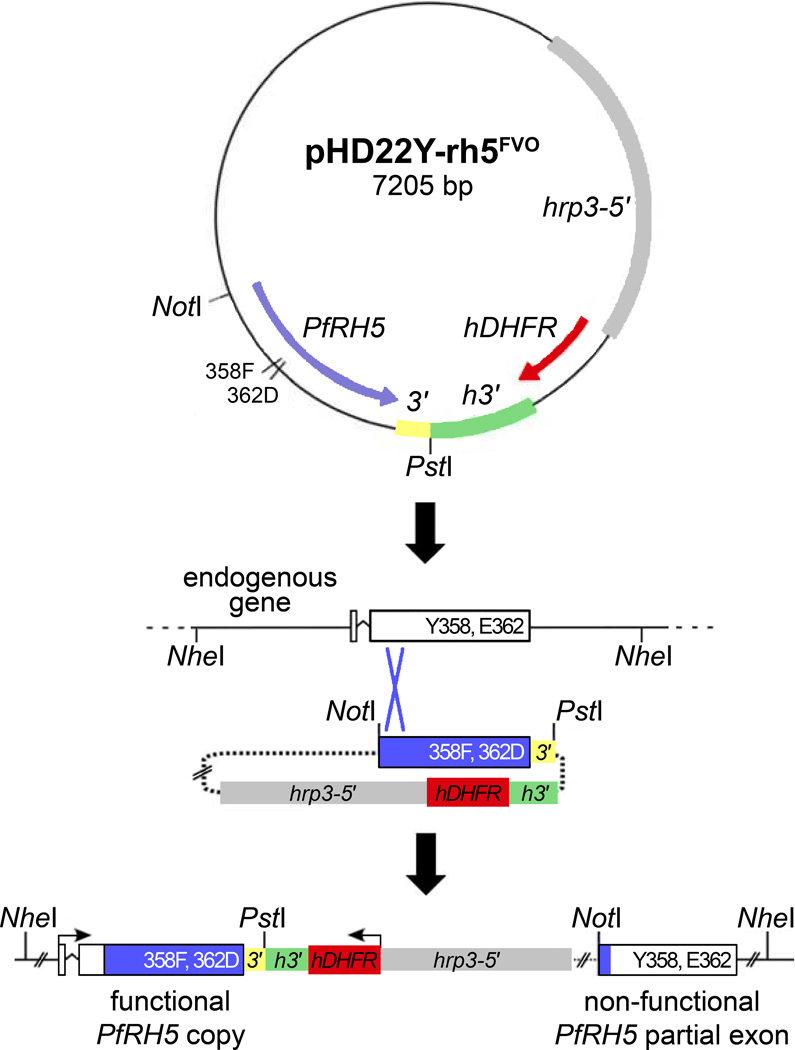
Fig 1.
Illustration of the strategy to generate PfRH5-modified 7G8 parasites by homologous recombination. A 1396-bp fragment of PfRH5 containing codon polymorphisms of the FVO parasite was PCR-amplified and cloned into the pHD22Y vector [45]. Following transfection of 7G8 parasites with the desired construct, a portion of the FVO PfRH5 gene along with the 0.2 kb 3′ flanking sequence spontaneously recombined by single-site crossover and replaced the endogenous 7G8 PfRH5 sequence with a modified allele. The remainder of the construct, including the hDHFR drug selection marker, and a remnant of the endogenous 7G8 PfRH5 sequence lie downstream of the modified PfRH5 allele. Similar strategies were used for the other constructs employed in this study. 3′, 3′ terminal flanking region of PfRH5; hrp3-5′, promoter region from P. falciparum histidine-rich protein 3; hDHFR, human dihydrofolate reductase coding segment; h3′3′ regulatory region of P. falciparum histidine-rich protein 2.
