Abstract
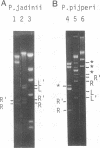
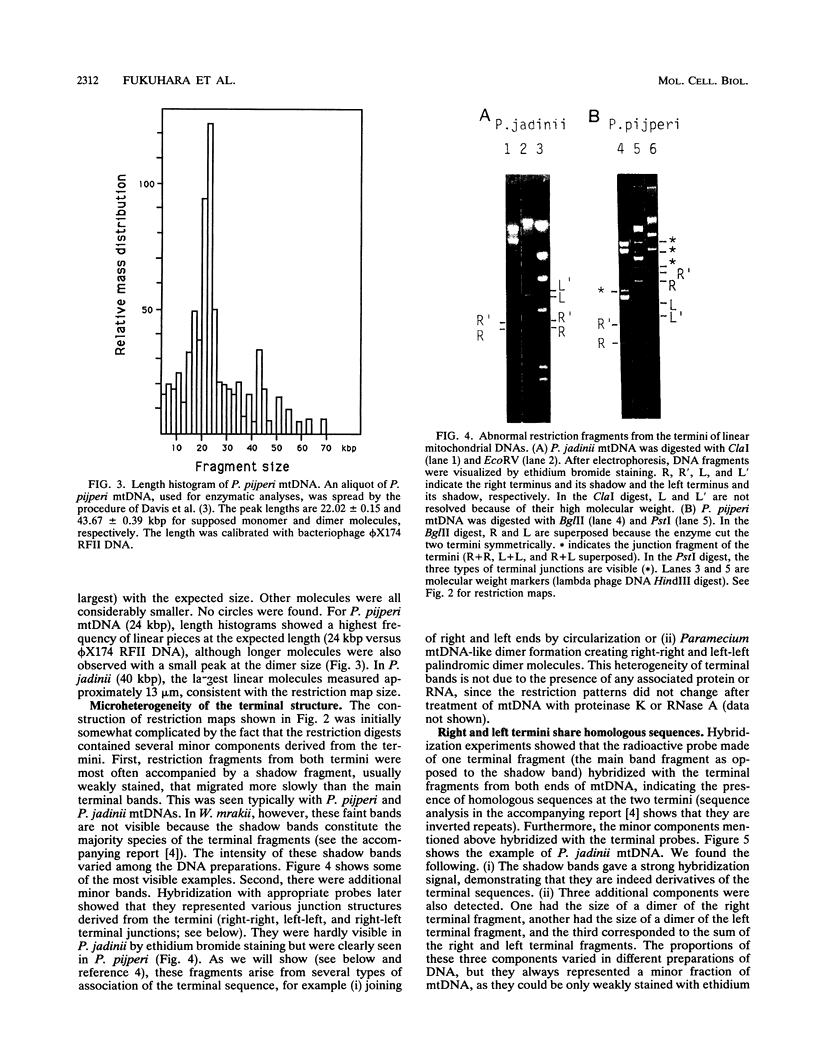
In most yeast species, the mitochondrial DNA (mtDNA) has been reported to be a circular molecule. However, two cases of linear mtDNA with specific termini have previously been described. We examined the frequency of occurrence of linear forms of mtDNA among yeasts by pulsed-field gel electrophoresis. Among the 58 species from the genera Pichia and Williopsis that we examined, linear mtDNA was found with unexpectedly high frequency. Thirteen species contained a linear mtDNA, as confirmed by restriction mapping, and labeling, and electron microscopy. The mtDNAs from Pichia pijperi, Williopsis mrakii, and P. jadinii were studied in detail. In each case, the left and right terminal fragments shared homologous sequences. Between the terminal repeats, the order of mitochondrial genes was the same in all of the linear mtDNAs examined, despite a large variation of the genome size. This constancy of gene order is in contrast with the great variation of gene arrangement in circular mitochondrial genomes of yeasts. The coding sequences determined on several genes were highly homologous to those of the circular mtDNAs, suggesting that these two forms of mtDNA are not of distant origins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camougrand N., Mila B., Velours G., Lazowska J., Guérin M. Discrimination between different groups of Candida parapsilosis by mitochondrial DNA restriction analysis. Curr Genet. 1988 May;13(5):445–449. doi: 10.1007/BF00365667. [DOI] [PubMed] [Google Scholar]
- Casey J. W., Hsu H. J., Rabinowitz M., Getz G. S., Fukuhara H. Transfer RNA genes in the mitochondrial DNA of cytoplasmic petite mutants of Saccharomyces cerevisiae. J Mol Biol. 1974 Oct 5;88(4):717–733. doi: 10.1016/0022-2836(74)90395-7. [DOI] [PubMed] [Google Scholar]
- Dinouël N., Drissi R., Miyakawa I., Sor F., Rousset S., Fukuhara H. Linear mitochondrial DNAs of yeasts: closed-loop structure of the termini and possible linear-circular conversion mechanisms. Mol Cell Biol. 1993 Apr;13(4):2315–2323. doi: 10.1128/mcb.13.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinouël N., Sor F., Fukuhara H. Nucleotide sequence of transfer RNA genes from the linear mitochondrial DNA of the yeast Williopsis mrakii and Pichia pijperi. Nucleic Acids Res. 1992 Jul 11;20(13):3509–3509. doi: 10.1093/nar/20.13.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Kovác L., Lazowska J., Slonimski P. P. A yeast with linear molecules of mitochondrial DNA. Mol Gen Genet. 1984;197(3):420–424. doi: 10.1007/BF00329938. [DOI] [PubMed] [Google Scholar]
- Kurtzman C. P. DNA relatedness among saturn-spored yeasts assigned to the genera Williopsis and Pichia. Antonie Van Leeuwenhoek. 1991 Jul;60(1):13–19. doi: 10.1007/BF00580436. [DOI] [PubMed] [Google Scholar]
- Ragnini A., Fukuhara H. Genetic instability of an oligomycin resistance mutation in yeast is associated with an amplification of a mitochondrial DNA segment. Nucleic Acids Res. 1989 Sep 12;17(17):6927–6937. doi: 10.1093/nar/17.17.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnini A., Fukuhara H. Mitochondrial DNA of the yeast Kluyveromyces: guanine-cytosine rich sequence clusters. Nucleic Acids Res. 1988 Sep 12;16(17):8433–8442. doi: 10.1093/nar/16.17.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset S., Nocentini S., Revet B., Moustacchi E. Molecular analysis by electron microscopy of the removal of psoralen-photoinduced DNA cross-links in normal and Fanconi's anemia fibroblasts. Cancer Res. 1990 Apr 15;50(8):2443–2448. [PubMed] [Google Scholar]
- Sor F., Chéret G., Fabre F., Faye G., Fukuhara H. Sequence of the HMR region on chromosome III of Saccharomyces cerevisiae. Yeast. 1992 Mar;8(3):215–222. doi: 10.1002/yea.320080307. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Analysis of chromosomal DNA patterns of the genus Kluyveromyces. Yeast. 1989 Jan-Feb;5(1):1–10. doi: 10.1002/yea.320050103. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. Linear mitochondrial deoxyribonucleic acid from the yeast Hansenula mrakii. Mol Cell Biol. 1981 May;1(5):387–393. doi: 10.1128/mcb.1.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]