Relative invasion rates into A. nancymaae erythrocytes were determined for each of the transformed 7G8 parasite lines (Table 1). Transformants expressing PfRH5 alleles with the 204R codon of Palo Alto, the 347D codon of Santa Lucia, the 347Y/429N codons of Malayan Camp (MalCamp), or the 358F/362D codons of FVO had gained the ability to invade A. nancymaae erythrocytes. The 7G8 transformant expressing 358F and 362D codons (FVO allele) exhibited the highest A. nancymaae erythrocyte invasion rate (71.6 ± 5.7%), whereas the 204R (Palo Alto), 347D (Santa Lucia) and 347Y/429N (Malayan Camp) transformants invaded at lower rates of 29.4 ± 6.6%, 16.8 ± 2.6% and 26.8 ± 2.0%, respectively. These rates compare to 38.7 ± 7.6% for the previously reported 7G8204K/407V transformant and to 55.4± 4.2%, 43.2 ± 7.3% and 34.6 ± 5.4% for the LC12 progeny clone and its LC12204K/407V and LC12204K/407I transformants, all of which contain PfRH5 204K (Table 1). The replacement of 204K by 204I in the LC12204I/407I transformant was associated with dramatically reduced invasion of A. nancymaae erythrocytes, as expected (4.6 ± 1.0%; Table 1).
In contrast to the A. nancymaae erythrocyte invasion rate of 26.8 ± 2.0% for the 7G8347Y/429N transformant, little or no increased invasion was observed for the 7G8429N line containing the single PfRH5 429N polymorphism (2.2 ± 1.4% vs. 0.4 ± 0.2% for the untransformed 7G8 line or 2.1 ± 0.4% for control 204I/407I-transformed 7G8 parasites; Table 1). These data suggest that the PfRH5 347Y amino acid substitution is a key determinant of A. nancymaae invasion by 7G8 transformants expressing the Malayan Camp allele. Also, the 347Y amino acid change occurs at exactly the same position as the 347D substitution that enables invasion of the Santa Lucia-transformed parasites. 7G8 transformants expressing the 410M polymorphism of the noninfective Dd2 parasite showed no substantial change from the noninvasive phenotype of untransformed 7G8 or control 204I/407I-transformed-7G8 parasites (2.0 ± 0.2% vs. 0.4 ± 0.2% or 2.1 ± 0.4%, respectively; Table 1).
In previous work with neuraminidase-treated cells, we found that the I204K polymorphism was linked to sialic acid-sensitive binding of PfRH5 at the surface of A. nancymaae as well as human erythrocytes [23]; neuraminidase treatment also abrogated the invasion of A. nancymaae (but not human) erythrocytes by the LC12 and I204K-transformed 7G8 parasites. To test the sialic acid dependence of additional PfRH5 polymorphisms listed in Table 1, we compared the invasion rates of the corresponding 7G8 transformants into A. nancymaae erythrocytes treated with neuraminidase. Results (Fig. 2) showed that the neuraminidase-treated A. nancymaae erythrocytes were in all cases resistant to the otherwise invasive transformants: 7G8204R, 7G8347D, 7G8347Y/429N, and 7G8358F/362D.
Fig. 2.
Relative invasion rates of PfRH5 allele-modified P. falciparum lines into A. nancymaae erythrocytes and neuraminidase-treated A. nancymaae erythrocytes. AnRBC, A. nancymaae erythrocytes; HsRBC, human erythrocytes; Neu, neuraminidase.
3.2. A positive charge mutation at PfRH5 position 204 supports parasite invasion of rat erythrocytes
Previous studies noted that Malayan Camp but not 7G8 parasites could invade rat as well as A. nancymaae erythrocytes [50]. To investigate whether an ability to invade rat erythrocytes was PfRH5-dependent, we tested the invasion of rat erythrocytes by the 7G8 transformants listed in Table 1. Of the A. nancymaae-invasive transformants, only LC12 and 7G8 transformants that expressed a PfRH5 allele encoding 204K or 204R invaded rat erythrocytes (invasion rates of 23.5 ± 2.3% for 7G8204K/407V, 20.5 ± 3.6% for LC12204K/407V, 20.4 ± 2.7% for LC12204K/407I, and 26.7 ± 4.0% for 7G8204R). The other A. nancymaae-invasive transformants that retained 204I and expressed PfRH5 polymorphisms at different positions did not invade rat erythrocytes, including the highly A. nancymaae-invasive 7G8358F/362D (FVO) transformant. A potential paradox was that the 7G8347Y/427N (Malayan Camp) transformant was among our lines that did not invade rat erythrocytes, in contrast to the previous report that Malayan Camp parasites could invade rat erythrocytes [50]. We therefore confirmed the PfRH5 sequence of our untransformed Malayan Camp parasites, tested their invasion rates and found that their invasion of rat erythrocytes was negligible (1.7 ± 0.3%). These data suggest that our Malayan Camp parasites and their PfRH5 sequence are not the same as those of Klotz et al. [50], perhaps because of a change during the long laboratory history of Malayan Camp and its use for various experimental purposes including Aotus monkey challenges.
We tested whether the invasion rates of rat erythrocytes are sialic acid dependent. Various 7G8 transformants containing 204K or 204R were assayed with rat erythrocytes that had been treated with neuraminidase. The 204K- or 204R-containing transformants showed different degrees of sialic acid sensitivity, invading neuraminidase-treated rat erythrocytes with invasion rates of 19.1 ± 5.4% for 7G8204K/407V, 13.8 ± 2.4% for LC12204K/407V, 4.5 ± 1.0% for 7G8204R and 16.3 ± 0.6% for LC12204K/407I (Fig. 3). Compared to the rates of invasion into untreated rat erythrocytes, these results demonstrate greater dependence of the 204R-containing than 204K-containing PfRH5 transformants on the presence of rat erythrocyte sialic acid (Fig. 3).
Fig. 3.
Relative invasion rates of PfRH5 allele-modified P. falciparum lines into rat erythrocytes and neuraminidase-treated rat erythrocytes. RnRBC, rat (Rattus norvegicus) erythrocytes; HsRBC, human erythrocytes; Neu, neuraminidase.
3.3. PfRH5-transformed 7G8 and LC12 parasites exhibit sialic acid-sensitive invasion of mouse erythrocytes
The 7G8 and Malayan Camp parasites were previously reported to invade mouse erythrocytes [50]. In our evaluations of the 7G8 and LC12 transformants, no lines failed to invade normal mouse erythrocytes; invasion rates ranged from 22.8 ± 0.5% for the 7G8358F/362D transformant to 58.4 ± 29.4% for 7G8204I/407I (Table 1). Neuraminidase treatment of the mouse cells greatly decreased invasion rates and eliminated invasion by the 7G8358F/362D transformant (Fig. 4).
Fig. 4.
Relative invasion rates of PfRH5 allele-modified P. falciparum lines into mouse erythrocytes and neuraminidase-treated mouse erythrocytes. MmRBC, mouse (Mus musculus) erythrocytes; HsRBC, human erythrocytes; Neu, neuraminidase.
3.4. A. nancymaae human, rat and mouse erythrocytes release sialic acid to different degrees upon neuraminidase treatment
Sialic acids are N- or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone, and are typically found as the outermost units of cell surface and secreted glycans such as the glycophorins. Substitutions at the 4, 5, 7, 8, and 9-carbon positions, with different α-glycosidic linkages at the 2-carbon position, result in over 50 variations [52, 53]. These sialic acids constitute the majority of the negative charge in a glycocalyx at the erythrocyte surface, supplying a repelling force that helps to prevent adherence between erythrocytes and of erythrocytes to blood vessels, and they also provide important surface attachments and entry functions for a variety of host-pathogen interactions [54]. In addition to the role of sialylated erythrocyte surface receptors recognized by P. falciparum merozoites [12, 14, 23, 33, 38], examples include the binding of influenza hemagglutinin to host cells and of Helicobacter pylori hemagglutinin to gastric mucins [55, 56].
In view of the various degrees of complete or partial reduction of invasion into neuraminidase-treated A. nancymaae, mouse or rat erythrocytes by the PfRH5-transformed parasites, we investigated the extent of sialic acid removal from erythrocyte membranes by neuraminidase cleavage. A. nancymaae, human, rat and mouse erythrocyte ghosts were treated with neuraminidase and the released sialic acids were collected, DMB-derivatized and quantitatively analyzed by HPLC. After neuraminidase treatment, the erythrocyte ghost membranes were subjected to acid hydrolysis to release residual uncleaved sialic acids, which were also collected, DMB-derivatized and analyzed by HPLC. As expected, the large proportion of sialic acid released from human and A. nancymaae erythrocytes was 5-N-acetlyneuraminic acid (Neu5Ac) [51]: only 10–20% of this sialic acid remained on the human and A. nancymaae membranes after neuraminidase treatment (Table 2). Neu5Ac release from rat and mouse erythrocytes was less efficient: 25–35% remained on the membranes after neuraminidase treatment (Table 2).
Table 2.
Sialic acid release from A. nancymaae, human, rat, and mouse erythrocyte membranes
| Erythrocyte membrane sample |
Sialic acid release stepa | Neu5Ac (pmol) |
% remaining after neuraminidase treatment |
Neu5Gc (pmol) |
% remaining after neuraminidase treatment |
|---|---|---|---|---|---|
| A. nancymaae | Neuraminidase treatment Residual release by acid treatment |
2876.2 584.4 |
16.9% | 0.5 4.3 |
N.D.b |
| Human | Neuraminidase treatment Residual release by acid treatment |
2599.3 327.4 |
11.2% | 0.8 0.9 |
N.D. |
| Rat | Neuraminidase treatment Residual release by acid treatment |
768.9 374.9 |
32.8% | 16.8 32.2 |
65.7% |
| Mouse | Neuraminidase treatment Residual release by acid treatment |
664.6 243.9 |
26.8% | 40.5 79.7 |
66.3% |
Residual sialic acids remaining on the membranes after Arthrobacter ureafaciensneuraminidase treatment were released by exposure to 2 M acetic acid at 80°C for 3 h.
N.D., not determined because of low signal levels. A. nancymaae and humans carry a mutated, non-functional DMPNeu5Ac hydroxylase gene that is incapable of Neu5Gc synthesis; the small amounts of Neu5Gc on the erythrocytes of these species probably originate from dietary sources [51].
5-N-glycolylyneuraminic acid (Neu5Gc) was released from rat and mouse erythrocyte membranes in smaller amounts and was less sensitive to neuraminidase treatment than Neu5Ac (65–70% remaining after treatment; Table 2). Very low levels of Neu5Gc were obtained from A. nancymaae and human erythrocytes as these species carry a mutated, non-functional DMP-Neu5Ac hydroxylase gene that is incapable of Neu5Gc synthesis; the small amounts of Neu5Gc on the erythrocytes of these species probably originate from dietary sources [51].
4. Discussion
Polymorphisms of PfRH5 can confer different erythrocyte invasion abilities to P. falciparum parasites. In the present and previous studies [23], we have shown that the inability of 7G8 parasites to invade A. nancymaae erythrocytes can be transformed to a highly invasive phenotype by the introduction of polymorphisms from P. falciparum lines such as Santa Lucia (204R), GB4 (204K/407V), Palo Alto (347D), Malayan Camp (347Y/429N), or FVO (358F/362D). Further, PfRH5 polymorphisms 204K and 204R enable the 7G8 transformants to invade rat erythrocytes whereas the other polymorphisms fail to promote the invasion of rat erythrocytes at all. A positive charge at PfRH5 position 204 may therefore serve as an important enabler for the invasion of rat erythrocytes by 7G8 transformants. All transformants showed little or no ability to invade neuraminidase-treated A. nancymaae erythrocytes, suggesting that the 204K, 204R, 347D, 347Y/429N, and 358F/362D polymorphisms may support the binding of PfRH5 to neuraminidaseremovable sialic acid residues on proteins at the A. nancymaae erythrocyte surface.
Neuraminidase treatment of rat erythrocytes also reduced the invasion rates of 7G8204K and 7G8204R PfRH5 transformants but to different extents, producing a particularly strong effect on the invasion ability of the 7G8204R transformant. It is not yet clear how these different levels of reduction relate to incomplete removal of Neu5Ac and Neu5Gc by the action of neuraminidase. Neuraminidase stripped less Neu5Ac from rat than from A. nancymaae erythrocytes (~67% vs. 83% removed; Table 3), and rat erythrocytes carried levels of Neu5Gc that A. nancymaae could not synthesize. One possibility is that the Neu5Ac and Neu5Gc residues remaining after neuraminidase treatment were sufficient to support the observed levels of 7G8204K and 7G8204R invasion. In this scenario, lower invasion of neuraminidase-treated rat erythrocytes by the 7G8204R vs. 7G8204K transformants might have resulted from differential ability of the modified PfRH5 molecules to bind residual sialic acids on the erythrocyte surface. A similar explanation might account for the reduced invasion rates of transformed 7G8 and LC12 lines into neuraminidase-treated mouse erythrocytes, including complete inability of 7G8358F/362D parasites to invade the treated mouse erythrocytes.
In previous work (ref [23] Fig. S3), we showed that the 204K form of PfRH5 expressed by LC12 parasites did not bind to neuraminidase-treated human cells in erythrocyte binding assays, even though these parasites were able to invade the neuraminidase-treated human erythrocytes at 60–70% of the rate they invaded untreated human erythrocytes. In comparison, the 7G8 form of PfRH5, which contains no 204K mutation, showed efficient binding of PfRH5 to the neuraminidase-treated erythrocytes and a 60–70% relative invasion rate similar to LC12. These findings are consistent with the observation that neuraminidase treatment of recombinant basigin or obliteration of its glycosylation motifs does not affect binding to PfRH5 from 3D7 parasites [37] (PfRH5 from 3D7 and 7G8 both contain the 204I residue [23]). Although the mutant 204K or 204R forms of PfRH5 expressed by LC12 and other A. nancymaae-invasive parasites remain to be tested for basigin binding ability, unsuccessful efforts in two laboratories to knock-out the PfRH5 gene from P. falciparum [23, 37], and the ability of LC12 to invade neuraminidase-treated human erythrocytes despite loss of PfRH5 binding in erythrocyte binding assays, support suggestions that the PfRH5-PfRipr complex may serve in an important role apart from or in addition to a mechanical attachment function [40, 57].
The PfRH5 polymorphisms reported here demonstrate the flexibility of P. falciparum parasites to invade the erythrocytes of primate and rodent species that present different surface receptors. Interestingly, a search of SNPs and alleles available in the MalariaGEN database [58] did not show prevalence in naturally-infected human populations of any of the Aotus-competent PfRH5 polymorphisms identified here (204K, 204R, 347D, 347Y, 358F or 362D). Other PfRH5 polymorphisms were instead reported from deep sequencing studies of P. falciparum samples from 227 patients in Africa, Asia and Oceania, including N88D, Y147H, H148D, S197Y, C203Y, A233E, I364I, H365N, V371I, I407V, I410M, Q477H AND I493V (http://www.malariagen.net/data) [58]. The specificity of particular PfRH5 polymorphisms to Aotus-infective parasites suggests that these polymorphisms were selected during adaptation of P. falciparum isolates to the monkeys. Deep sequencing data from additional surveys may be useful in further searches for the presence of Aotus-competent PfRH5 polymorphisms in human parasite populations. PfRH5 polymorphisms may also differ among the P. falciparum-like parasites that occur in apes [59–62]; indeed, a 204K polymorphism is present in the PrRH5 sequence of a single isolate of the chimpanzee malaria parasite P. reichenowi [23].
Interactions of PfRH5, PfRipr and basigin are thought to be essential to human erythrocyte invasion [37, 40]. The polymorphisms of PfRH5 at amino acid positions 204, 347 and 358/362 occur within a region proposed to recognition domain that binds erythrocytes [23, 25], and this region shares conserved features with the sialic acid-dependent binding region of PfRH1 as well as the sialic acid-independent binding region of PfRH4 [39]. PfRH1 binding may involve α-helices in coiled coils so that, following release from the rhoptries, they project out from the merozoite surface to interact with the red blood cell surface [38, 41]. Further characterization of the structure of PfRH5, the features of its binding to human and non-human receptors, and the functional roles of its polymorphisms will advance our understanding of the events that underlie erythrocyte invasion and perhaps inform new strategies for molecular approaches against malaria.
Research Highlights.
Only some P. falciparum (Pf) lines are able to infect Aotus nancymaae (owl) monkeys
Various PfRH5 alleles in these lines support infection of monkey erythrocytes (RBC)
Non-invasive Pf parasites (7G8) become invasive when transformed by these alleles
Invasion of owl monkey RBC by the 7G8 transformants is sialic acid-dependent
Rat RBC can be invaded by 7G8 transformants expressing PfRH5 I204K or I204R
Acknowledgements
We thank Lynn Lambert, Angela Lunger, and Sachy Orr-Gonzalez for the provision of blood samples; Osamu Kaneko for helpful discussions; and NIAID intramural editor Brenda Marshall for assistance. This research was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- EBA
erythrocyte binding antigen
- EBL
erythrocyte binding-like
- RBL
reticulocyte binding-like
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34(13–14):1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124(4):755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa M, Miller LH, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 5.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011;9(1):9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Bannister LH, Mitchell GH. The malaria merozoite, forty years on. Parasitology. 2009;136(12):1435–1444. doi: 10.1017/S0031182009990734. [DOI] [PubMed] [Google Scholar]
- 7.Baum J, Papenfuss AT, Baum B, Speed TP, Cowman AF. Regulation of apicomplexan actin-based motility. Nat Rev Microbiol. 2006;4(8):621–628. doi: 10.1038/nrmicro1465. [DOI] [PubMed] [Google Scholar]
- 8.Iyer J, Gruner AC, Renia L, Snounou G, Preiser PR. Invasion of host cells by malaria parasites: a tale of two protein families. Mol Microbiol. 2007;65(2):231–249. doi: 10.1111/j.1365-2958.2007.05791.x. [DOI] [PubMed] [Google Scholar]
- 9.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230(4725):553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 10.Mayer DC, Kaneko O, Hudson-Taylor DE, Reid ME, Miller LH. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A. 2001;98(9):5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JK, Triglia T, Reed MB, Cowman AF. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Microbiol. 2001;41(1):47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 12.Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA, et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9(1):87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem. 2003;278(16):14480–14486. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- 14.Mayer DC, Mu JB, Kaneko O, Duan J, Su XZ, Miller LH. Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc Natl Acad Sci U S A. 2004;101(8):2518–2523. doi: 10.1073/pnas.0307318101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson DS, Wellems TE. EBL-1, a putative erythrocyte binding protein of Plasmodium falciparum, maps within a favored linkage group in two genetic crosses. Mol Biochem Parasitol. 2000;105(1):105–113. doi: 10.1016/s0166-6851(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 16.Mayer DC, Cofie J, Jiang L, Hartl DL, Tracy E, Kabat J, et al. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc Natl Acad Sci U S A. 2009;106(13):5348–5352. doi: 10.1073/pnas.0900878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayner JC, Vargas-Serrato E, Huber CS, Galinski MR, Barnwell JW. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med. 2001;194(11):1571–1581. doi: 10.1084/jem.194.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol. 2005;55(1):162–174. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 19.Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci U S A. 2000;97(17):9648–9653. doi: 10.1073/pnas.160469097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triglia T, Thompson J, Caruana SR, Delorenzi M, Speed T, Cowman AF. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun. 2001;69(2):1084–1092. doi: 10.1128/IAI.69.2.1084-1092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duraisingh MT, Triglia T, Ralph SA, Rayner JC, Barnwell JW, McFadden GI, et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22(5):1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko O, Mu J, Tsuboi T, Su X, Torii M. Gene structure and expression of a Plasmodium falciparum 220-kDa protein homologous to the Plasmodium vivax reticulocyte binding proteins. Mol Biochem Parasitol. 2002;121(2):275–278. doi: 10.1016/s0166-6851(02)00042-7. [DOI] [PubMed] [Google Scholar]
- 23.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4(1):40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez M, Lustigman S, Montero E, Oksov Y, Lobo CA. PfRH5: a novel reticulocyte-binding family homolog of plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS One. 2008;3(10):e3300. doi: 10.1371/journal.pone.0003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, et al. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum . Int J Parasitol. 2009;39(3):371–380. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69(7):1213–1226. doi: 10.1016/0092-8674(92)90642-p. [DOI] [PubMed] [Google Scholar]
- 27.Iyer JK, Amaladoss A, Genesan S, Preiser PR. Variable expression of the 235 kDa rhoptry protein of Plasmodium yoelii mediate host cell adaptation and immune evasion. Mol Microbiol. 2007;65(2):333–346. doi: 10.1111/j.1365-2958.2007.05786.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell GH, Hadley TJ, McGinniss MH, Klotz FW, Miller LH. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood. 1986;67:1519–1521. [PubMed] [Google Scholar]
- 29.Hadley TJ, Klotz FW, Pasvol G, Haynes JD, McGinniss MH, Okubo Y, et al. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest. 1987;80(4):1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan SA, Miller LH, Wellems TE. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990;86(2):618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopaticki S, Maier AG, Thompson J, Wilson DW, Tham WH, Triglia T, et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun. 2011;79(3):1107–1117. doi: 10.1128/IAI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264(5167):1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 33.Tolia NH, Enemark EJ, Sim BK, Joshua-Tor L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell. 2005;122(2):183–193. doi: 10.1016/j.cell.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 34.Mayer DC, Jiang L, Achur RN, Kakizaki I, Gowda DC, Miller LH. The glycophorin C N-linked glycan is a critical component of the ligand for the Plasmodium falciparum erythrocyte receptor BAEBL. Proc Natl Acad Sci U S A. 2006;103(7):2358–2362. doi: 10.1073/pnas.0510648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier AG, Baum J, Smith B, Conway DJ, Cowman AF. Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infect Immun. 2009;77(4):1689–1699. doi: 10.1128/IAI.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tham WH, Wilson DW, Lopaticki S, Schmidt CQ, Tetteh-Quarcoo PB, Barlow PN, et al. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A. 2010;107(40):17327–17332. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Yeo KP, Aw SS, Kuss C, Iyer JK, Genesan S, et al. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog. 2008;4(7):e1000104. doi: 10.1371/journal.ppat.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaur D, Singh S, Jiang L, Diouf A, Miller LH. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc Natl Acad Sci U S A. 2007;104(45):17789–17794. doi: 10.1073/pnas.0708772104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Lopaticki S, Riglar DT, Dekiwadia C, Uboldi AD, Tham WH, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7(9):e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayner JC. The merozoite has landed: reticulocyte-binding-like ligands and the specificity of erythrocyte recognition. Trends Parasitol. 2009;25(3):104–106. doi: 10.1016/j.pt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg. 1984;78(3):339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- 43.Haynes JD, Diggs CL, Hines FA, Desjardins RE. Culture of human malaria parasites Plasmodium falciparum. Nature. 1976;263(5580):767–769. doi: 10.1038/263767a0. [DOI] [PubMed] [Google Scholar]
- 44.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 45.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A. 1997;94(20):10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Koning-Ward TF, Speranca MA, Waters AP, Janse CJ. Analysis of stage specificity of promoters in Plasmodium berghei using luciferase as a reporter. Mol Biochem Parasitol. 1999;100(1):141–146. doi: 10.1016/s0166-6851(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 47.Deitsch KW, Driskill CL, Wellems TE. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 2001;29(3):850–853. doi: 10.1093/nar/29.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aley SB, Sherwood JA, Howard RJ. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984;160(5):1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gysin J. Animal models: primates. In: Sherman IW, editor. Malaria: parasite biology, pathogenesis and protection. Washington, D.C.: ASM Press; 1998. pp. 419–441. [Google Scholar]
- 50.Klotz FW, Chulay JD, Daniel W, Miller LH. Invasion of mouse erythrocytes by the human malaria parasite, Plasmodium falciparum. J Exp Med. 1987;165(6):1713–1718. doi: 10.1084/jem.165.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280(6):4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 52.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87(9):851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schauer R, de Freese A, Gollub M, Iwersen M, Kelm S, Reuter G, et al. Functional and biosynthetic aspects of sialic acid diversity. Indian J Biochem Biophys. 1997;34(1–2):131–141. [PubMed] [Google Scholar]
- 54.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Aspholm M, Olfat FO, Norden J, Sonden B, Lundberg C, Sjostrom R, et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2006;2(10):e110. doi: 10.1371/journal.ppat.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wellems TE, Fairhurst RM. An evolving picture of the interactions between malaria parasites and their host erythrocytes. Cell Res. 2012;22(3):453–456. doi: 10.1038/cr.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487(7407):375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, et al. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci U S A. 2010;107(23):10561–10566. doi: 10.1073/pnas.1005435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krief S, Escalante AA, Pacheco MA, Mugisha L, Andre C, Halbwax M, et al. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos. PLoS Pathog. 2010;6(2):e1000765. doi: 10.1371/journal.ppat.1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, et al. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467(7314):420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. A plethora of Plasmodium species in wild apes: a source of human infection? Trends Parasitol. 2011;27(5):222–229. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]